Giant cell arteriitis – a changing entity
DOI: https://doi.org/10.4414/smw.2011.13272
F
Kesten, M
Aschwanden, P
Gubser, K
Glatz, T
Daikeler
Summary
Giant cell arteriitis (GCA) is the most common of the vasculitis syndromes and, being a disease of the elderly, its incidence is increasing with the general ageing of the population. GCA is most feared for its early complications, namely blindness and stroke, resulting from inflammation and subsequent occlusion of ocular and extra cranial arteries, respectively. More recently, however, GCA has been recognised to also affect limb arteries and the aorta with a high prevalence. These newly recognised features of GCA pose diagnostic, therapeutic and prognostic challenges to treating physicians. Here, recent developments in the field of GCA are summarised and discussed.
Introduction
Giant cell arteriitis (GCA) affects large and medium sized arteries and is the most prevalent of the systemic vasculitis syndromes [1, 2]. Inflammation of the vessel wall is characterised by infiltration of T-cells and macrophages, presence of eponymous giant cells, granulomatous lesions, intimal hyperplasia and destruction of elastic fibres (fig. 1) [3].
While a stone relief from ancient Egypt very likely depicts a blind harp player suffering from GCA (fig. 2), the first official case was published in 1932 by Horton, Magath and Brown. In their report an individual with blindness, tongue necrosis and jaw claudication is described, and the authors conclude that their patient may suffer from “a new form of arteriitis affecting the temporal vessels ... which probably represents a new clinical syndrome” (Morbus Horton) [4].
GCA predominantly affects individuals over 50 years of age, with a current prevalence of approximately 20/100000 in Central Europe. Women are affected more frequently than men (3:1), and the incidence increases with age [5]. The triggering events of GCA remain unknown. Geographical differences as well as occasional familial clustering suggest environmental, infectious and/or genetic factors to play a role [2].
Clinical presentation and diagnostic approach
In its best known form, GCA affects the external carotid artery and its major extra cranial branches, leading to the classical symptoms of temporal headache, scalp tenderness, jaw claudication and tender temporal arteries. A feared complication – occurring in up to 20% of patients – is ocular ischaemia with ensuing anterior ischaemic optic neuropathy, leading to irreversible vision loss in 10–15% [6]. Another potentially catastrophic manifestation is cerebral ischaemia with infarction, occurring with predilection in the vertebro-basilar territory [7].
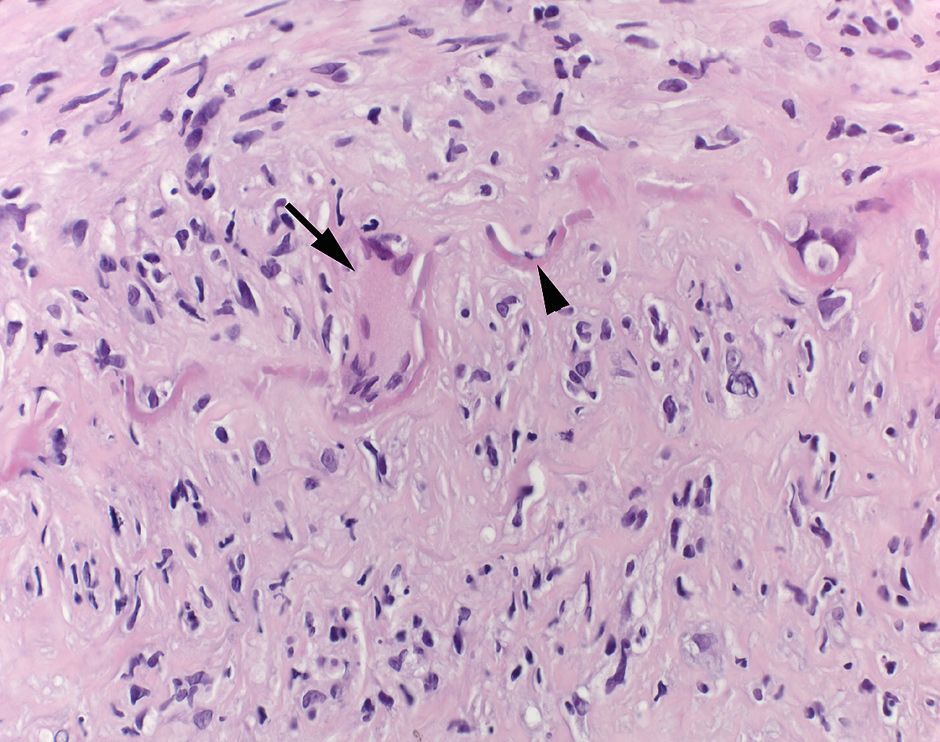
Figure 1
Histopathology picture from a GCA-patient. Temporal artery biopsy with characteristic histological findings of active giant cell arteriitis: The arterial wall shows a dense chronic lymphohistiocytic inflammatory infiltrate. A multinucleated giant cell (arrow) lies next to the disrupted elastic lamina (arrowhead). The intima in the lower half of the image is thickened. H&E, 400x

Figure 2
Detail of the tomb relief “the blind harp-player”. In this stone relief from the 14th century B.C., a blind harp player (eyes closed) of advanced age, with a very prominent temporal artery and seemingly in pain (Polymyalgia rheumatica?) is depicted (reproduced from Toellner R: Historia Medicinae, 1983, with permission of the publisher [Andreas und Andreas, Salzburg, Austria]).
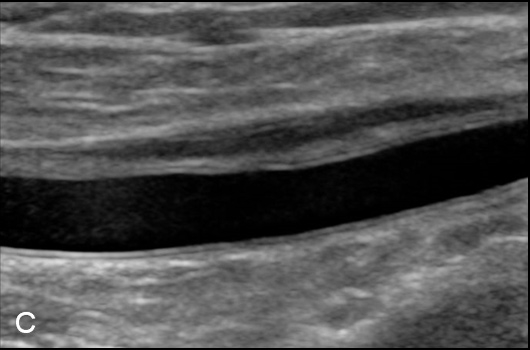
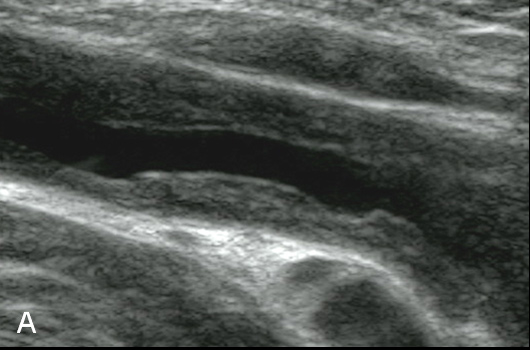
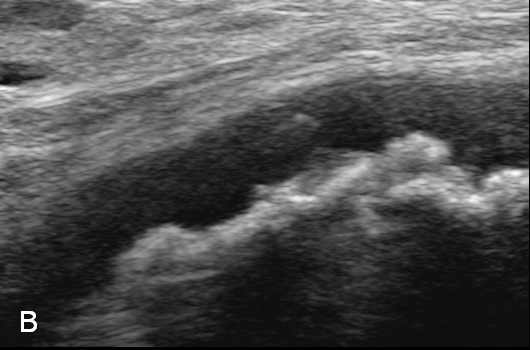
Figure 3
Duplex Sonographic (DS) images from three distinct arterial segments. Arterial segment (A) is classified as “vasculitis”, with homogenous, hypoechoic wall broadening, segment (B) as “arteriosclerosis”, with eccentric, irregular plaques and acoustic shadowing, and segment (C) as “normal”, showing a thin homogenous intima/media layer.
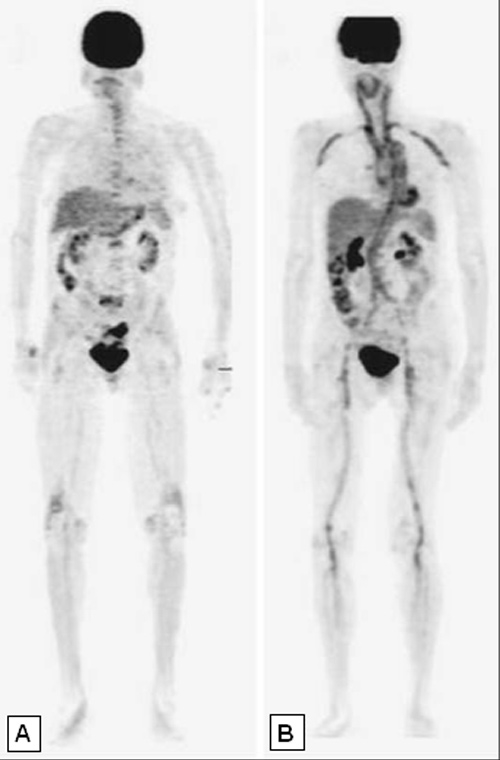
Figure 4
Whole body [18F]-FDG-PET studies. In Panel (A) a normal PET study is shown, in Panel (B) intense FDG uptake in the wall of numerous large vessels is indicative of extensive vasculitis.
A systemic inflammatory response is characteristic in patients with GCA, variably reflected by fever, malaise, anorexia, weight loss, anaemia, and thrombocytosis. High erythrocyte sedimentation rates (ESR)- and C-reactive protein (CRP)-levels are typically seen in patients with GCA, yet a normal ESR/CRP does not exclude the disease, particularly when other clinical findings support the diagnosis [8, 9]. Occurring in up to 60%, polymyalgia rheumatica is the most common musculoskeletal manifestation in patients with GCA [10, 11]. Inversely, GCA may be present more often (9–20%) than previously appreciated in patients with signs and symptoms characteristic of polymyalgia rheumatica [12]. The current view is that GCA and PMR are two different but frequently overlapping conditions [11]. However, patients with PMR associated with GCA often have more severe disease, reflected by significant laboratory abnormalities and pronounced constitutional symptoms [13]. A retrospective study aimed to define the characteristics that are predictive for evidence of vasculitis in temporal artery biopsy samples in patients presenting with isolated PMR. In that study, among patients <70 years of age and without cranial features of GCA, the risk of GCA was so low, that temporal artery biopsy could be safely avoided and patients treated with low dose corticosteroids as is done in patients with isolated (“pure”) PMR [14].
According to the Chapel Hill Consensus Conference, large vessel vasculitis syndromes include GCA and Takayasu arteriitis [15]. ACR (American College of Rheumatology) criteria used to classify Takayasu arteriitis are: age at disease onset <40 years, claudication of extremities, decreased brachial artery pulse, blood pressure difference >10 mm Hg between arms, bruit over subclavian arteries or aorta and arteriogram abnormality. ACR criteria used to classify GCA are summarised in table 1 [17]. As reflected by these criteria, important differences between the two entities clearly exist. Whilst Takayasu arteriitis is typically seen in young women of Asian ancestry and predominantly affects the aorta and its first branches, GCA largely affects older patients with a Caucasian background, showing a clinical predominance for cranial symptoms [16]. However, and discussed in detail below, recent findings also suggest important overlap.
It is important to realize that both sensitivity and specificity of the ACRcriteria in an unselected population (i.e. when used as diagnostic criteria) is very low. Only very recently, the ACR and European League against Rheumatism (EULAR) have launched an exciting prospective observational study with the goal to develop, for the first time, diagnostic criteria – and at the same time reassess the classification criteria – for primary vasculitis (Diagnostic and Classification Criteria for Primary Systemic Vasculitis [DCVAS]). For the time being, a positive biopsy remains the gold standard for diagnosing GCA, and temporal artery biopsy (TAB) is recommended in all suspected cases. The sensitivity and specificity of TAB has been reported to be around 75% and 90%, respectively [18]. Due to the skip-lesion character of inflammation, sensitivity depends on a sufficient length of the biopsy sample [19]. Importantly, sensitivity and specificity of TAB has not been re-assessed in light of recent developments indicating forms of GCA sparing cranial vessels to be a rather common variant of the disease (see below) [14].
Against this background – and thus confronted with the reality of lacking a straightforward diagnostic approach for a potentially dreadful disease – modern imaging has taken centre stage over the last years in search of broadly applicable tools to establish or exclude the diagnosis of GCA and will most likely find its way into coming diagnostic criteria:
Ultrasound Imaging
In 1995, Schmidt et al. were the first to indentify hypoechogenic wall thickening on duplex sonography (DS) around the temporal artery – termed halo-sign – as indicative of vessel wall inflammation [20]. This publication generated considerable interest in DS as a non-invasive tool for diagnosing large vessel vasculitis, yet operator-dependency of the procedure emerged as a limitation. Recently, applying a simple and robust set of DS-criteria to the screening of 2x11 arterial regions in patients with suspected GCA, we were able to confirm high specificity of DS for diagnosing GCA (fig. 3) [21]. Unfortunately, sensitivity of DS in detecting GCA remained rather low [21–23].
Inflammation of limb arteries in patients with GCA – sometimes the only manifestation of the disease– has been described sporadically since the 1970s [24]. Yet, only with the advent of modern imaging techniques such as DS, systematic evidence became available indicating that GCA is a systemic vasculitis affecting vessels well beyond the cranial arteries. Indeed, we and others have shown that DS-defined large vessel vasculitis of lower limb arteries is present in about 30% (!) of patients with newly diagnosed GCA [21, 25]. Thus, GCA can be a (near-)perfect mimic of atherosclerosis induced intermittent claudication [26, 27].
Positron emission tomography (PET)
PET-studies, and more recently PET/Computer Tomography (CT) studies have become a major tool for diagnosing large vessel vasculitis. Specifically, assessing 18F-fluoro-2-deoxy-D-glucose (FDG) uptake by PET is extremely helpful to assess vasculitic involvement of intrathoracic vessels (that cannot be visualized by DS)(fig. 4), and in cases of non-specific signs and symptoms compatible with large vessel vasculitis [28, 29]. Sensitivity and specificity of PET/CT to detect large vessel vasculitis in untreated patients is around 85% and 95%, respectively [28]. In steroid-treated patients sensitivity of PET studies decreases [30]. However, no data is available regarding the dose and time dependency of this effect, nor about the subgroup of patients that remains “PET-positive” despite long-term steroid-exposure.
Importantly, FDG-PET/CT-scan studies revealed aortitis – a feature classically ascribed to Takayasu arteriitis – as being present in >80% of newly diagnosed GCA patients [31], and also identified GCA as a prominent cause for fever of unknown origin (FUO) in elderly patients [32, 33]. Limitations of PET studies include its low resolution (approx. 4 mm), and the fact that pronounced FDG uptake by the CNS prevents visualisation of cranial arteries.
Magnetic resonance imaging (MRI)
One Tesla MRI is not useful for the diagnosis of GCA because of its low sensitivity [34], but advances of MRI technique, with systems operating at field strengths of 3 Tesla and more, has increased its sensitivity and specificity for diagnosing vascular inflammation [35]. MRI has been shown to reliably identify cervical, extra- and intracranial vasculitis, as well as aortitis [36]. To date, however, the clinical usefulness of high field-strength MRI in the context of large vessel vasculitis remains restricted by its limited availability and high costs.
Aside these important methodological advances, careful clinical and translational findings, as well as exciting basic research data hold great promise to soon impact the management of patients with suspected or defined GCA:
On a population level, ischaemic complications were shown to more often occur in patients with a low systemic inflammatory response [37, 38]. In line with this clinical observation, in the subset of patients with ischaemic events, levels of the acute-phase inflammatory cytokine IL-6 – both within the temporal artery biopsy specimens and in the circulation – are decreased [39]. These data indicate that inflammation as reflected by an acute-phase response and vascular occlusion can be dissociated and may be characteristics of distinct variants among a GCA-spectrum of disease. Understanding the regulation of vascular inflammation in GCA at the molecular level and the genetic susceptibility of individual patients (see Refs. [40] and [41] for more detailed information) may therefore help to identify subgroups of patients, and eventually permit to develop more specific treatment modalities.
Consensus exists with regard to a key role of dendritic cells (DCs) – a cell type resident within the adventitia of the vessel wall and centrally involved in initiating adaptive immune responses – in triggering GCA [42]. DCs are thought to become activated by molecules that bind receptors that are recognising a group of “danger signals” – so called toll-like receptor ligands- that gain access to the vascular wall via the vasa vasorum. Upon activation, DCs secrete inflammatory mediators that are recruiting and activating CD4+ T-cells and macrophages. At least two distinct CD4+ T-cell subsets, namely Th17 and Th1 cells, promote vascular inflammation in GCA [43, 44]. Recently, we have identified a third subset of T-cells with a phenotype linked to immunosuppressive cellular characteristics (CD4+ FoxP3+) as being part of the inflammatory infiltrate in a subset of patients with GCA (Cantoni N., Hess C. et al., unpublished observation). Macrophages, activated by T-cells within the vessel wall, secrete pro-inflammatory cytokines such as IL-1 and IL-6, as well as tissue-destructing enzymes and reactive oxygen intermediates. Together this inflammatory process leads to intimal hyperplasia and disruption of vessel wall integrity. Whether CD4+ FoxP3+ T-cells promote fibrosis and occlusion of vessels – while inhibiting acute-phase cytokine secretion (such as IL-6) – remains to be determined. It is, however, tempting to speculate that GCA-patients with defined histopathological characteristics may eventually benefit from distinct treatment strategies.
|
Table 1: ACR classification criteria for giant cell arteriitis [13]. |
| – Age ≥50 years at disease onset |
| – New onset of localised headache |
| – Temporal artery tenderness or decreased temporal artery pulse |
| – ESR ≥50 mm/hour |
| – Biopsy: necrotising arteriitis; mononuclear cell infiltrates or a granulomatous process with multinucleated giant cells |
| Presence of ≥3/5: sensitivity of 93% and specificity of 91% for distinguishing GCA from other primary vasculitis syndromes
|
Therapy
Glucocorticoids (unfortunately) still are the mainstay of therapy in patients with GCA. Failure to rapidly respond to steroid treatment in terms of resolution of symptoms and normalisation of inflammation should raise the question of an alternative diagnosis. While highly efficient in inducing remission, glucocorticoids cause severe side effects [47], and studies more precisely defining dose and duration of glucocorticoid based therapies in GCA should be a priority. Based on a metaanalysis of the published evidence, current EULAR guidelines suggest that the initially prescribed dose of prednisolone/prednisone should be 1 mg/kg body weight (not exceeding 60 mg/day) maintained for four weeks and tapered gradually thereafter [45]. If severe ischaemic complications – such as visual impairment or stroke – are present, 0.5–1 g of methylprednisolone is recommended to be administered intravenously for three days, followed by oral dosage/tapering. Due to the risk of irreversible ischaemic events, treatment with glucocorticoids should be started immediately upon strong clinical suspicion of GCA. Initiating treatment prior to a tamporal artery biopsy is unlikely to significantly influence results if the biopsy is not delayed beyond 1–2 weeks into therapy [46]. Based on our experience the same may well be true for DS (unpublished observation).
In the absence of evidence for relapsing disease, the prednisolone/prednisone-dose should reach 10–15 mg/d by month 3, and 5 mg/d by month 6 after diagnosis. Prednisolone/prednisone should then be maintained at 5 mg/d for at least 12 months, whereupon further tapering – guided by symptoms, signs of inflammation and possibly secondary adrenal insufficiency – is indicated [47]. Importantly, however, GCA takes a relapsing course in approx. 40% of cases within the first two years after diagnosis. As at initial presentation, relapses are usually associated with a rise in ESR/CRP. Beyond two years after diagnosis recurrence of GCA becomes less likely, and five years after initial diagnosis recurrence is exceedingly rare. Thus – at least in its classic inflammatory form – GCA appears to eventually follow a self-limiting course [48].
A frustrating 86% (!) of all patients with GCA suffer from therapy (i.e. glucocorticoid) related adverse effects [47]. Steroid-sparing/Disease Modifying anti-Rheumatic Drugs (DMARD) have been tested in various studies aiming to optimise outcome while reducing glucocorticoid toxicity. However, with the exception of methotrexate (MTX), no DMARD has been shown to be unequivocally effective in the treatment of GCA [49–51]. MTX – if used at a proper dose and ensuring bioavailability (10–15 mg/week, preferably subcutaneously) confers a modest benefit in terms of reducing the cumulative steroid dose and is indicated in relapsing disease [49]. Of note, while the value of TNF-α blocking agents – such as etanercept or infliximab – in treating resistant cases remains to be determined, these biologicals were not generally successful in inducing or maintaining disease remission [52, 53]. Hopes are up for therapies targeting cytokines that have been firmly established to play a role in the inflammatory process of GCA, and first promising results from a series of GCA-cases treated with IL-6 blocking antibodies have recently been reported [54].
Once the diagnosis of GCA is established, disease activity should be monitored clinically as well as based on serial measurements of ESR/CRP. The role of imaging in assessing the course of the disease remains to be determined. In case of a suspected relapse, increasing the daily prednisolone/prednisone dose by 10–15 mg for a few weeks may be sufficient to control disease activity again. Patients who have discontinued therapy and experience a relapse (which is rather rare) [48], should probably be treated analogous to newly diagnosed patients (evidence lacking).
Importantly, all patients should receive osteoporosis prophylaxis consisting of calcium, vitamin D3 and – if indicated based on a DEXA examination – bisphosphonates. Although no positive impact of anti-aggregation therapy on the risk of severe ischaemic complications has yet been formally demonstrated [9, 55–57], low dose aspirin (75–150 mg/day) is generally recommended for patients with GCA in the absence of contraindications [45, 55].
Prognosis
Visual loss and stroke occur (if so) early in the course of the disease [48]. Visual loss is usually irreversible, while the prognosis of cerebral ischaemia depends on the size and localisation of the affected area.
The fact that inflammation of the aorta is a prominent feature of GCA has only recently become evident. Formation of aortic aneurysms seems to correlate with the initial inflammatory involvement of the aorta [36] and the presence of hypertension at the time of diagnosis [58]. Therefore, after diagnosis, patients should be periodically monitored for the existence of aneurysmal disease, in particular in those with hypertension and severe inflammatory response at the time of diagnosis. It is important for the management of patients to realise that aneurysms can arise several years after diagnosis – i.e. when other signs and symptoms of GCA have subsided [59]. Of note, this fact does not rule out the possibility of ongoing subclinical vessel wall inflammation. Irrespective of its pathophysiology, in a large population based cohort, thoracic aneurysms of the aorta were 17 times (!) more frequent in patients with GCA as compared to non-affected controls [60]. Patients in this cohort were not actively screened for aneurysms and the prevalence of aortic aneurysms in patients with a history of GCA might be even higher. Despite the increased risk of strokes generally early after the diagnosis of GCA and aortic aneurysms several years later (in the extended follow up of these patients), most population-based studies have not shown significantly increased mortality in GCA patients [61, 62].
8-point summary
-> The prevalence of GCA is increasing
-> GCA is an important cause of FUO among individuals >50 years of age
-> GCA often affects the aorta and limb arteries
-> Given the high prevalence of aortic and limb artery inflammation – and its impact on the management of patients – DS and PET/CT screening are valid options
-> Due to the probably increased incidence of (mainly thoracic) aneurysms of the aorta, GCA has an impact on patient care beyond the early phase of the disease
-> Immediate glucocorticoid treatment remains the only means to prevent irreversible visual loss and other catastrophic ischaemic events in GCA
-> Glucocorticoid treatment of patients with GCA is associated with dramatic morbidity (add MTX on first relapse), prevention/therapy of osteoporosis is imperative and low-dose aspirin recommended
-> Careful treatment of co-morbidity also impacting the cardiovascular risk (i.e. hypertension, diabetes mellitus, hypercholesterolaemia) is of paramount importance
Closing remarks
Novel aspects of GCA have emerged – yet many long standing problems remain. In order to meet the challenges posed by the changing entity “GCA”, prospective cohorts – such as the “Basel Riesenzell-Arteritis Kohorte” (BARK) study – are important. Only through a systematic and prospective approach can we examine precisely how clinical and biological features of GCA relate to presentation, complications, treatment response and prognosis of this complex, feared and at the same time fascinating disease.
Acknowledgments: We would like to thank Martin Walter (University Hospital Bern, Switzerland) for providing PET study images.
References
1 Hunder GG. Epidemiology of giant-cell arteriitis. Cleve Clin J Med. 2002;69(Suppl 2):SII79–82.
2 Gonzalez-Gay MA, Vazquez-Rodriguez TR, Lopez-Diaz MJ, Miranda-Filloy JA, Gonzalez-Juanatey C, Martin J, et al. Epidemiology of giant cell arteriitis and polymyalgia rheumatica. Arthritis Rheum. 2009; 61(10):1454–61.
3 Björnsson J. Histopathology of primary vasculitic disorders. In: Hoffman GS WC, ed. Inflammatory Diseases of Blood Vessels. New York: Marcel Dekker; 2002:255–65.
4 Horton BT MT, Brown GE. An undescribed form of arteriitis of the temporal vessels. Proc Staff Meet Mayo Clin. 1932;7:700.
5 Salvarani C, Gabriel SE, O’Fallon WM, Hunder GG. The incidence of giant cell arteriitis in Olmsted County, Minnesota: apparent fluctuations in a cyclic pattern. Ann Intern Med. 1995;123(3):192–4.
6 Gonzalez-Gay MA, Garcia-Porrua C, Llorca J, Hajeer AH, Branas F, Dababneh A, et al. Visual manifestations of giant cell arteriitis. Trends and clinical spectrum in 161 patients. Medicine (Baltimore). 2000;79(5):283–92.
7 Gonzalez-Gay MA, Vazquez-Rodriguez TR, Gomez-Acebo I, Pego-Reigosa R, Lopez-Diaz MJ, Vazquez-Trinanes MC, et al. Strokes at time of disease diagnosis in a series of 287 patients with biopsy-proven giant cell arteriitis. Medicine (Baltimore). 2009;88(4):227–35.
8 Salvarani C, Hunder GG. Giant cell arteriitis with low erythrocyte sedimentation rate: frequency of occurrence in a population-based study. Arthritis Rheum. 2001;45(2):140–5.
9 Berger CT, Wolbers M, Meyer P, Daikeler T, Hess C. High incidence of severe ischaemic complications in patients with giant cell arteriitis irrespective of platelet count and size, and platelet inhibition. Rheumatology (Oxford). 2009;48(3):258–61.
10 Salvarani C, Hunder GG. Musculoskeletal manifestations in a population-based cohort of patients with giant cell arteriitis. Arthritis Rheum. 1999;42(6):1259–66.
11 Gonzalez-Gay MA. Giant cell arteriitis and polymyalgia rheumatica: two different but often overlapping conditions. Semin Arthritis Rheum. 2004;33(5):289–93.
12 Blockmans D, De Ceuninck L, Vanderschueren S, Knockaert D, Mortelmans L, Bobbaers H. Repetitive 18-fluorodeoxyglucose positron emission tomography in isolated polymyalgia rheumatica: a prospective study in 35 patients. Rheumatology (Oxford). 2007;46(4):672–7.
13 Gonzalez-Gay MA, Garcia-Porrua C, Vazquez-Caruncho M. Polymyalgia rheumatica in biopsy proven giant cell arteriitis does not constitute a different subset but differs from isolated polymyalgia rheumatica. J Rheumatol. 1998;25(9):1750–5.
14 Rodriguez-Valverde V, Sarabia JM, Gonzalez-Gay MA, Figueroa M, Armona J, Blanco R, et al. Risk factors and predictive models of giant cell arteriitis in polymyalgia rheumatica. Am J Med. 1997;102(4):331–6.
15 Jennette JC, Falk RJ, Andrassy K, Bacon PA, Churg J, Gross WL, et al. Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum. 1994;37(2):187–92.
16 Maksimowicz-McKinnon K, Clark TM, Hoffman GS. Takayasu arteriitis and giant cell arteriitis: a spectrum within the same disease? Medicine (Baltimore). 2009;88(4):221–6.
17 Hunder GG, Bloch DA, Michel BA, Stevens MB, Arend WP, Calabrese LH, et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteriitis. Arthritis Rheum. 1990;33(8):1122–8.
18 Gonzalez-Gay MA, Garcia-Porrua C, Llorca J, Gonzalez-Louzao C, Rodriguez-Ledo P. Biopsy-negative giant cell arteriitis: clinical spectrum and predictive factors for positive temporal artery biopsy. Semin Arthritis Rheum. 2001;30(4):249–56.
19 Mahr A, Saba M, Kambouchner M, Polivka M, Baudrimont M, Brocheriou I, et al. Temporal artery biopsy for diagnosing giant cell arteriitis: the longer, the better? Ann Rheum Dis. 2006;65(6):826–8.
20 Schmidt WA, Kraft HE, Vorpahl K, Volker L, Gromnica-Ihle EJ. Color duplex ultrasonography in the diagnosis of temporal arteriitis. N Engl J Med. 1997;337(19):1336–42.
21 Aschwanden M, Kesten F, Stern M, Thalhammer C, Walker UA, Tyndall A, et al. Vascular involvement in patients with giant cell arteriitis determined by duplex sonography of 2x11 arterial regions. Ann Rheum Dis. 2010;69(7):1356–9.
22 Perez Lopez J, Solans Laque R, Bosch Gil JA, Molina Cateriano C, Huguet Redecilla P, Vilardell Tarres M. Colour-duplex ultrasonography of the temporal and ophthalmic arteries in the diagnosis and follow-up of giant cell arteriitis. Clin Exp Rheumatol. 2009;27(1 Suppl 52):S77–82.
23 Karassa FB, Matsagas MI, Schmidt WA, Ioannidis JP. Meta-analysis: test performance of ultrasonography for giant-cell arteriitis. Ann Intern Med. 2005;142(5):359–69.
24 Klein RG, Hunder GG, Stanson AW, Sheps SG. Large artery involvement in giant cell (temporal) arteriitis. Ann Intern Med. 1975;83(6):806–12.
25 Lie JT. Aortic and extracranial large vessel giant cell arteriitis: a review of 72 cases with histopathological documentation. Semin Arthritis Rheum. 1995;24(6):422–31.
26 Affolter B, Thalhammer C, Aschwanden M, Glatz K, Tyndall A, Daikeler T. Difficult diagnosis and assessment of disease activity in giant cell arteriitis: a report on two patients. Scand J Rheumatol. 2009;38(5):393–4.
27 Kermani TA, Matteson EL, Hunder GG, Warrington KJ. Symptomatic lower extremity vasculitis in giant cell arteriitis: a case series. J Rheumatol. 2009;36(10):2277–83.
28 Meller J, Sahlmann CO, Gurocak O, Liersch T, Meller B. FDG-PET in patients with fever of unknown origin: the importance of diagnosing large vessel vasculitis. Q J Nucl Med Mol Imaging. 2009;53(1):51–63.
29 Walter MA, Melzer RA, Schindler C, Muller-Brand J, Tyndall A, Nitzsche EU. The value of [18F]FDG-PET in the diagnosis of large-vessel vasculitis and the assessment of activity and extent of disease. Eur J Nucl Med Mol Imaging. 2005;32(6):674–81.
30 Papathanasiou ND, Du Y, Menezes LJ, Al-Muhaideb A, Shastry M, Beynon H, et al. 18F-Fluorodeoxyglucose PET/CT in the evaluation of large-vessel vasculitis: diagnostic performance and correlation with clinical and laboratory parameters. Br J Radiol. 2011 Mar 8.
31 Blockmans D, de Ceuninck L, Vanderschueren S, Knockaert D, Mortelmans L, Bobbaers H. Repetitive 18F-fluorodeoxyglucose positron emission tomography in giant cell arteriitis: a prospective study of 35 patients. Arthritis Rheum. 2006;55(1):131–7.
32 Knockaert DC, Vanneste LJ, Bobbaers HJ. Recurrent or episodic fever of unknown origin. Review of 45 cases and survey of the literature. Medicine (Baltimore). 1993;72(3):184–96.
33 Knockaert DC, Vanderschueren S, Blockmans D. Fever of unknown origin in adults: 40 years on. J Intern Med. 2003;253(3):263–75.
34 Ghinoi A, Zuccoli G, Nicolini A, Pipitone N, Macchioni L, Bajocchi GL, et al. 1T magnetic resonance imaging in the diagnosis of giant cell arteriitis: comparison with ultrasonography and physical examination of temporal arteries. Clin Exp Rheumatol. 2008;26(3 Suppl 49):S76–80.
35 Blockmans D, Bley T, Schmidt W. Imaging for large-vessel vasculitis. Curr Opin Rheumatol. 2009;21(1):19–28.
36 Both M, Ahmadi-Simab K, Reuter M, Dourvos O, Fritzer E, Ullrich S, et al. MRI and FDG-PET in the assessment of inflammatory aortic arch syndrome in complicated courses of giant cell arteriitis. Ann Rheum Dis. 2008;67(7):1030–3.
37 Cid MC, Font C, Oristrell J, de la Sierra A, Coll-Vinent B, Lopez-Soto A, et al. Association between strong inflammatory response and low risk of developing visual loss and other cranial ischemic complications in giant cell (temporal) arteriitis. Arthritis Rheum. 1998;41(1):26–32.
38 Hernandez-Rodriguez J, Garcia-Martinez A, Casademont J, Filella X, Esteban MJ, Lopez-Soto A, et al. A strong initial systemic inflammatory response is associated with higher corticosteroid requirements and longer duration of therapy in patients with giant-cell arteriitis. Arthritis Rheum. 2002;47(1):29–35.
39 Hernandez-Rodriguez J, Segarra M, Vilardell C, Sanchez M, Garcia-Martinez A, Esteban MJ, et al. Elevated production of interleukin-6 is associated with a lower incidence of disease-related ischemic events in patients with giant-cell arteriitis: angiogenic activity of interleukin-6 as a potential protective mechanism. Circulation. 2003;107(19):2428–34.
40 Gonzalez-Gay MA, Amoli MM, Garcia-Porrua C, Ollier WE. Genetic markers of disease susceptibility and severity in giant cell arteriitis and polymyalgia rheumatica. Semin Arthritis Rheum. 2003;33(1):38–48.
41 Rueda B, Lopez-Nevot MA, Lopez-Diaz MJ, Garcia-Porrua C, Martin J, Gonzalez-Gay MA. A functional variant of vascular endothelial growth factor is associated with severe ischemic complications in giant cell arteriitis. J Rheumatol. 2005;32(9):1737–41.
42 Weyand CM, Goronzy JJ. Medium- and large-vessel vasculitis. N Engl J Med. 2003;349(2):160–9.
43 Deng J, Younge BR, Olshen RA, Goronzy JJ, Weyand CM. Th17 and Th1 T-cell responses in giant cell arteriitis. Circulation. 2010;121(7):906–15.
44 Weyand CM, Younge BR, Goronzy JJ. IFN-gamma and IL-17: the two faces of T-cell pathology in giant cell arteriitis. Curr Opin Rheumatol. 2011;23(1):43–9.
45 Mukhtyar C, Guillevin L, Cid MC, Dasgupta B, de Groot K, Gross W, et al. EULAR recommendations for the management of large vessel vasculitis. Ann Rheum Dis. 2009;68(3):318–23.
46 Narvaez J, Bernad B, Roig-Vilaseca D, Garcia-Gomez C, Gomez-Vaquero C, Juanola X, et al. Influence of previous corticosteroid therapy on temporal artery biopsy yield in giant cell arteriitis. Semin Arthritis Rheum. 2007;37(1):13–9.
47 Proven A, Gabriel SE, Orces C, O’Fallon WM, Hunder GG. Glucocorticoid therapy in giant cell arteriitis: duration and adverse outcomes. Arthritis Rheum. 2003; 49(5):703–8.
48 Martinez-Lado L, Calvino-Diaz C, Pineiro A, Dierssen T, Vazquez-Rodriguez TR, Miranda-Filloy JA, et al. Relapses and recurrences in giant cell arteriitis: a population-based study of patients with biopsy-proven disease from northwestern Spain. Medicine (Baltimore). 2011;90(3):186–93.
49 Mahr AD, Jover JA, Spiera RF, Hernandez-Garcia C, Fernandez-Gutierrez B, Lavalley MP, et al. Adjunctive methotrexate for treatment of giant cell arteriitis: an individual patient data meta-analysis. Arthritis Rheum. 2007;56(8):2789–97.
50 De Silva M, Hazleman BL. Azathioprine in giant cell arteriitis/polymyalgia rheumatica: a double-blind study. Ann Rheum Dis. 1986;45(2):136–8.
51 Nuenninghoff DM, Matteson EL. The role of disease-modifying antirheumatic drugs in the treatment of giant cell arteriitis. Clin Exp Rheumatol. 2003;21(6 Suppl 32):S29–34.
52 Martinez-Taboada VM, Rodriguez-Valverde V, Carreno L, Lopez-Longo J, Figueroa M, Belzunegui J, et al. A double-blind placebo controlled trial of etanercept in patients with giant cell arteriitis and corticosteroid side effects. Ann Rheum Dis. 2008;67(5):625–30.
53 Hoffman GS, Cid MC, Rendt-Zagar KE, Merkel PA, Weyand CM, Stone JH, et al. Infliximab for maintenance of glucocorticosteroid-induced remission of giant cell arteriitis: a randomized trial. Ann Intern Med. 2007;146(9):621–30.
54 Seitz M, Reichenbach S, Bonel HM, Adler S, Wermelinger F, Villiger PM. Rapid induction of remission in large vessel vasculitis by IL-6 blockade. A case series. Swiss Med Wkly. 2011;141:w13156.
55 Lee MS, Smith SD, Galor A, Hoffman GS. Antiplatelet and anticoagulant therapy in patients with giant cell arteriitis. Arthritis Rheum. 2006;54(10):3306–9.
56 Gonzalez-Gay MA, Pineiro A, Gomez-Gigirey A, Garcia-Porrua C, Pego-Reigosa R, Dierssen-Sotos T, et al. Influence of traditional risk factors of atherosclerosis in the development of severe ischemic complications in giant cell arteriitis. Medicine (Baltimore). 2004;83(6):342–7.
57 Salvarani C, Della Bella C, Cimino L, Macchioni P, Formisano D, Bajocchi G, et al. Risk factors for severe cranial ischaemic events in an Italian population-based cohort of patients with giant cell arteriitis. Rheumatology (Oxford). 2009;48(3):250–3.
58 Gonzalez-Gay MA, Garcia-Porrua C, Pineiro A, Pego-Reigosa R, Llorca J, Hunder GG. Aortic aneurysm and dissection in patients with biopsy-proven giant cell arteriitis from northwestern Spain: a population-based study. Medicine (Baltimore). 2004;83(6):335–41.
59 Marie I, Proux A, Duhaut P, Primard E, Lahaxe L, Girszyn N, et al. Long-term follow-up of aortic involvement in giant cell arteriitis: a series of 48 patients. Medicine (Baltimore). 2009;88(3):182–92.
60 Nuenninghoff DM, Hunder GG, Christianson TJ, McClelland RL, Matteson EL. Incidence and predictors of large-artery complication (aortic aneurysm, aortic dissection, and/or large-artery stenosis) in patients with giant cell arteriitis: a population-based study over 50 years. Arthritis Rheum. 2003;48(12):3522–31.
61 Matteson EL, Gold KN, Bloch DA, Hunder GG. Long-term survival of patients with giant cell arteriitis in the American College of Rheumatology giant cell arteriitis classification criteria cohort. Am J Med. 1996;100(2):193–6.
62 Gonzalez-Gay MA, Blanco R, Abraira V, Garcia-Porrua C, Ibanez D, Garcia-Pais MJ, et al. Giant cell arteriitis in Lugo, Spain, is associated with low longterm mortality. J Rheumatol. 1997;24(11):2171–6.