Frequency of and predictors for patient-reported medical and medication errors in Switzerland
DOI: https://doi.org/10.4414/smw.2011.13262
Summary
OBJECTIVES: To analyse the frequency of and identify risk factors for patient-reported medical errors in Switzerland. The joint effect of risk factors on error-reporting probability was modelled for hypothetical patients.
METHODS: A representative population sample of Swiss citizens (n = 1306) was surveyed as part of the Commonwealth Fund’s 2010 lnternational Survey of the General Public’s Views of their Health Care System’s Performance in Eleven Countries. Data on personal background, utilisation of health care, coordination of care problems and reported errors were assessed. Logistic regression analysis was conducted to identify risk factors for patients’ reports of medical mistakes and medication errors.
RESULTS: 11.4% of participants reported at least one error in their care in the previous two years (8% medical errors, 5.3% medication errors). Poor coordination of care experiences was frequent. 7.8% experienced that test results or medical records were not available, 17.2% received conflicting information from care providers and 11.5% reported that tests were ordered although they had been done before. Age (OR = 0.98, p = 0.014), poor health (OR = 2.95, p = 0.007), utilisation of emergency care (OR = 2.45, p = 0.003), inpatient-stay (OR = 2.31, p = 0.010) and poor care coordination (OR = 5.43, p <0.001) are important predictors for reporting error. For high utilisers of care that unify multiple risk factors the probability that errors are reported rises up to p = 0.8.
CONCLUSIONS: Patient safety remains a major challenge for the Swiss health care system. Despite the health related and economic burden associated with it, the widespread experience of medical error in some subpopulations also has the potential to erode trust in the health care system as a whole.
Introduction
Adverse events and medical errors cause considerable harm. Initially, to patients but also to health care professionals involved in errors (“second victims”) [1–3]. Chart review studies of adverse events have been conducted in many European countries recently. In the Netherlands, the incidence of one or more adverse event was 5.7% of all hospital admissions of which 40% were deemed preventable [4]. In Sweden, the adverse events occurred in 12.3% of hospital admissions with 70% being judged preventable [5]. The Spanish adverse event study reported an incidence of patients with adverse events relating directly to hospital care of 8.4% and 9.3% if events from the pre-hospitalisation period were included [6]. No adverse event study using chart review methodology is currently available for Switzerland. Schwappach et al. surveyed 4000 patients in eight Swiss hospitals for adverse events during their hospital stay [7]. 21.4% reported at least one definite safety event, for example, infection or omitted drug doses. 3.2% were very concerned and 14.7% were somewhat concerned about their safety. In a recent survey of Swiss cancer patients, 16% reported having experienced an error in their care and 11% were currently very concerned about errors [8]. However, these studies concentrate on single episodes of hospital care. The accumulated risk for patients that utilise various types of health care over time, for example, emergency care, outpatient care, medication, may be even higher. For example, many patients suffer from adverse events after discharge and are therefore not identified in record based studies [9]. While there is less evidence relating to outpatient care, the available studies suggest that primary care, and in particular drug use, is associated with considerable risk [10–13].
Surveying patients about error experiences may be a particularly valuable source in order to estimate patients’ total risk. As patients are the only individuals physically present during every treatment and consultation, they carry with them important contextualised information in particular with relation to transition between different settings [14, 15]. Surveying patients about their experience of medical error across the specific types of health care consumed, e.g. hospital care, can help to identify risk factors for error along the care continuum, and relative to specific patient-level factors and the amount and type of health care utilised. These data can inform health policy about common risk factors and those populations at highest risk. The main objective of this study was an analysis of the frequency and the identification of risk factors for patient-reported medical error in Switzerland. To evaluate the joint effects of the identified risk factors, the probability that hypothetical patients with different personal and health-related profiles and health care utilisation patterns would report error in their care was modelled.
Methods
Design
This analysis is based on data from “The Commonwealth Fund's 2010 lnternational Survey of the General Public's Views of their Health Care System's Performance in Eleven Countries”, which was conducted in Australia (AUS), Canada (CAN), France (FRA), Germany (GER), the Netherlands (NETH), New Zealand (NZ), Norway (NOR), Sweden (SWE), Switzerland (SWITZ), the United Kingdom (UK), and the United States (US) in 2010. Computer assisted telephone interviews were conducted with nationally representative samples of adults age 18 and over in each of these countries. The interviews were conducted by professional interviewing staff and took on average 18–21 minutes. Response rates varied from 13% in Norway to 54% in Switzerland. In this investigation, only data obtained in Switzerland are analysed.
Survey
The 2010 International Health Policy Survey assessed public confidence in the health care system including access to care, cost and quality of care. Methods and results of earlier versions of the survey have been published previously [16–18]. For the purpose of this analysis, the following items relating to medical error experience are of particular relevance: whether respondents had ever been given the wrong medication or wrong dose by a doctor, nurse, hospital or pharmacist in the past two years (referred to as “medication error” hereinafter); whether there was a time in the past two years when the responder thought a medical mistake was made in her treatment or care (referred to as “medical error” hereinafter). Items that ask about laboratory errors (delay in communicating laboratory results; errors in laboratory tests) are not included in this analysis because we anticipated that predictors of these errors may be others than for medical and medication errors.
The response categories were yes, no, not sure and decline to answer. Participants who reported any of the above errors were also asked whether the error occurred while they were hospitalised (yes, in the hospital; no; not sure; decline to answer). Participants were also asked several questions related to demographics, their health and utilisation of health care services. Responses to three items that asked for experience of poor coordination of care in the past two years were also included in the analysis: whether subjects reported a) test results or medical records were unavailable at the time of a scheduled appointment; b) receiving conflicting information from different providers; c) doctors ordered medical tests that had already been done.
These items indicate poor quality of care and can be regarded as process errors or communicative failures though they do not immediately cause harm.
Data analysis
Raw survey data were weighted for age, sex, education and region according to the most recent national census to reflect demographic distributions. To dichotomise data for analysis, “not sure” and “decline to answer” responses were recoded to missing.
An aggregate measure was computed that captures a positive response to any of the two error items. To identify potential predictors, several demographic, health-related and heath care utilisation variables were tested for their association with error experience in bivariate analyses: Age, gender, education, income, general health status, presence of chronic conditions (out of a specified list of conditions), having a regular doctor, number of doctors seen in the past 12 months, specialist care in the past two years, elective surgery in the past two years, hospital stay in the past two years, emergency care use in the past two years, medical tests (lab, x-ray, etc.) in the past two years, and current regular use of prescription drugs. Responses to three coordination of care items were used to compute an indicator variable indicating experience of none vs. any of these three events. Variables that were significantly associated with error experience in bivariate analyses at the 0.1 level were entered into the logistic regression model. Logistic regression was conducted for the aggregate measure, i.e. report of "any medical or medication error as dependent variable. Multicollinearity of the predictor variables was assessed with the variance inflation factor (VIF). VIFs >10 were inspected and multicollinear variables were omitted from the models. Model fit was assessed using the Archer-Lemeshow goodness-of-fit statistic, a F-adjusted mean residual goodness-of-fit test under complex sampling [19]. To evaluate the joint effects of the identified risk factors, we estimated the predicted probability that hypothetical patients with different health-related profiles and health care utilisation patterns would report error in their care. Data were analysed with the software package STATA v11.2 [20].
Results
Interviews in Switzerland were completed with 1306 adults aged 18 and above.
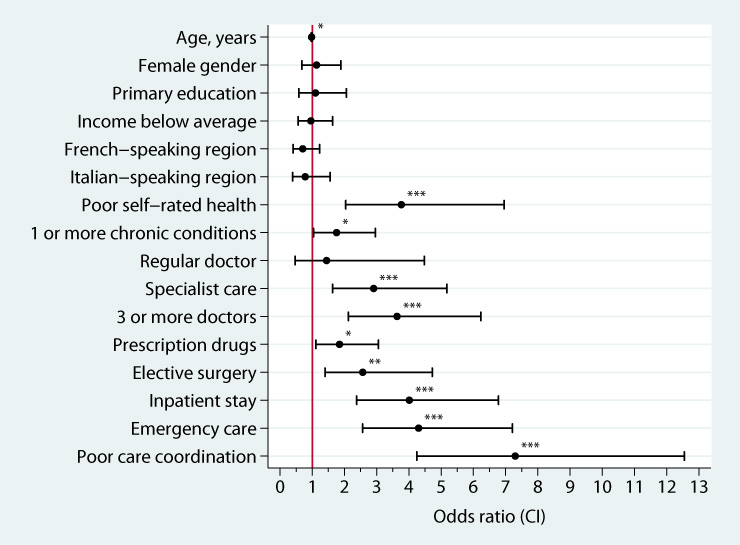
Figure 1
Bivariate associations between demographic, health and health care utilisation variables and report of any medical or medication error among Swiss respondents, weighted data.
Stars indicate significant associations (*p <0.05; **p <0.01; ***p <0.001).
Sample characteristics are provided in table 1. Poor coordination of care experiences were frequent among respondents: 7.8% experienced that test results or medical records were not available, 17.2% received conflicting information by care providers and 11.5% reported that tests were ordered though they had been done before. Nearly a quarter of citizens (24.0%) reported any one of these coordination problems in the past two years. Experience of error was also common among Swiss patients. 8% reported a medical error and 5.3% a medication error. Overall, more than one out of ten citizens experienced a medical or medication error during the past two years (11.4%). The fraction of respondents that reported both types of error was 1.8%. Of those respondents that experienced errors, 32.9% reported that the last error in their care occurred in hospital. A number of variables were associated with experience of error in bivariate analysis (fig. 1). Health status and health care utilisation variables were associated with self-reported errors. Poor coordination of care experiences, emergency care, inpatient stay, and poor self-reported health had the strongest associations with risk for self-reported error while having a regular doctor was not linked to error. Higher age was inversely related to errors (OR = 0.98, p = 0.02). Neither gender, education, income nor linguistic regions were significantly associated with patient-reported error.
Results of the final regression model are presented in table 2. Inspection of the variance inflation factors and the goodness-of-fit statistic indicate that the 5 factor model fit the observed data well. Regression results show that experience of poor care coordination is the single most important risk factor, associated with a five-fold increase in reporting error. Utilisation of care, namely, emergency care and hospitalisation are important predictors for reporting error. Responders with poor health are at considerably higher risk for errors in their care, even after adjusting for care utilisation.
The joint influence of the risk factors on the probability that patients report error in their care is substantial (illustrated in fig. 2). For example, the differences between hypothetical patients D and A (poor vs. good health, any emergency care vs. none, any inpatient-stay vs. none, any coordination of care problems vs. none) account for a nearly 20-fold increase in probability of reporting error keeping age constant at 35 years (pA= 0.039 pD= 0.784, p <0.001).
|
Table 1: Characteristics of the Swiss sample, weighted data (n = 1306). |
|
Characteristic
|
% of participants
|
| Female gender |
51.8 |
| Age, mean |
47.7 years |
| 18–29 years |
16.8 |
| 30–49 years |
41.8 |
| 50–64 years |
21.5 |
| 65 years and above |
19.9 |
| Linguistic regions |
|
| German |
67.6 |
| French |
21.4 |
| Italian |
10.9 |
| Rhaeto-Roman |
0.2 |
| Education (recoded from nation-specific response codes) |
| Primary education |
26.0 |
| Secondary education |
60.1 |
| Tertiary education |
13.9 |
| Income (relative to Swiss national average) |
| Much below average |
20.6 |
| Somewhat below average |
23.9 |
| Average |
27.0 |
| Somewhat above average |
20.9 |
| Much above average |
7.7 |
| Self-rated health |
|
| Excellent/very good |
51.3 |
| Good |
39.1 |
| Fair/poor |
9.6 |
| Chronic conditions |
|
| None |
51.6 |
| 1 condition |
26.0 |
| 2 or more conditions |
22.4 |
| Has a regular doctor |
93.5 |
| Specialist doctor seena
|
43.7 |
| Number of doctors seenb
|
|
| None |
17.5 |
| 1–2 doctors |
64.4 |
| 3 or more |
18.1 |
| Current regular use of prescription drugs |
| None |
59.8 |
| 1 prescription drug |
14.9 |
| 2 or more |
25.2 |
| Had medical tests (lab, x-ray)a
|
75.7 |
| Had elective surgerya
|
12.3 |
| Hospital stay a
|
21.5 |
| Emergency care a
|
22.2 |
|
a in the past two years
b in the past 12 months |
|
Table 2: Results of robust logistic regression analysis, weighted data. Predictors for self-reported error (medical or medication error). |
|
Variable
|
OR
|
CI
|
p
|
| Age, years |
0.98 |
0.97–0.99 |
0.014 |
| Fair / poor self-rated health (vs. excellent / good) |
2.95 |
1.35–6.47 |
0.007 |
| Hospital stay (vs. no) |
2.31 |
1.22–4.36 |
0.010 |
| Emergency care (vs. no) |
2.45 |
1.36–4.41 |
0.003 |
| Poor coordination of care experiences (vs. no) |
5.43 |
3.08–9.58 |
<0.001 |
| n |
1,293 |
| Archer-Lemeshow test statistic |
0.974 |
0.459 |
Discussion
This study reports about patients' perceptions of error in Switzerland and identified a number of important risk factors. The result that a quarter of surveyed patients experienced coordination of care problems and more than one in ten patients reported either medical or medication errors in their care is alarming. Utilisation of hospital and emergency care significantly increased the risk for reporting error. Patients with poor health are at significant risk even after adjusting for health care utilisation. While the majority of Swiss citizens have a regular doctor, this had no protective effects in terms of patient reported safety. It is not surprising that poor care coordination experience is the most important single risk factor for reporting errors. Unavailable records, conflicting information and repetition of tests can signal, cause or coincide with safety events, and can themselves be regarded as “error”, even if they may not cause harm. Screening for poor care coordination may be a useful approach to help identify areas or processes of care associated with error.
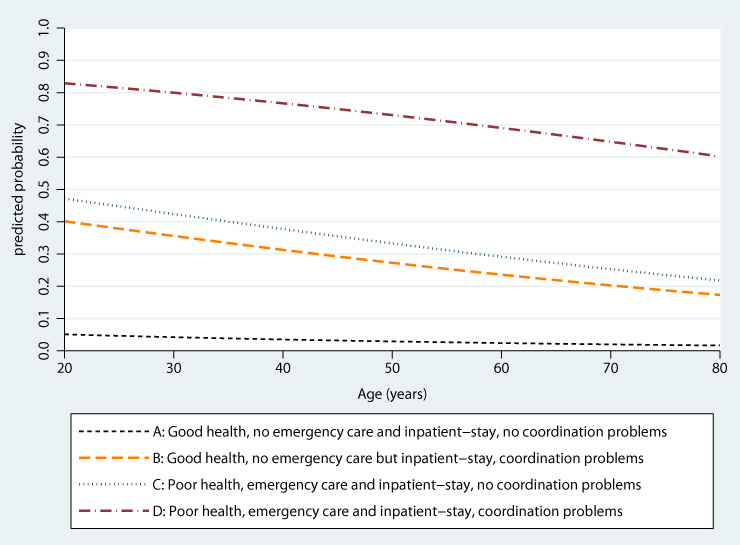
Figure 2
Predicted probability for any patient-reported error for four hypothetical patients (A-D), weighted data.
This study has some limitations. Firstly, due to the limited sample size we used an aggregate measure of error as outcome variable in regression analyses. Distinct associations with specific types of errors, i.e. medication errors, may thus have gone undetected. Secondly, due to the nature of the data we cannot demonstrate causal or temporal relationship between health care utilisation and error. While responders were asked to consider the past two years in most of the questions, we do not know whether health care was utilised before or after the reported events occurred and how they are connected. For example, utilisation of hospital and emergency may both be an outcome of adverse events that occurred in primary care rather than their underlying cause. However, nearly one third of responders reported that the last error they experienced occurred in hospital. Finally, this study is based on patients’ self-reports regarding their health, health care utilisation and experience of errors.
We have no direct “objective data” to validate patients’ reports. Responses relating to patients’ health status and presence of chronic diseases are in good concordance with other representative surveys conducted in Switzerland [21]. To the authors knowledge, no investigations into the accuracy of Swiss patients’ self-reports of care utilisation have been conducted. Validation studies conducted in other countries reveal that patients accurately report inpatient hospitalisations but tend to underreport emergency department and physician visits [22, 23]. Validation of patient-reported medical errors is hardly possible as errors are not documented in medical records. However, several studies investigated the accuracy of adverse events reported by patients. Evidence shows that patients' reports of adverse events are often well in concordance with other detection methods, e.g. record review, [24–29]. Indeed, a considerable fraction of incidents reported by patients are not documented in records but can be validated by clinician review. It is, however, unclear whether the degree of concordance is similar across the care continuum. For example, the public may be more aware towards safety in hospitals as compared to safety of primary care. As a result, patients may be more or less vigilant and educated about safety and have different abilities or motivation to detect errors. In addition, patients' reports of errors are likely to be affected by institutional standards and cultural norms among health care workers on how openly to communicate errors towards patients. As a result, it may be more or less likely for a patient to be informed if error occurs. Thus, patients’ reports of error do reflect not only incidence of error but are also “contaminated” by identification and reporting biases. Reporting effects, rather than differences in true frequency may also help to explain why younger patients were systematically more likely to report errors compared to respondents aged 65 and above, a finding that has been reported in several previous studies [18, 24, 29, 30]. In a recent survey study among Swiss hospital patients, the likelihood for reporting adverse events during hospital stay decreased significantly with age by a comparable magnitude [7]. Zhu et al. report that patients who reported at least one negative effect or complication associated with hospitalisation were significantly younger and that younger patients’ reports were more likely to be classified as adverse events by physician reviewers [29]. Younger patients may be more aware of safety problems, may recall events better and may be less reluctant to report these.
Besides younger age, poorer health was an independent predictor for self-reported errors. Again, this relationship has also been observed for adverse events reported by Swiss hospital patients [7]. Several interpretations may help to explain this finding. Firstly, poor health is often associated with excess utilisation of health care and the data available for our model may not have sufficiently adjusted for this influence. Secondly, patients with poor health who utilise various types of health care in different settings can also often be regarded as experts on their health and the care they receive. They may thus simply be more vigilant and perform better in identifying errors. In effect, this would result in underestimation of the true incidence of errors in the entire sample. Thirdly, patients in poor health may be less tolerant to poor treatment results and unmet needs, even if these do not constitute errors.
Despite the limitations, the results of this study are worrying. Our modelling of hypothetical patients show that for high-utilisers of health care that unify multiple risk factors it is nearly the rule rather than the exception that errors occur. For example, the probability that a 35 year old patient with poor health who utilised hospital and emergency care and perceived at least one coordination of care problem is estimated as p = 0.78. Despite the potential for health-related harm that may be caused by errors, the common experience of error in these populations may also erode trust in the health care system as a whole. These results emphasize that patient safety remains a major challenge for the Swiss health care system.
Acknowledgments:The author thanks the Commonwealth Fund for permission to analyze the data. The support by Markus Weber (Swiss Federal Office of Public Health, BAG) is highly appreciated. The contents are the sole responsibility of the author and do not represent the views of the Commonwealth Fund or local agencies of the participating countries.
References
1 von Laue NC, Schwappach DL, Koeck CM. The epidemiology of medical errors: a review of the literature. Wien Klin Wochenschr. 2003;115(10):318–25.
2 Institute of Medicine. To err is human. Building a safer health system. Washington, DC: National Academy Press; 2000.
3 Schwappach D, Boluarte T. The emotional impact of medical error involvement on physicians: A call for leadership and organizational accountability. Swiss Med Wkly. 2008;139(10):9–15.
4 Zegers M, de Bruijne MC, Wagner C, Hoonhout LHF, Waaijman R, Smits M, et al. Adverse events and potentially preventable deaths in Dutch hospitals: results of a retrospective patient record review study. Qual Saf Health Care. 2009;18(4):297–302.
5 Soop M, Fryksmark U, Koster M, Haglund B. The incidence of adverse events in Swedish hospitals: a retrospective medical record review study. Int J Qual Health Care. 2009;21(4):285–91.
6 Aranaz-Andres JM, Aibar-Remon C, Vitaller-Murillo J, Ruiz-Lopez P, Limon-Ramirez R, Terol-Garcia E, et al. Incidence of adverse events related to health care in Spain: results of the Spanish National Study of Adverse Events. J Epidemiol Community Health. 2008;62(12):1022–9.
7 Schwappach DLB, Frank O, Hochreutener MA. “New perspectives on well-known issues”: Patients’ experiences and perceptions of safety in Swiss hospitals. Zeitschrift für Evidenz, Fortbildung und Qualität im Gesundheitswesen 2011;In Press, Corrected Proof. doi:10.1016/j.zefq.2010.07.002.
8 Schwappach DLB, Wernli M. Chemotherapy Patients’ Perceptions of Drug Administration Safety. J Clin Oncol. 2010;28(17):2896–901.
9 Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–7.
10 Thomsen LA, Winterstein AG, Sondergaard B, Haugbolle LS, Melander A. Systematic Review of the Incidence and Characteristics of Preventable Adverse Drug Events in Ambulatory Care. Ann Pharmacother. 2007;41(9):1411–26.
11 Miller GC, Britth HC, Valenti L. Adverse drug events in general practice patients in Australia. Med J Aust. 2006;184(7):321–4.
12 Gurwitz JH, Field TS, Harrold LR, Rothschild J, Debellis K, Seger AC, et al. Incidence and Preventability of Adverse Drug Events Among Older Persons in the Ambulatory Setting. JAMA. 2003;289(9):1107–16.
13 Rubin G, George A, Chinn DJ, Richardson C. Errors in general practice: development of an error classification and pilot study of a method for detecting errors. Qual Saf Health Care. 2003;12(6):443–7.
14 Unruh KT, Pratt W. Patients as actors: the patient’s role in detecting, preventing, and recovering from medical errors. Int J Med Inform. 2007;76(Suppl 1):S236–S244.
15 Schwappach DL. Engaging patients as vigilant partners in safety: a systematic review. Med Care Res Rev. 2010;67:119–48.
16 Schoen C, Osborn R, Huynh PT, Doty M, Zapert K, Peugh J, et al. Taking The Pulse Of Health Care Systems: Experiences Of Patients With Health Problems In Six Countries. Health Aff. 2005;Suppl Web Exclusives(W5):509–25.
17 Schoen C, Osborn R, How SKH, Doty MM, Peugh J. In Chronic Condition: Experiences Of Patients With Complex Health Care Needs, In Eight Countries, 2008. Health Aff. 2009;28(1):w1–w16.
18 Scobie A. Self-reported medical, medication and laboratory error in eight countries: risk factors for chronically ill adults. Int J Qual Health Care. 2011;23(2):182–6.
19 Archer KJ, Lemeshow S, Hosmer DW. Goodness-of-fit tests for logistic regression models when data are collected using a complex sampling design. Computational Statistics & Data Analysis. 2007;51(9):4450–64.
20 StataCorp. Stata Statistical Software: Release 11.2. College Station, TX: Stata Corporation; 2010.
21 Estoppey D, Paccaud F, Vollenweider P, Marques-Vidal P. Trends in self-reported prevalence and management of hypertension, hypercholesterolemia and diabetes in Swiss adults, 1997–2007. BMC Public Health. 2011;11(1):114.
22 Wolinsky FD, Miller TR, An H, Geweke JF, Wallace RB, Wright KB, et al. Hospital Episodes and Physician Visits: The Concordance Between Self-Reports and Medicare Claims. Med Care. 2007;45(4):300–7.
23 Zuvekas SH, Olin GL. Validating Household Reports of Health Care Use in the Medical Expenditure Panel Survey. Health Serv Res. 2009;44(5p1):1679–700.
24 Fowler FJ, Epstein A, Weingart SN, Annas CL, Bolcic-Jankovic D, Clarridge B, et al. Adverse Events During Hospitalization: Results of a Patient Survey. Jt Comm J Quality Safety. 2008;34:583–90.
25 Weingart SN, Pagovich O, Sands DZ, Li JM, Aronson MD, Davis RB, et al. What can hospitalized patients tell us about adverse events? Learning from patient-reported incidents. J Gen Intern Med. 2005;20(9):830–6.
26 King A, Daniels J, Lim J, Cochrane DD, Taylor A, Ansermino JM. Time to listen: a review of methods to solicit patient reports of adverse events. Qual Saf Health Care. 2010;19(2):148–57.
27 Massó Guijarro P, Aranaz Andres JM, Mira JJ, Perdiguero E, Aibar C. Adverse events in hospitals: the patient's point of view. Qual Saf Health Care. 2010;19(2):144–7.
28 Schwappach DL. “Against the silence”: Development and first results of a patient survey to assess experiences of safety-related events in hospital. BMC Health Serv Res. 2008;8(1):59.
29 Zhu J, Stuver SO, Epstein AM, Schneider EC, Weissman JS, Weingart SN. Can We Rely on Patients’ Reports of Adverse Events? Med Care 2011;Publish Ahead of Print. doi: 10.1097/MLR.0b013e31822047a8.
30 Weissman JS, Schneider EC, Weingart SN, Epstein AM, David-Kasdan J, Feibelmann S, et al. Comparing patient-reported hospital adverse events with medical record review: do patients know something that hospitals do not? Ann Intern Med. 2008;149(2):100–8.

