Computed tomography angiography (CTA) to prove circulatory arrest for the diagnosis of brain death in the context of organ transplantation
DOI: https://doi.org/10.4414/smw.2011.13261
Summary
QUESTION UNDER STUDY: For the determination of brain death (BD) in potential organ donors, confirmatory tests that show cessation of cerebral circulation are used in many countries. Conventional angiography is considered the golden standard among these ancillary examinations. In recent years other angiographic techniques such as CT angiography (CTA) have been increasingly employed to establish the diagnosis of BD. We report our experience with CTA in this setting.
MATERIAL AND METHODS: From 2007 to 2010, 29 patients were examined in order to determine BD using CTA. The studies consisted of an unenhanced head scan, a CT angiogram of the brain supplying vessels in the head and neck and a second head scan 80 seconds after contrast injection (venous phase). The studies were retrospectively re-evaluated by two experienced neuroradiologists according to the criteria accepted by the Swiss Academy of Medical Sciences.
RESULTS: In 22 patients, cessation of cerebral circulation was confirmed in the venous phase CT. In seven patients, cessation of brain circulation was not confirmed due to residual contrast enhancement in the relevant cerebral vessels, i.e. the M4-segments of the middle cerebral artery and/or the internal cerebral veins. In these patients, clinical re-evaluation after a minimum of six hours confirmed the diagnosis of BD. Using the clinical examination as the “golden standard,” CTA achieved a sensitivity of 75.9%.
CONCLUSION: CTA is a useful additional tool for the confirmation of the diagnosis of brain death. Pooling of contrast in the relevant cerebral vessels, however, can be detected in up to 25% of CTAs in clinically brain dead patients.
Abbreviations:
BD Brain Death
CTA Computed Tomography Angiography
DSA Digital Subtraction Angiography
MDCT Multi-Detector Computed Tomography
TCD Trans-Cranial Doppler
Introduction
In many countries confirmatory tests are required for the diagnosis of brain death in patients who meet the clinical criteria of death. In Switzerland, the current Swiss Federal Law on the Transplantation of Organs, Tissues, and Cells, implemented in July 2007, defines death as follows: A human being is dead “if the functions of his or her brain, including the brainstem, have irreversibly ceased” [1].
In Switzerland, 103 deceased donors were recorded in 2009. Cerebral haemorrhage was the most common cause of death [2]. Twenty one of these donors were treated at the authors’ institution.
The current medical ethical guidelines issued by the Swiss Academy of Medical Sciences in May 2005 [3] state that brain death (defined as “Demonstration of irreversible cessation of brain function in the context of intended organ transplant”) cannot be determined by a single clinical observation. A second clinical exam six hours later or an additional test to confirm the cerebrovascular flow arrest is required. Approved ancillary tests are DSA [4] (fig. 1), TCD [5, 6], or 99m-Tc-HMPAO-SPECT [7, 8]. In addition, hypothermia, metabolic disorders, intoxication or medication must be ruled out as a possible cause of coma. In patients with suspected cranial polyradiculitis and in those whose cranial nerve function cannot be tested clinically, ancillary tests are mandatory.
Another test that is explicitly mentioned in the current guidelines is “CT angiography (for the visualisation of blood vessels)“ [3]. This is usually performed as CT angiography of the supraaortic and intracranial vessels. As we have been confronted with nonspecific and ambiguous findings, such as only slight opacification of basal intracranial vessels to a varying extent, we have systematically and retrospectively studied all CTA performed since October 2007 (n = 37) at the authors’ institution for the confirmation of brain death. We compare our protocol and results to those published by Dupas [9] and Frampas [10], which were used to define the French and Swiss guidelines for determining brain death [3, 11].
The purpose of our study was, firstly, to test the applicability of the Dupas and Frampas criteria in a clinical setting, and secondly, to show that the standardised protocol used here for performing and evaluating a CT brain death study is feasible with any modern MDCT scanner, which might help to further propagate this method in other hospitals in Switzerland.
Material and methods
Patient characteristics and study design
Over a period of 30 months (10/2007 – 4/2010), CT of the head and CTA of the supraaortic and intracranial vessels was performed in 37 eligible donors (consent by either patient [organ donor card] or relatives was given) who fulfilled the clinical criteria as defined in the 2007 guidelines, i.e. in all patients, all clinical signs of brain death had been found by a neurological exam including a positive apnoea test.
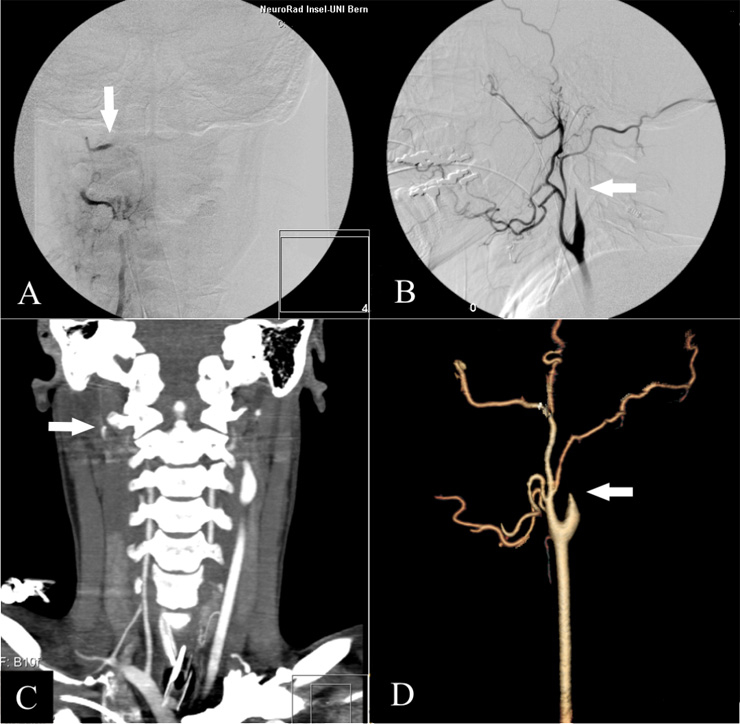
Figure 1
“Gold Standard” DSA: AP view of the right vertebral artery (A) and lateral view of the right carotid artery (B). The stop of contrast in the intersection between extra- and intradural segment of the vertebral artery and the cervical segment of the internal carotid artery are typical angiographic findings (white arrows); in DSA, they are rated as proof of cerebral circulatory arrest.
From the axial CT-Dataset similar views as DSA can be obtained in 2D- (C; vertebral artery) or 3D-reformations (D; carotid artery). For the evaluation of brain death we only use the axial source images. Doing reformations is time consuming, operator dependent and not established in the literature for the determination of brain death. They are shown here only for exemplary purposes and nicely illustrate the lack of intracranial blood supply.
After detailed retrospective re-evaluation of the 37 CT exams, eight had to be excluded because of deviations from the CTA protocol that later became standard. Although these variations still allowed sufficient diagnostic safety, we decided to focus on the 29 patients who were examined according to the same, still current protocol.
The analysis of these patients’ clinical and imaging data for this study was approved by the institutional review board as required for retrospective studies.
Imaging protocol
Patients were examined in either an 8-slice GE LightSpeed Ultra (General Electric, Milwaukee, USA) or a 16-slice Somatom Sensation 16 CT scanner (Siemens Medical Systems, Erlangen, Germany). The standard protocol consisted of an unenhanced CT study of the head, a CT angiography of the cervical and intracranial vessels with application of 70 ml contrast agent (Iomeron® 400, Bracco SA, Milan, Italy) into a cubital vein using an automated injector with a flow rate of 4 ml/s (MedRad, Volkach, Germany), and a late venous-phase contrast-enhanced series of the skull with the same parameters as the unenhanced scan (table 1). The CTA was started automatically with the appearance of contrast agent in the aortic arch, the late-phase series was started manually one minute after the CTA was finished.
Image analysis
For this study, the axial source images of the CT angiography and the venous-phase CT were re-evaluated in comparison to the unenhanced study. Two experienced neuroradiologists classified the image findings according to two published criteria lists in consensus reading by comparing the CTA and venous-phase CT with the unenhanced CT of each patient. Firstly, opacification of the A3-segments of the anterior cerebral artery, the M4-segments of the middle cerebral artery, the vein of Galen and the internal cerebral veins were considered as signs of a still perfused brain according to a 7 point scale proposed by Dupas et al. [9]. Then, we analysed only four criteria that had to be met to make the diagnosis of circulatory arrest in the late-phase study according to the 4 point scale proposed by Frampas et al. [10]. Namely, no contrast in the M4-segments of both middle cerebral arteries and no contrast in the internal cerebral veins on both sides (table 2). In both evaluations, bilateral opacification of the superficial temporal artery was used as an indicator of a technically successful study (fig. 2).
During the study all patients were intubated, ventilated and monitored by the Intensive Care Unit team. As mandatory the mean arterial blood pressure was recorded. Clinical diagnosis of brain death was made by staff neurologists. When the staff neuroradiologist found intracranial circulatory arrest the patient was declared legally dead.
All patients in our study were clinically diagnosed as brain dead, so the sensitivity for CTA has been calculated for having a positive result.
|
Table 1: CT study protocols on the two scanners used. |
|
GE LightSpeed Ultra |
| |
|
Unenhanced study
|
CT-Angiography
|
Enhanced (late-phase) study
|
| Exam volume |
|
Entire brain |
Aortic arch to vertex |
Entire brain |
| Gantry angulation |
|
Cantho-meatal |
No tilt (0°) |
Cantho-meatal |
| mAs |
Posterior fossa |
180 |
380* |
180 |
| Supratentorial |
160 |
380* |
160 |
| keV |
|
120 |
140 |
120 |
| Field of view [mm] |
Men |
220 |
200 |
220 |
| Women |
200 |
200 |
200 |
| Slice thickness / table feed [mm] |
|
1.25 / 1.25 |
1.25 / 0.625 |
1.25 / 1.25 |
| |
*: Preset value; automatic dose adjustment |
|
Siemens sensation 16 |
| |
|
Unenhanced study |
CT-Angiography |
Enhanced (late-phase) study |
| Exam volume |
|
Entire brain |
Aortic arch
to vertex |
Entire brain |
| Gantry angulation |
|
Cantho-meatal |
No tilt (0°) |
Cantho-meatal |
| mAs |
|
200 |
130–500* |
200 |
| keV |
|
120 |
120 |
120 |
| Field of view [mm] |
|
200 |
220 |
200 |
| Slice thickness / table feed [mm] |
|
1.5 / 1.5 |
0.75 / 0.75 |
1.5 / 1.5 |
| |
*: Automatic dose adjustment |
|
Table 2: Scored vessel evaluation for the determination of circulatory arrest. |
|
1 point each for
non-opacification of the
|
7 point score
(Dupas et al., 1998)
|
4 point score
(Frampas et al., 2009)
|
| terminal braches (M4) of the right middle cerebral artery |
1 point |
1 point |
| terminal braches (M4) of the left middle cerebral artery |
1 point |
1 point |
| right pericallosal artery (A3) |
1 point |
|
| left pericallosal artery (A3) |
1 point |
|
| right internal cerebral vein |
1 point |
1 point |
| left internal cerebral vein |
1 point |
1 point |
| great cerebral vein |
1 point |
|
|
sum
|
7 points
|
4 points
|
Results
There were 15 men and 14 women with a mean age of 49.2 years (range 16.2–85.7) in the study. Detailed demographic and clinical data are given in table 3. The leading causes of death were trauma and cerebrovascular diseases followed by intracranial haemorrhage (table 4).
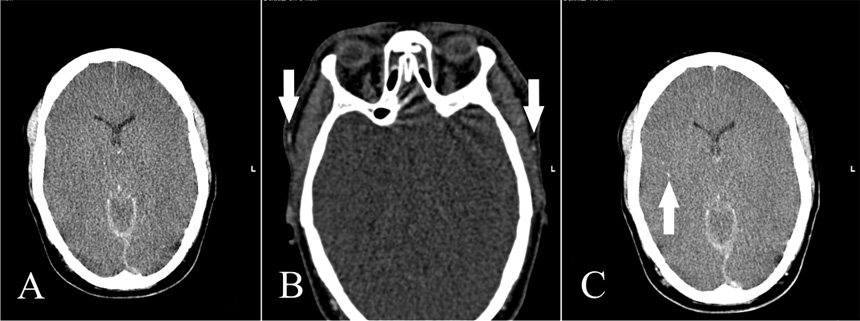
Figure 2
A: Non-contrast CT shows subarachnoid and subdural haemorrhage; gray matter/white matter differentiation is not discernible. B: CTA with contrast media in the superficial temporal artery on both sides indicating a technically sufficient study (white arrows). There is no intracranial arterial opacification. C: Venous-phase CT shows opacification of an M3 segmental artery on the right (white arrow) but no contrast in the M4 segments of the middle cerebral artery and/or the internal cerebral veins. Cerebral circulatory arrest was confirmed.
There were no problems in performing the CT studies according to our protocol. No toxic side effects to the intravenously injected contrast agent were observed. On average, the mean arterial blood pressure was 85.3 mm Hg.
CT exam time (scoutview to final late-phase series) was 8:39 (median, ± 2:43) minutes. This duration was independent from the time of day when the study was performed as an experienced team of technicians and nurses was available around the clock.
According to our protocol, the late-phase study has to be performed one minute after the start of the angio series. In our patients, the mean time between CTA and late-phase series was 1:04 (median, ± 0:22) minutes.
All studies could be used for evaluation because bilateral opacification of the superficial temporal arteries was clearly visible indicating sufficient intravenous bolus injection of the contrast, sufficient cardiac output and correct timing of the CTA examination (table 3).
Vessel-Evaluation (table 2) of the early arterial phase CTA revealed two patients in whom the criteria of brain circulatory arrest were not met. In one patient, A3 segments of the anterior cerebral artery were opacified on both sides (according to the 7 point scale), in another patient, all arterial vessels up to and including A3 and M4-segments as well as the deep cerebral veins were opacified (table 3).
In the analysis of the relevant venous-phase CT, the number of patients who could not be declared brain dead according to CTA criteria rose to seven (24.1%). In three of these patients, A3 and M4-segments were contrasted (fig. 3), in one patient, only deep cerebral veins showed opacification and in three patients both arterial and deep venous vessels were opacified. The sensitivity for CTA was 75.9%. Evaluation according to the 7 point and 4 point scales yielded identical results. In the seven patients who did not show circulatory arrest a second clinical observation within 6 to 12 hours finally confirmed brain death.
Of these seven patients, three had some form of potentially pressure relieving defect of the skull (one craniectomy, gunshot and fracture each). Among the patients declared as brain dead, there were also three patients with similar conditions where the bony skull was not intact.
|
Table 3: Patient data and CT imaging findings. |
|
|
| 1 |
m |
16.2 |
s |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
y |
y |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
y |
y |
| 2 |
m |
47.3 |
a |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
y |
y |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
y |
y |
| 3 |
f |
69.0 |
h |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
y |
y |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
y |
y |
| 4 |
m |
28.0 |
t |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
y |
y |
|
|
|
|
|
|
|
|
|
n |
n |
| 5 |
m |
44.9 |
c |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
y |
y |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
y |
y |
| 6 |
m |
19.1 |
t |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
y |
y |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
y |
y |
| 7 |
f |
71.7 |
h |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
y |
y |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
y |
y |
| 8 |
m |
35.5 |
a |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
y |
y |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
y |
y |
| 9 |
m |
85.7 |
h |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
y |
y |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
y |
y |
| 10 |
f |
26.3 |
t |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
y |
y |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
y |
y |
| 11 |
f |
59.4 |
t |
1 |
1 |
|
|
1 |
1 |
1 |
|
|
n |
y |
|
|
|
|
1 |
1 |
1 |
|
|
n |
n |
| 12 |
f |
41.7 |
c |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
y |
y |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
y |
y |
| 13 |
f |
52.7 |
a |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
y |
y |
1 |
1 |
1 |
1 |
|
|
|
|
|
n |
n |
| 14 |
m |
40.1 |
t |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
y |
y |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
y |
y |
| 15 |
f |
53.0 |
c |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
y |
y |
|
|
|
|
1 |
1 |
1 |
|
|
n |
n |
| 16 |
f |
70.9 |
h |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
y |
y |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
y |
y |
| 17 |
f |
53.7 |
h |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
y |
y |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
y |
y |
| 18 |
m |
47.8 |
t |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
y |
y |
|
|
1 |
1 |
|
|
|
|
|
n |
n |
| 19 |
m |
33.1 |
a |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
y |
y |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
y |
y |
| 20 |
f |
62.7 |
c |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
y |
y |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
y |
y |
| 21 |
m |
35.8 |
c |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
y |
y |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
y |
y |
| 22 |
m |
74.2 |
c |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
y |
y |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
y |
y |
| 23 |
f |
64.0 |
c |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
y |
y |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
y |
y |
| 24 |
f |
34.6 |
o |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
y |
y |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
y |
y |
| 25 |
f |
61.7 |
c |
|
|
|
|
|
|
|
|
|
n |
n |
|
|
|
|
|
|
|
|
|
n |
n |
| 26 |
m |
63.5 |
h |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
y |
y |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
y |
y |
| 27 |
f |
82.1 |
h |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
y |
y |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
y |
y |
| 28 |
m |
16.6 |
t |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
y |
y |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
y |
y |
| 29 |
m |
34.2 |
t |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
|
y |
y |
|
|
|
|
1 |
1 |
1 |
|
|
n |
n |
| Legend:
1) Cause of death:
a = anoxia; c = cerebrovascular disease; h = haemorrhage; o = other; s = suicide; t = trauma
2) Opacification: 1: none; 0: vessel opacified
3) 7-point- and 4-point-scale:
According to Ref. [7, 8]; y: criteria for brain death fulfilled, n: criteria not fulfilled |
|
Table 4: Cause of death. |
| |
Number
|
% of all 29 patients
|
| Anoxia |
4 |
13.8 |
| Cerebrovascular disease |
8 |
27.6 |
| Haemorrhage |
7 |
24.1 |
| Suicide |
1 |
3.4 |
| Trauma |
8 |
27.6 |
| Other |
1 |
3.4 |
Discussion
The introduction of CTA as a means of determining circulatory arrest in the brain was intended to simplify and to accelerate the definite diagnosis of brain death [9–14].
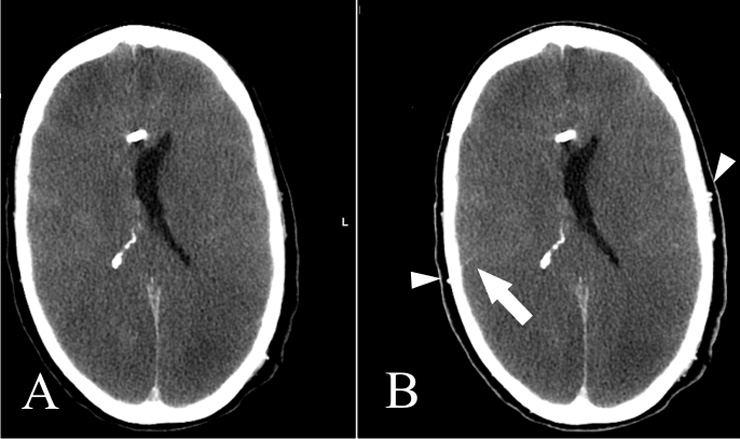
Figure 3
Non-contrast CT (A) and venous-phase CT (B): Opacification of the superficial temporal artery indicates sufficient contrast injection (Arrowheads). Note also opacification of an M4 segment artery on the right, a marker of some residual cortical perfusion. In this case, circulatory arrest could not be confirmed.
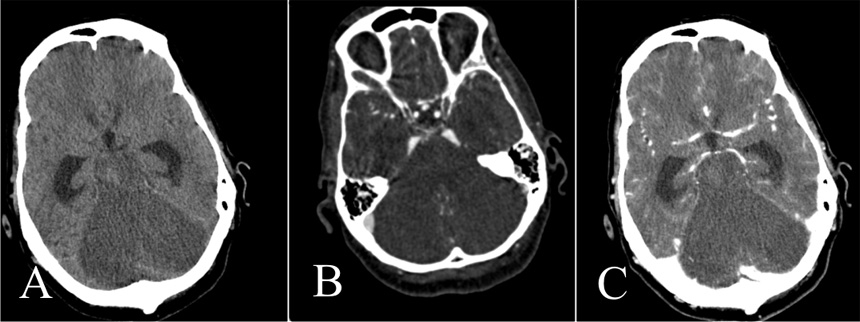
Figure 4
Unenhanced CT (A), CTA (B), and venous-phase CT (C).
Note normal opacification of the supratentorial vessels. Brain death could not be confirmed although there is extensive infarction of the cerebellum, pons and brainstem. The supratentorial ventricles are dilated.
Several studies have compared CTA with other approved ancillary tests:
– Quesnel et al. [12] compared CTA with EEG. He found a sensitivity of only 52.4% for CTA determining BD by using the 7 point score of Dupas.
– Combes et al. [13] compared CTA with venous injected cerebral DSA and showed sensitivity for CTA of 69.7%.
– Berenguer et al. [14] recently compared CTA with nuclear medicine perfusion scans. They used only the arterial phase for the diagnosis of BD and found a sensitivity of 86%.
Technically, CTA is far easier to perform than conventional catheter DSA. It can also be performed in smaller hospitals wherever a CT scanner is available.
The protocol is not demanding with regard to scanner speed, although a modern MDCT allows for thinner slices and thereby simplifies evaluation of more peripheral and smaller branches of the cerebral vessels. It is important to precisely reproduce the technical and geometric parameters of the non-enhanced series in the late enhancement study [9, 10] as the evaluation of, for example, M4 opacification requires high spatial and contrast resolution. This is especially important in the presence of subarachnoid haemorrhage where very slight opacification may be hard to detect.
Our protocol slightly differs from the protocol suggested by Dupas and Frampas [9, 10] as we start the CTA at the level of the aortic arch. We consider this approach justified as the additional X-ray exposure is minimal while we obtain supplementary information on the cervical section of the brain supplying arteries. If, for example, opacification of a temporal artery is missing, a study of the cervical vessels may show the underlying pathology (traumatic tear or haemorrhagic compression of the external carotid artery). Whilst Dupas and Frampas [9, 10] report results from series that were acquired with fixed time intervals of 20 and 60 seconds after injection, we use a bolus triggering technique to start the CTA and perform a late enhancement series 60 seconds after CTA start. Furthermore, we use only 70 ml of contrast agent with a flow rate of 4 ml/s in comparison to the published 120 ml / 3 ml/s which reduces total injection time in our protocol from 40 to 17.5 seconds.
In brain dead patients, a phenomenon known as “stasis filling” may occur in the late venous phase. This term was initially used in conventional angiography [15] and describes a prolonged filling of subarachnoid intracranial vessels. In the meantime, it is also used to describe this phenomenon in CTA [9]. To evaluate parenchymal blood flow, it is therefore best to look for opacification of distal cerebral arteries as close as possible to the capillary bed, e.g. M4 segments of the middle cerebral artery. On the other side of the capillary bed, an additional prove of persistent cerebral blood flow is the opacification of the internal cerebral venous system in the venous-phase of CTA [16].
Arterial-phase CTA alone is probably not suitable for the determination of brain death because venous enhancement can only be visualised in a venous phase CT. This is an important issue in determining perfusion of the cortex and basal ganglia. In 1998, Dupas [9] found a large increase of opacified cervical and intracranial vessels in the late venous-phase CT (60 seconds) compared to the early arterial phase (20 seconds). Our results confirm this finding. The initial series is suitable to study gross vascular anatomy in the neck and a stop of the contrast bolus but not for the clear delineation of residual cerebral circulation.
Opacification of distal M4 branches of the middle cerebral artery is a parameter for cortical perfusion, the internal cerebral veins are an indicator of basal ganglia perfusion [9, 10, 16].
One patient (table 2, no. 25) had extensive ischaemia of the cerebellum, pons and brainstem, the supratentorial territory, however, was nearly normally opacified in the arterial and venous phase (fig. 4) meaning that the criteria of circulatory arrest of the whole brain were not fulfilled. This is an example where the supratentorial brain may still be viable in context of law but the infarction of the brainstem leads to a clinically unreactive coma.
While Frampas [10] found a sensitivity of 62.8% for the 7 point scale and 85.7% for the improved 4 point scale in the venous-phase CTA study, our results from both methods were comparable (75.9%). A possible explanation is the timing of our protocol. Due to the use of the manual start of the venous phase CT, this starts approximately 80 seconds after the beginning of the contrast injection. The “stasis filling” [9, 15] therefore might have a longer effect and so there might be more opacification of the distal vessels. In addition, the mean interval between clinical examination and CTA might be shorter so that the intracranial pressure might not be sufficiently high.
Cessation of brain circulation due to BD is a continuous process. During this process the clinical and technical ancillary exams are performed. The clinical examination targets the brain stem. When the patient is clinically brain dead there might still be insufficient oedema of the brain to raise the intracranial pressure above the blood pressure. In this case there might still be an opacification of intracranial vessels, as illustrated in seven of the 29 cases in this study. This, however, does not mean that the brain is sufficiently perfused and viable. The longer the waiting period between clinical BD observation and CTA, the higher the sensitivity of CTA [17].
Nevertheless, even with standardised protocols and proven evaluation scores there remains a noticeable difference of about 25% between the initial clinical diagnosis of brain death and opacification of significant brain supplying or draining vessels in the following CT. This is not surprising, because CTA (as DSA) is not a method for measuring brain perfusion, which is defined as steady state delivery of blood to the brain parenchyma. Using CTA, we investigate and detect with high sensitivity whether contrast agent reaches blood vessels far from the capillary bed, i.e. arteries and veins with diameters greater than 200 µm.
This may happen by stasis filling, as described above or via the numerous and well known collaterals from the external carotid artery, which can transmit small amounts of contrast to the intracranial blood vessels. In addition, retrograde intracranial venous inflow can occur through similar mechanisms. Contrast enhancement of dural sinuses, which are not compressed by increased intracranial pressure, may be preserved in BD. In our series of 29 patients we found opacification of the superior sagittal sinus in more than 50%, which is in accordance with published data [8].
In many countries, including Switzerland, there is a defined waiting time before confirming death with a second clinical examination. Alternatively and to avoid this time delay, CTA may be performed.
Thanks to recent developments in CT technology [18, 19], using one single intravenously injected contrast bolus, 320-row MDCT can simultaneously reconstruct a digitally subtracted whole brain 4D-CTA with direct visualisation of the blood flow through the arterial, parenchymal and venous phase with a temporal resolution of one second. In addition, perfusion parameters such as cerebral blood flow and blood volume can be calculated from the same data set.
The combination of both – the morphological information from CTA and the functional information obtained by perfusion CT – should increase the sensitivity of a brain death CT study. Even if small amounts of contrast invade proximal cerebral vessels, arrest of cerebral circulation and lack of brain perfusion can be detected and visualised directly. To date, however, this concept has not been studied for the determination of brain death.
In conclusion, arterial and venous phase CTA is an easy to perform method to prove circulatory arrest of the whole brain and confirm clinically diagnosed brain death as required by current Swiss legislation. In about 75% brain death can be confirmed by this ancillary test in order to avoid the time delay of six hours which is mandatory if brain death has to be confirmed by two clinical neurological examinations only. TCD as another ancillary test might have technical difficulties (no bone window in up to 10% of cases) and is skill dependent [6, 20]. CTA, if performed according to a standardised protocol, is fast, can be performed on any MDCT scanner, is usually available around the clock in larger hospitals and is, last but not least, less invasive and less expensive than DSA. This might help to further propagate this method in other hospitals in Switzerland.
Evaluation should be performed by a radiologist with experience in neurovascular CT studies as the exact delineation of opacified vessels may be difficult if, for example, subarachnoid haemorrhage is present.
This study shows that the Dupas [9] and the new Frampas [10] protocol and criteria, as suggested in the SAMW guidelines, are sufficiently robust to be used clinically.
A limitation is that, as this is a retrospective study that focuses on the actual clinical use of one method, we did not compare MDCT to other ancillary test or imaging modalities. According to the procedures established at our institution, we cannot evaluate the possible use of a second MDCT study as in cases where CT did not clearly show brain death, we generally opt for a second clinical examination.
The 4 point evaluation score should probably be included in future editions of the Swiss guidelines as it provides a reliable method that can easily be established and standardised in all Swiss hospitals involved in organ extraction and transplantation.
Addendum: While we were preparing this manuscript, a revised version of the SAMW guidelines was prepared and discussed and passed the internal commission in late May 2011. Pending approval by the Federal Council, it may come into effect in 2011. These new guidelines rely on only one clinical determination of brain death using the well approved criteria for this examination. According to these revised guidelines, CT (or other ancillary tests) will only be required in cases where a clinical exam is not appropriate because the reason for the loss of cerebral function is unclear.
References
1 Schweizerische Eidgenossenschaft. Bundesgesetz vom 8. Oktober 2004 über die Transplantation von Organen, Geweben und Zellen (Transplantationsgesetz). SR-Number 810.21, enacted July, 1st 2007. (German text)
2 Swiss National Foundation for organ donation and transplantation (Swiss Transplant), annual report 2009. German. Available from: http://www.swisstransplant.org
3 Swiss Academy of Medical Sciences. The Determination of Death in the Context of Organ Transplantation – Medical Ethical Guidelines of the SAMS. 2005
4 Vatne K, Nakstad P, Lundar T. Digital subtraction angiography (DSA) in the evaluation of brain death. A comparison of conventional cerebral angiography with intravenous and intraarterial DSA. Neuroradiology. 1985;27(2):155–7.
5 Monteiro LM, Bollen CW, van Huffelen AC, Ackerstaff RG, Jansen NJ, van Vught AJ. Transcranial Doppler ultrasonography to confirm brain death: a meta-analysis. Intensive Care Med. 2006; 32(12):1937–44.
6 De Freitas GR, Andre C. Sensitivity of transcranial Doppler for confirming brain death: a prospective study of 270 cases. Acta Neurol Scand. 2006;113(6):426–32.
7 Joffe AR, Lequier L, Cave D. Specificity of radionuclide brain blood flow testing in brain death: case report and review. J Intensive Care Med. 2010;25(1):53–64.
8 Lee VW, Hauck RM, Morrison MC, Peng TT, Fischer E, Carter A. Scintigraphic evaluation of brain death: significance of sagittal sinus Visualization. J Nucl Med. 1987;28(8):1279–83.
9 Dupas B, Gayet-Delacroix M, Villers D, Antonioli D, Veccherini MF, Soulillou JP. Diagnosis of brain death using two-phase spiral CT. AJNR Am J Neuroradiol. 1998;19(4):641–7.
10 Frampas E, Videcoq M, de Kerviler E, Ricolfi F, Kuoch V, Mourey F, et al. CT angiography for brain death diagnosis. AJNR Am J Neuroradiol. 2009;30(8):1566–70.
11 Leclerc X, Boulin A, Bracard S, Chiras J, Cognard Ch, Courtheoux P, et al. CT angiography for the diagnosis of brain death: recommendations of the French Society of Neuroradiology (SFNR). J Neuroradiol. 2007;34(4):217–9.
12 Quesnel C, Fulgencio JP, Adrie C, Marro B, Payen L, Lembert N, et al. Limitations of computed tomographic angiography in the diagnosis of brain death. Intensive Care Med. 2007;33(12):2129–35.
13 Combes J-C, Chomel A, Ricolfi F, D’Athis P, Freysz M. Reliability of Computed Tomographic Angiography in the Diagnosis of Brain Death. Transplantation Proc. 2007;39(1):16–20.
14 Berenguer CM, Davis FE, Howington JU. Brain death confirmation: comparison of computed tomographic angiography with nuclear medicine perfusion scan. J Trauma. 2010;68(3):553–9.
15 Kricheff II, Pinto RS, George AE, Braunstein P, Korein J. Angiographic findings in brain death. Ann N Y Acad Sci. 1978;315(1):168–83.
16 Andeweg J. Consequences of the anatomy of deep venous outflow from the brain. Neuroradiology. 1999;41(4):233–41.
17 Brocas E, Thierry S, Le Roy C, et al. Délai entre le diagnostic clinique et angioscannographique d’état de mort encéphalique (EME). A propos de 10 cas. 46ème congrès national d’anesthésie et de réanimation 2004, Paris. Editions scientifiques et médicales Elsevier SAS et SFAR. R239
18 Salomon E, Barfett J, Willems P, Geibrasert S, Bacigaluppi S, Krings T. Dynamic CT Angiography and CT Perfusion Employing a 320-Detector Row CT. Protocol and Current Clinical Applications. Clin Neuroradiol. 2009;19(3):187–96.
19 Klingebiel R, Siebert E, Diekmann S, Wiener E, Masuhr F, Wagner M, et al. 4-D Imaging in Cerebrovascular Disorders by Using 320-Slice CT: Feasibility and Preliminary Clinical Experience. Acad Radiol. 2009;16(2):123–9.
20 McMahon CJ, McDermott P, Horsfall D, Selvarajah JR, King AT, Vail A. The reproducibility of transcranial Doppler middle cerebral artery velocity measurements: implications for clinical practice. Br J Neurosurg. 2007;21(1):21–7.



