Mandibular advancement splints for the treatment of sleep apnoea syndrome
DOI: https://doi.org/10.4414/smw.2011.13276
Summary
Oral devices, in particular Mandibular Advancement Splints (MAS), which hold the mandible in a protruded position during sleep, are increasingly used for the treatment of Obstructive Sleep Apnoea (OSA). These devices can be effective in treating OSA across a range of severity. Complete resolution of OSA (Apnoea-Hypopnoea Index [AHI] reduced <5/hr) with use of an MAS occurs in around 40% of patients. Overall two thirds of patients experience some clinical benefit (≥50% AHI reduction AHI) however others will not objectively respond to this form of treatment, despite improvement in symptoms. Although MAS are less efficacious in reducing polysomnographic indices of OSA than the standard treatment, Continuous Positive Airway Pressure (CPAP), improvements in health outcomes appear to be comparable. Therefore, the superiority of CPAP in improving oxygen desaturations and reducing AHI may be extenuated by its low compliance, resulting in both treatments having similar effectiveness in clinical practice. MAS are now recommended as a first line treatment for mild to moderate OSA, as well as in more severe patients who are unable to tolerate or refuse CPAP. Success with MAS treatment has been associated with factors such as female gender, younger age, supine-dependent OSA, lower BMI, smaller neck circumference and craniofacial factors, however a reliable, validated method for prediction in the clinical setting has yet to be established. MAS are well tolerated, however short-term side effects are common although generally minor and transient. Long-term dental changes are for the most part subclinical, but can be problematic for a minority of patients. MAS are a dental-based treatment for a medical sleep disorder and, as such, an interdisciplinary care model is considered important for the attainment of optimal patient outcomes.
Introduction
Obstructive Sleep Apnoea (OSA) is a disorder characterised by repetitive closure of the upper airway during sleep, resulting in sleep fragmentation and nocturnal oxygen desaturation, leading to daytime sleepiness and neurocognitive impairment. Long-term consequences of OSA include increased risk of cardiovascular morbidity and all cause mortality [1]. Thus with the estimated prevalence of OSA at 4% of men and 2% of women in the middle-aged population [2] and the associated symptoms and increased risk of adverse long-term consequences, OSA is a significant public health problem. Therefore implementation and sustentation of effective treatment is vital. The current gold standard treatment, Continuous Positive Airway Pressure (CPAP), involves delivering positive pressure generated by a machine to the upper airway via tubing and a facial/nasal mask interface to pneumatically splint open the airway at night. Although CPAP is highly efficacious in preventing upper airway collapse, its obtrusive nature makes adherence to treatment suboptimal [3–4]. Over the last decade or so, oral devices have emerged as a viable alternative to CPAP therapy for the treatment of OSA. Oral devices are worn during sleep to alter the upper airway configuration thereby reducing the propensity to collapse. Some less commonly used designs do this by holding the tongue forward in a protruded position outside the lips thus preventing tongue movement into the posterior airway space (tongue stabilising/retaining devices). However most commonly used and investigated are Mandibular Advancement Splints (MAS) which are worn intra-orally to protrude and hold the mandible in a forward position (fig. 1). This anatomical adjustment alters upper airway structure and function such that airway collapsibility during sleep is reduced [5] (fig. 2). The advantages of this form of treatment compared to CPAP include simplicity and portability, no requirement for a power supply and better patient acceptance. The therapeutic use of oral devices for treatment of OSA has been validated over the last decade by a substantial number of randomised-controlled trials supporting their efficacy. The mandibular advancement type of oral devices are now recommended as a first line therapy for mild to moderate OSA and in more severe cases where CPAP is refused or is not able to be tolerated [6].
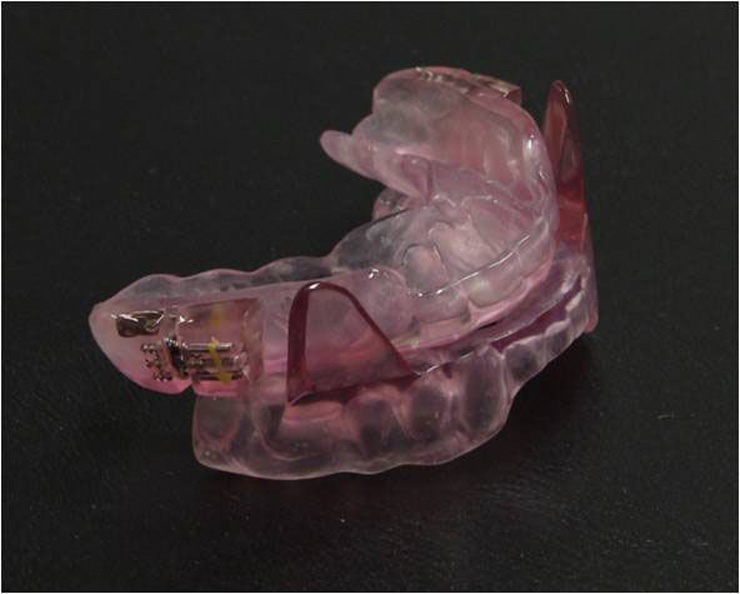
Figure 1
Mandibular Advancement Splint (MAS). Example of a customised two-piece device with plates that attach to the upper and lower dental arches (SomnoMed MASTM). With this device the level of mandibular advancement is adjusted via the lateral screws on the upper plate. The acrylic extensions on the lower plate provide the coupling to the upper plate to maintain the jaw in the protruded position.
The mechanisms of action of oral devices
Oral devices for the treatment of OSA in use in clinical practice today are predominantly MAS that attach to the upper and lower dental arches to protrude and retain the mandible in a forward position, and they are the oral devices referred to in this review. The objective of any treatment for OSA is to prevent the repetitive collapse of the upper airway during sleep. It has been demonstrated that MAS increase upper airway closing pressure, or in other words reduce collapsibility [5]. The mandibular protrusion produced by the device is intrinsic to the action of reducing upper airway collapse as inactive devices (which are worn intra-orally but do not advance the mandible) are ineffective in reducing the Apnoea-Hypopnoea Index (AHI) [7–8]. However the precise mechanisms by which these devices promote stabilisation of the airway through advancement of the mandible are still not well understood. The predominant effect of MAS on upper airway structure has been found to be enlargement of the velopharyngeal (retropalatal) airway space, mediated via an increase in lateral diameter (fig. 3) [9–12]. This perhaps counterintuitive effect of the MAS is presumably caused by the stretching of soft tissue connections that lie within the palatoglossal and palatopharyngeal arches and connect the mandible and tongue to the soft palate and lateral pharyngeal walls [13]. The resulting increase in airway volume provides a more patent airway with reduced probability of collapse. In addition to altering upper airway structure, MAS may also influence upper airway neuromuscular function. Research studies have demonstrated increased genioglossus muscle activity when the device is worn [14–15]. Stimulation of upper airway dilator muscles may therefore be an additional mechanism by which MAS stabilise the airway. The relative importance of anatomical versus neuromuscular responses to MAS may also vary between individual patients.
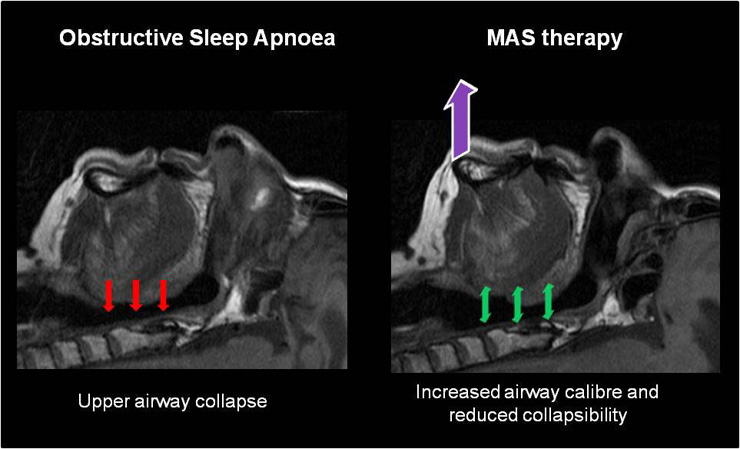
Figure 2
MAS treatment for Obstructive Sleep Apnoea. In OSA collapsing forces on the upper airway result in complete or partial upper airway obstruction. MAS worn during sleep hold the mandible and tongue in a protruded position resulting in an increase in upper airway calibre and a reduction in the propensity to collapse.
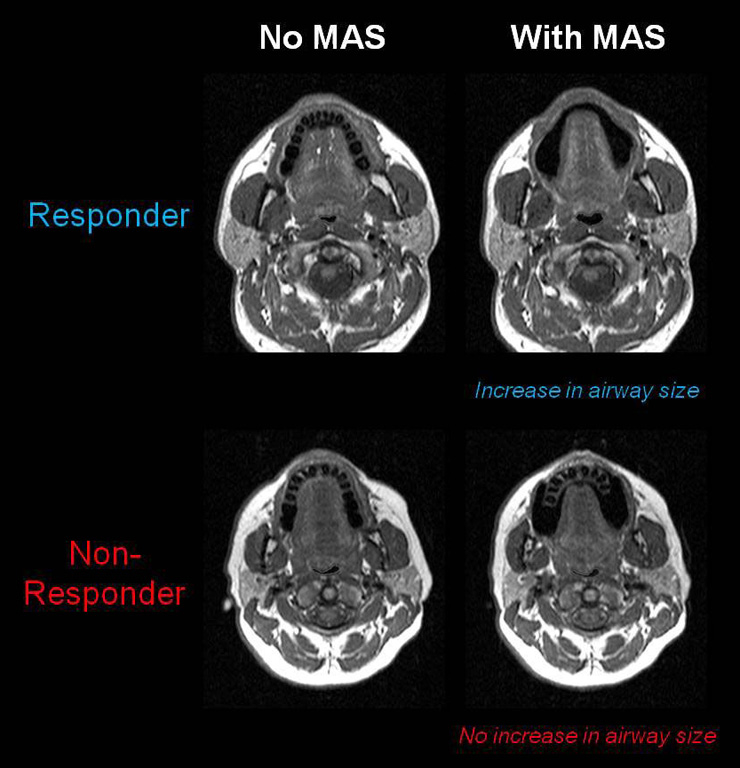
Figure 3
Comparison of upper airway responses to MAS in a treatment responder and non-responder using MRI. Differences in the upper airway response to MAS can be seen between patients who respond to this form of therapy and those who do not. In the MAS responder there is an increase in the cross-sectional area of the velopharynx, particularly in the lateral dimension. However in the MAS non-responder no increase in upper airway dimensions is evident.
Clinical efficacy of oral devices for the treatment of OSA
There are now a substantive number of published randomised controlled trials comparing oral devices to either placebo or CPAP treatment which have objectively established the efficacy of these devices for the treatment of OSA across the full range of disease severity [16–19]. Ultimately the goal of any OSA treatment is to improve symptoms and outcomes by preventing the occurrence of complete or partial upper airway collapse during sleep (apnoeas or hypopnoeas, respectively). The severity of OSA is measured by the Apnoea Hypopnoea Index (AHI, the number of apnoeas and hypopnoeas per hour of sleep) during overnight sleep monitoring by polysomnography. Success rates in reducing AHI vary with the definition of success used. Using the most stringent definition of treatment response (reduction of AHI to less than 5/hour or the absence of OSA), 35–40% of patients can be successfully treated with an oral device. However there are a further 25% of patients who still show a significant improvement, reducing AHI by 50% or more. The remaining 35–40% of patients will not respond (defined as less than 50% reduction in AHI) and some patients will even show a worsening of OSA with an oral device. Overall around two-thirds of OSA patients will experience a clinical benefit using an oral device [7–8, 20–21]. Apart from decreasing AHI, oral devices improve other polysomngraphic measures of OSA, including oxygen desaturation (although rarely to normal levels) [22–23], sleep architecture [8] and arousal indices [7, 23–24].
In terms of health outcomes, oral devices appear to lead to improvement in subjective daytime sleepiness as measured by the Epworth Sleepiness Scale [25–27], although the magnitude is small and may include a placebo effect [7–8]. However a limited number of studies using objective tests of sleepiness and simulated driving performance report improvements equivalent to those with CPAP [25–26, 28–29]. Reports on the effect of oral devices on neuro-physiological function are also limited but show improved performance in some neurocognitive assessments after oral device treatment but not others [25–26, 28, 30].
As OSA is associated with increased cardiovascular morbidity and mortality, modifying this risk is an important goal of treatment. Modest reductions in blood pressure (2 to 4 mm Hg) following treatment with an oral device have been reported both in uncontrolled and randomised placebo-controlled trials [20, 25, 31–33]. Currently long-term studies into the effect of oral devices on cardiovascular endpoints are lacking. However there is some preliminary evidence that oral devices may improve intermediate endpoints such as oxidative stress and endothelial function [34–35].
Improvements in long-term adverse consequences are of course dependent on sustained efficacy of the treatment over time. Less is known about the effectiveness of MAS therapy long term. However studies re-evaluating patients from 1–5 years after treatment initiation indicate that there is a reasonably high rate of sustained control even in severe OSA [23, 36–38]. Reductions in efficacy can be attributed to issues such as weight gain [39] or device wear and tear [37] and highlight the need for long-term dental and medical follow-up to sustain effectiveness.
In direct comparison to CPAP, oral devices are consistently found to be less efficacious in improving polysomnographic measures of OSA (AHI, oxygen saturation) [25, 28, 40–41]. These studies are summarised in table 1. However reports of similar improvements in health outcomes (e.g. blood pressure [25, 33], sleepiness [25–26, 28]) suggest that there may not be such a discrepancy in clinical practice. Specifically, suboptimal compliance to CPAP would reduce effective treatment time, impinging on its superior efficacy and potentially equilibrating the overall effectiveness of oral devices and CPAP in the clinical setting.
Surgical interventions, such as uvulopalatopharyngoplasty (UPPP), tonsillectomy and maxillomandibular advancement, are another treatment option for OSA [42]. Studies directly comparing surgical treatments for OSA to oral device therapy are rare. However one randomised trial comparing MAS with surgery (UPPP) found that MAS had the higher success rate in improving OSA at both 1 and 5 years follow-up [38].
|
Table 1: Efficacy of MAS compared to CPAP (standard treatment): published randomised crossover studies. |
|
Study
(First Author, Year)
|
Treatment interval
|
OSA SEVERITY
AHI (/hr)
|
Most efficacious treatment
(MAS vs. CPAP p <0.05) |
Patient treatment preference
|
|
BASELINE
|
CPAP
|
MAS
|
| Clark, 1996 [70] |
2 weeks |
38.9 ± 14.3 |
11.2 ± 3.9 * |
19.9 ± 12.7* |
N/A |
MAS |
| Ferguson, 1996 [69] |
4 months |
19.7 ± 13.8 |
3.5 ± 1.6 * |
9.7 ± 7.3* |
CPAP |
MAS |
| Ferguson, 1997 [68] |
4 months |
25.3 ± 15.0 |
4.0 ± 2.2 * |
14.2 ± 14.7* |
CPAP |
MAS |
| Engleman, 2002 [28] |
8 weeks |
31 ± 26 |
8 ± 6 * |
15 ± 16* |
CPAP |
CPAP |
| Randerath, 2002 [40] |
6 weeks |
17.5 ± 7.7 |
3.2 ± 2.9 * |
13.8 ± 11.1* |
CPAP |
MAS |
| Tan, 2002 [41] |
2 months |
22.2 ± 9.6 |
3.1 ± 2.8 * |
13.8 ± 11.1* |
NS |
MAS |
| Barnes, 2004 [25] |
3 months |
21.3 ± 1.3 |
4.8 ± 0.5 * |
14.0 ± 1.1* |
CPAP |
CPAP |
| Hoekema, 2008 [67] |
2-3 months |
39 ± 4.3 |
2.4 ± 4.2 * |
7.8 ± 14.4* |
NS |
N/A |
| Gagnadoux, 2009 [26] |
8 weeks |
34 ± 13 |
2 (1-8) |
6 (1-8) |
CPAP |
MAS |
| AHI – Apnoea Hypopnoea Index (OSA defined as AHI >5/hr), NS – No significant difference between treatments. *p <0.05 compared to baseline. AHI values = Mean ± SD except Gagnadoux, 2009 = median (interquartile range) |
Patient selection
As of 2006, clinical practice parameters of the American Academy of Sleep Medicine (AASM) state that MAS are indicated as a first line therapy for patients with mild to moderate OSA who prefer an oral device over CPAP, who fail treatment attempts or are inappropriate candidates for CPAP [6]. MAS can effectively treat OSA across a range of disease severity, however it is recommended that patients with severe OSA initially trial CPAP before considering an oral device due to its superior efficacy [6]. Similarly CPAP therapy is preferential in severely symptomatic patients who require urgent treatment (such as in cases of sleepy drivers) and those with medical co-morbidities as CPAP is immediately effective, whereas oral device therapy requires an extended acclimatisation period until the optimal therapeutic benefit is achieved. The dental status of the patient must also be considered initially and can preclude a patient from this treatment modality. To be suitable for a MAS, a patient must have enough teeth to allow the device to be adequately retained in position in the mouth. The precise number of teeth required will depend on the device design. Some devices can even be used in patients who have an edentulous upper arch, however the presence of at least 10 teeth per dental arch has been suggested. The patient must also have a sufficient level of dental health and be free of temporomandibular joint problems. It has also been suggested that a limited maximal mandibular protrusive distance (<6 mm) is a contraindication. It is estimated that up to a third of OSA patients are excluded from treatment with this device on the basis of dental factors alone [43]. Patient indications and contraindications for MAS therapy are summarised in table 2.
|
Table 2: Indications and Contraindications for MAS therapy. |
|
Indications
|
Contraindications
|
| • Mild to moderate Obstructive Sleep Apnoea |
• Central Sleep Apnoea |
| • Severe Obstructive Sleep Apnoea with refusal or intolerance of CPAP |
• Severe symptomatic OSA requiring immediate treatment (e.g. Sleepy drivers, severe hypoxemia) |
| • Good dental health with <10 teeth per dental arch |
• Exaggerated gag reflex
• Temporomandibular jaw problems
• Periodontal disease |
| CPAP – Continuous Positive Airway Pressure |
Optimising treatment outcome
Once oral device therapy is implemented, treatment efficacy should be objectively assessed by overnight monitoring to ensure that the occurrence of respiratory events has ceased. Patients should also be monitored in terms of dental hygiene throughout treatment to prevent development of dental contraindications, such as periodontitis, which may reduce effectiveness. However not all OSA patients will experience clinical benefit from this form of therapy which can lead to considerable wastage of health resources and delay the implementation of an efficacious treatment. Therapy should be implemented using a device with proven efficacy, specific dental expertise and to patients most likely to respond to this treatment modality in order to enhance patient outcomes. However, exactly what the parameters are that will ensure a successful treatment outcome are not yet clearly established and remain a high priority for research in the field. It seems likely that a combination of patient-specific, device-specific and titration-specific factors will influence treatment outcome.
Device specific factors
Although the key design feature of these devices is the provision of mandibular advancement, there are a numerous and diverse assortment of available designs. Most notably, the device can be configured as a single piece (monobloc) or consist of separate upper and lower plates in a two-piece (duobloc) design. Additionally devices can differ in size, construction material, customisation to the individual’s dentition, amount of occlusal coverage and the allowance of vertical and lateral jaw movement. Two-piece appliances permit more adjustability resulting in a greater range of achievable mandibular protrusion at greater levels of comfort. Again there are differences between designs in the mechanisms by which two plate devices are coupled together, including elastic or plastic connectors, hook connectors, metallic pin and tube connectors, acrylic extensions and magnets, which may influence adjustability and comfort. Not a lot is known about the effect of different design features on clinical outcomes. However, devices that are custom-made for the individual patient are associated with more comfort and better retention in the mouth and result in greater improvement in OSA compared to non-customised, prefabricated “boil and bite” type devices [44]. Device design has the potential to influence efficacy through patient compliance by relative comfort and experience of adverse effects, and therefore careful attention to device selection is warranted. A few randomised trials have compared efficacy of different mandibular advancement appliance designs [23, 45–46]. Initial assessments of treatment have found greater improvements in both symptoms and polysomnographic indices with appliances that do not allow mouth opening (monobloc or two piece appliance) than with two-piece appliances with limited mouth and jaw movement [23, 45]. However 2 year follow-up assessments showed no differences in long-term efficacy between the two appliance designs [23]. Factors in addition to protrusion of the mandible appear to have some influence on treatment response and future comparative studies are essential to further define the design features that optimise outcomes.
Titration-specific factors
Two-piece devices are generally incrementally titrated to provide increasing levels of protrusion, under the direction of a dentist, until the optimal level of protrusion is reached. The standard approach is to provide an initial four week period of acclimatisation to the device followed by an additional eight-to-twelve weeks in which the level of advancement is gradually increased. This lengthy period before full implementation of treatment is considered a drawback in cases where full treatment is required urgently. There does not appear to be a direct relationship between the degree of mandibular advancement and therapeutic response [8, 47]. Therefore the degree or ‘dose’ of mandibular advancement required to prevent apnoeas and hypopnoeas varies between patients, analogous to different pressure requirements with CPAP, and generally ranges between 50 to 90% of the maximum protrusion. The titration method, including the initial and target amount of mandibular advancement and the time intervals at which increments are made are highly variable between dental practitioners. The effects of such differences in titration methodologies on treatment outcome are not known.
Single-night titration studies monitoring the effects of incremental mandibular advancement by polysomnogaphy, analogous to CPAP titration, have been investigated [26, 48–49]. In this set-up the patient sleeps wearing an oral device with a hydraulic or motorised advancement mechanism attached to the lower plate. The degree of mandibular protrusion is controlled remotely via a computer interface and the amount of protrusion which best eliminates respiratory events can then be determined over the course of the night without waking the patient. Such a single-night titration protocol has the potential benefit of not only determining if the patient will respond to treatment but also what degree of mandibular protrusion will be necessary to achieve this. However a single-night titration protocol may also have limitations in that, without a gradual build-up of tolerance to mandibular advancement over time, the required ‘dose’ may not be achievable within a single night without significant discomfort to the patient.
Patient specific factors
Selecting patients who will ultimately experience treatment success is not straight-forward. There is a general belief that patients with less severe OSA will experience greater success with this treatment modality, although this is certainly not always the case [7–8]. Patients with positional OSA, a higher AHI in the supine compared to lateral recumbent position, may particularly benefit from oral device therapy as MAS are reported to have a greater effect on supine AHI [39, 50]. Other patient characteristics reported to be associated with better treatment outcomes include younger age, lower body mass index (BMI) and smaller neck circumference [8, 39, 50]. Females are also thought to respond better to this form of treatment [39], however the issue of gender differences in treatment response to oral devices has not been adequately addressed with studies of treatment outcome prediction so far conducted in predominantly male samples.
Additionally individual craniofacial morphology and upper airway physiology appear to play a role in the potential therapeutic benefit achievable with a MAS. Analyses of craniofacial measurements from lateral cephalometric x-rays have identified anatomical parameters associated with MAS treatment response. Cephalometric variables such as a longer maxilla, smaller overjet, shorter soft palate, shorter facial height, reduced distance between the mandibular plance and hyoid bone, and a smaller retropalatal airway space have variably been demonstrated in OSA patients with a positive treatment outcome [8, 51–53]. In terms of physiological factors, OSA patients who collapse their upper airway primarily in the oropharyngeal region during sleep are more likely to respond to this treatment, whereas primary velopharyngeal airway collapse appears to be associated with treatment failure [54]. Lower nasal resistance in OSA patients is also associated with a favourable response to MAS therapy [55]. Patient specific factors related to better treatment responses are summarised in table 3.
Overall demographic, anthropometric, polysomnographic, physiologic and anatomical characteristics appear to be associated with oral device treatment success and such factors may aid in appropriately selecting OSA patients for this form of treatment. The challenge comes in how to assess physiologic predictors in individual patients in clinical practice. This requires simple, inexpensive and widely available techniques that would generally be limited to those that can be applied during wakefulness in an office-based setting. Techniques such as nasopharyngoscopy [9], flow-volume curves from spirometry [56] and observing the site of upper airway collapse in response to phrenic nerve stimulation during wakefulness [57] have been trialled as surrogate assessments of the site of upper airway collapse during sleep for treatment outcome prediction. However the use of known predictive factors and techniques for prediction of treatment outcome in the clinical practice setting have either not been assessed in prospective studies or degradation in performance of the prediction method in subsequent patient samples has occurred [58]. Therefore there is currently still considerable doubt about the validity and utility of relying solely on any of these individual parameters for patient selection. The upper airway response to mandibular advancement is complex and a single structural or functional assessment may prove to be inadequate to accurately predict treatment outcome in all patients. The relative importance of the factors contributing to response is also likely to vary between individuals. Potentially a combination of structural and functional assessments and patient characteristics may give the most accurate prediction of MAS treatment outcome in individual patients. The development of clinically-applicable and reliable methods to predict treatment outcome is a high priority for the field.
Side effects and compliance
The desired action of oral devices in moving and holding the mandible in a forward position for therapeutic benefit may equally exert reciprocal forces on the teeth, jaw and gums leading to discomfort and side effects. Indeed this mechanical pressure can result in acute symptoms, as well as long-term dental and skeletal changes. However side effects and complications are generally thought to be minor in nature. It is common and anticipated that side effects occur at the initiation of treatment and during the acclimatisation period but these are usually described as mild and temporary. A side-effect profile gleaned from multiple studies suggests that 6–86% of patients will experience adverse effects such as excessive salivation, mouth dryness, tooth pain, gum irritation, headaches, temporomandibular joint discomfort and morning after occlusal changes [16]. Differences in side effect frequencies reported by the various studies are likely to relate to the particular device used, the degree of mandibular advancement and the dental expertise involved. Symptoms generally resolve within days to weeks with regular use and appropriate adjustment of the device [17]. More severe and continuing adverse symptoms include temporomandibular joint pain, myofacial pain, tooth and gum pain, dry mouth and salivation, and again there is wide variation in the frequencies at which these are reported in published studies [16], although if severe and continuous these adverse effects may result in cessation of use of the device.
Longer term dental and skeletal changes are also evident with use of an oral device. These include changes in occlusal contact area, increased facial height, mouth opening and changes in the inclination of the incisors [59–63]. Common short- and long-term side effects are summarised in table 4. Duration of device wear appears to correlate with the extent of changes in the bite relationship and mandibular posture, however such changes are not necessarily undesirable with favourable occlusive changes reported in 41% of patients and some patients (14%) showing no change at all [59–60]. There is some evidence that daily jaw exercises improve the occlusal contact area and bite force and may be a potential method to help minimise occlusal changes in predisposed patients [64–65]. Generally, dental and skeletal changes due to oral device wear are minor or subclinical and it is uncommon that treatment will need to be ceased on this basis, although any such decision should be weighed against the degree of change versus the feasibility of alternative treatment such as CPAP.
Of course, overall, the effectiveness of treatment is dependent on regular and prolonged use of the device. As with all treatments, adherence to oral device therapy depends on the balance between the perceived benefit versus the side effects experienced. Unlike CPAP compliance, which can be objectively monitored via the machine, there is currently no commercially-available method for objectively measuring MAS adherence in clinical practice. Therefore this makes it difficult to objectively compare compliance between these two forms of treatment. However, self-reported compliance data from available studies suggests that, on average, 77% of patients still consistently used their device at one year [16]. A research study has used a MAS with a novel intra-oral temperature sensor in an attempt to objectively measure compliance [66], although this technology is not yet available in routine clinical practice. The monitoring data generated suggests that, on average, patients used the appliance for 6.8 hours per night (range 5.6–7.5 hours), matching usage figures from studies using a subjective report. In comparison, only 46% of CPAP users maintain at least 4 hours of therapy on more than 70% of nights [3]. However the development of technologies for objective compliance monitoring of nightly oral appliance use, as is possible for CPAP, will be essential for oral appliance research and in clinical practice.
|
Table 3: Patient factors associated with a better treatment outcome. |
|
Demographic/Anthropometric
|
Craniofacial morphology
|
| • ↓ age
• Female gender
• ↓ BMI
• ↓ neck circumference |
• ↓ soft palate length
• ↑ distance between hyoid bone and mandibular plane
• ↑ mandibular plane-cranial base angle
• Retrognathic mandible |
|
Polysomnography
|
Upper airway structure and function
|
| • ↓ baseline AHI
• Supine-dependent OSA
• Successful single night mandibular advancement titration |
• Oropharyngeal collapse
• ↓ nasal resistance
• ↑ airway calibre with mandibular advancement |
|
Table 4: Common side-effects associated with MAS use. |
|
Short-term
|
Long-term
|
| Mouth dryness
Salivation
Gum irritation
Dental discomfort
Temporomandibular joint pain |
Occlusal changes
Increased facial height
Increased mouth opening
Increased mandibular plane angle
Changes in inclination of incisors |
Conclusions
Oral devices can be an effective treatment for OSA across a range of disease severity and a strong evidence base now exists to support the use of these devices for improving polysomnographic indices as well as modifying the health risks associated with OSA. As oral devices are a dental-based treatment for a medical sleep disorder, a multidisciplinary care approach between sleep physician and dental practitioner are required to effectively implement this form of therapy for optimal patient outcomes. Ongoing review of care in both medical and dental settings is required to assess compliance, comfort and fit of the device as well as monitoring treatment efficacy and side-effects. Although, in terms of OSA treatments, CPAP has the greater overall efficacy, patients generally prefer MAS and therefore subsequent differences in adherence profiles between the two forms of treatment may equalise their therapeutic benefit in clinical practice. In any case, with this patient preference, the use of oral devices for the treatment of OSA is likely to increase in coming years, particularly if accurate methods to predict treatment outcomes are developed.
References
1 Marshall NS, Wong KK, Liu PY, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea as an independent risk factor for all-cause mortality: the Busselton Health Study. Sleep. 2008;31(8):1079–85.
2 Young T, Peppard PE, Taheri S. Excess weight and sleep-disordered breathing. J Appl Physiol. 2005;99(4):1592–9.
3 Kribbs NB, Pack AI, Kline LR, Smith PL, Schwartz AR, Schubert NM, et al. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am Rev Respir Dis. 1993;147(4):887–95.
4 Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;5(2):173–8.
5 Ng AT, Gotsopoulos H, Qian J, Cistulli PA. Effect of oral appliance therapy on upper airway collapsibility in obstructive sleep apnea. Am J Respir Crit Care Med. 2003;168(2):238–41.
6 Kushida CA, Morgenthaler TI, Littner MR, Alessi CA, Bailey D, Coleman J, Jr. et al. Practice parameters for the treatment of snoring and Obstructive Sleep Apnea with oral appliances: an update for 2005. Sleep. 2006;29(2):240–3.
7 Gotsopoulos H, Chen C, Qian J, Cistulli PA. Oral appliance therapy improves symptoms in obstructive sleep apnea: a randomized, controlled trial. Am J Respir Crit Care Med. 2002;166(5):743–8.
8 Mehta A, Qian J, Petocz P, Darendeliler MA, Cistulli PA. A randomized, controlled study of a mandibular advancement splint for obstructive sleep apnea. Am J Respir Crit Care Med. 2001;163(6):1457–61.
9 Chan AS, Lee RW, Srinivasan VK, Darendeliler MA, Grunstein RR, Cistulli PA. Nasopharyngoscopic evaluation of oral appliance therapy for obstructive sleep apnoea. Eur Respir J. 2010;35(4):836–42.
10 Chan AS, Sutherland K, Schwab RJ, Zeng B, Petocz P, Lee RW, et al. The effect of mandibular advancement on upper airway structure in obstructive sleep apnoea. Thorax. 2010;65(8):726–32.
11 Ryan CF, Love LL, Peat D, Fleetham JA, Lowe AA. Mandibular advancement oral appliance therapy for obstructive sleep apnoea: effect on awake calibre of the velopharynx. Thorax. 1999;54(11):972–7.
12 Sutherland K, Deane SA, Chan ASL, Schwab RJ, Ng AT, Darendeliler MA, et al. Comparative effects of two oral appliances on upper airway structure in obstructive sleep apnea. Sleep. 2011;In Press.
13 Isono S, Remmers JE, Tanaka A, Sho Y, Sato J, Nishino T. Anatomy of pharynx in patients with obstructive sleep apnea and in normal subjects. J Appl Physiol. 1997;82(4):1319–26.
14 Johal A, Gill G, Ferman A, McLaughlin K. The effect of mandibular advancement appliances on awake upper airway and masticatory muscle activity in patients with obstructive sleep apnoea. Clin Physiol Funct Imaging. 2007;27(1):47–53.
15 Tsuiki S, Ono T, Kuroda T. Mandibular Advancement Modulates Respiratory-Related Genioglossus Electromyographic Activity. Sleep Breath. 2000;4(2):53–8.
16 Ferguson KA, Cartwright R, Rogers R, Schmidt-Nowara W. Oral appliances for snoring and obstructive sleep apnea: a review. Sleep. 2006;29(2):244–62.
17 Hoekema A, Stegenga B, De Bont LG. Efficacy and co-morbidity of oral appliances in the treatment of obstructive sleep apnea-hypopnea: a systematic review. Crit Rev Oral Biol Med. 2004;15(3):137–55.
18 Hoffstein V. Review of oral appliances for treatment of sleep-disordered breathing. Sleep Breath. 2007;11(1):1–22.
19 Lim J, Lasserson TJ, Fleetham J, Wright J. Oral appliances for obstructive sleep apnoea. Cochrane Database Syst Rev. 2006;1):CD004435.
20 Gotsopoulos H, Kelly JJ, Cistulli PA. Oral appliance therapy reduces blood pressure in obstructive sleep apnea: a randomized, controlled trial. Sleep. 2004;27(5):934–41.
21 Pitsis AJ, Darendeliler MA, Gotsopoulos H, Petocz P, Cistulli PA. Effect of vertical dimension on efficacy of oral appliance therapy in obstructive sleep apnea. Am J Respir Crit Care Med. 2002;166(6):860–4.
22 Aarab G, Lobbezoo F, Hamburger HL, Naeije M. Effects of an oral appliance with different mandibular protrusion positions at a constant vertical dimension on obstructive sleep apnea. Clin Oral Investig. 2010;14(3):339–45.
23 Ghazal A, Sorichter S, Jonas I, Rose EC. A randomized prospective long-term study of two oral appliances for sleep apnoea treatment. J Sleep Res. 2009;18(3):321–8.
24 Deane SA, Cistulli PA, Ng AT, Zeng B, Petocz P, Darendeliler MA. Comparison of mandibular advancement splint and tongue stabilizing device in obstructive sleep apnea: a randomized controlled trial. Sleep. 2009;32(5):648–53.
25 Barnes M, McEvoy RD, Banks S, Tarquinio N, Murray CG, Vowles N, et al. Efficacy of positive airway pressure and oral appliance in mild to moderate obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170(6):656–64.
26 Gagnadoux F, Fleury B, Vielle B, Petelle B, Meslier N, N’Guyen XL, et al. Titrated mandibular advancement versus positive airway pressure for sleep apnoea. Eur Respir J. 2009;34(4):914–20.
27 Gindre L, Gagnadoux F, Meslier N, Gustin JM, Racineux JL. Mandibular advancement for obstructive sleep apnea: dose effect on apnea, long-term use and tolerance. Respiration. 2008;76(4):386–92.
28 Engleman HM, McDonald JP, Graham D, Lello GE, Kingshott RN, Coleman EL, et al. Randomized crossover trial of two treatments for sleep apnea/hypopnea syndrome: continuous positive airway pressure and mandibular repositioning splint. Am J Respir Crit Care Med. 2002;166(6):855–9.
29 Hoekema A, Stegenga B, Bakker M, Brouwer WH, de Bont LG, Wijkstra PJ, et al. Simulated driving in obstructive sleep apnoea-hypopnoea; effects of oral appliances and continuous positive airway pressure. Sleep Breath. 2007;11(3):129–38.
30 Naismith SL, Winter VR, Hickie IB, Cistulli PA. Effect of oral appliance therapy on neurobehavioral functioning in obstructive sleep apnea: a randomized controlled trial. J Clin Sleep Med. 2005;1(4):374–80.
31 Otsuka R, Ribeiro de Almeida F, Lowe AA, Linden W, Ryan F. The effect of oral appliance therapy on blood pressure in patients with obstructive sleep apnea. Sleep Breath. 2006;10(1):29–36.
32 Yoshida K. Effect on blood pressure of oral appliance therapy for sleep apnea syndrome. Int J Prosthodont. 2006;19(1):61–6.
33 Lam B, Sam K, Mok WY, Cheung MT, Fong DY, Lam JC, et al. Randomised study of three non-surgical treatments in mild to moderate obstructive sleep apnoea. Thorax. 2007;62(4):354–9.
34 Itzhaki S, Dorchin H, Clark G, Lavie L, Lavie P, Pillar G. The effects of 1-year treatment with a herbst mandibular advancement splint on obstructive sleep apnea, oxidative stress, and endothelial function. Chest. 2007;131(3):740–9.
35 Trzepizur W, Gagnadoux F, Abraham P, Rousseau P, Meslier N, Saumet JL, et al. Microvascular endothelial function in obstructive sleep apnea: Impact of continuous positive airway pressure and mandibular advancement. Sleep Med. 2009;10(7):746–52.
36 Lam B, Sam K, Lam JC, Lai AY, Lam CL, Ip MS. The efficacy of oral appliances in the treatment of severe obstructive sleep apnea. Sleep Breath. 2011;
37 Marklund M, Sahlin C, Stenlund H, Persson M, Franklin KA. Mandibular advancement device in patients with obstructive sleep apnea: long-term effects on apnea and sleep. Chest. 2001;120(1):162–9.
38 Walker-Engstrom ML, Tegelberg A, Wilhelmsson B, Ringqvist I. 4-year follow-up of treatment with dental appliance or uvulopalatopharyngoplasty in patients with obstructive sleep apnea: a randomized study. Chest. 2002;121(3):739–46.
39 Marklund M, Stenlund H, Franklin KA. Mandibular advancement devices in 630 men and women with obstructive sleep apnea and snoring: tolerability and predictors of treatment success. Chest. 2004;125(4):1270–8.
40 Randerath WJ, Heise M, Hinz R, Ruehle KH. An individually adjustable oral appliance vs continuous positive airway pressure in mild-to-moderate obstructive sleep apnea syndrome. Chest. 2002;122(2):569–75.
41 Tan YK, L’Estrange PR, Luo YM, Smith C, Grant HR, Simonds AK, et al. Mandibular advancement splints and continuous positive airway pressure in patients with obstructive sleep apnoea: a randomized cross-over trial. Eur J Orthod. 2002;24(3):239–49.
42 Maurer JT. Update on surgical treatments for sleep apnea. Swiss Med Wkly. 2009;139(43-44):624–9.
43 Petit FX, Pepin JL, Bettega G, Sadek H, Raphael B, Levy P. Mandibular advancement devices: rate of contraindications in 100 consecutive obstructive sleep apnea patients. Am J Respir Crit Care Med. 2002;166(3):274–8.
44 Vanderveken OM, Devolder A, Marklund M, Boudewyns AN, Braem MJ, Okkerse W, et al. Comparison of a custom-made and a thermoplastic oral appliance for the treatment of mild sleep apnea. Am J Respir Crit Care Med. 2008;178(2):197–202.
45 Bloch KE, Iseli A, Zhang JN, Xie X, Kaplan V, Stoeckli PW, et al. A randomized, controlled crossover trial of two oral appliances for sleep apnea treatment. Am J Respir Crit Care Med. 2000;162(1):246–51.
46 Lawton HM, Battagel JM, Kotecha B. A comparison of the Twin Block and Herbst mandibular advancement splints in the treatment of patients with obstructive sleep apnoea: a prospective study. Eur J Orthod. 2005;27(1):82–90.
47 Henke KG, Frantz DE, Kuna ST. An oral elastic mandibular advancement device for obstructive sleep apnea. Am J Respir Crit Care Med. 2000;161(2 Pt 1):420–5.
48 Petelle B, Vincent G, Gagnadoux F, Rakotonanahary D, Meyer B, Fleury B. One-night mandibular advancement titration for obstructive sleep apnea syndrome: a pilot study. Am J Respir Crit Care Med. 2002;165(8):1150–3.
49 Tsai WH, Vazquez JC, Oshima T, Dort L, Roycroft B, Lowe AA, et al. Remotely controlled mandibular positioner predicts efficacy of oral appliances in sleep apnea. Am J Respir Crit Care Med. 2004;170(4):366–70.
50 Chung JW, Enciso R, Levendowski DJ, Morgan TD, Westbrook PR, Clark GT. Treatment outcomes of mandibular advancement devices in positional and nonpositional OSA patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109(5):724–31.
51 Ng AT, Darendeliler MA, Petocz P, Cistulli PA. Cephalometry and prediction of oral appliance treatment outcome. Sleep Breath. 2011;
52 Lee CH, Kim JW, Lee HJ, Seo BS, Yun PY, Kim DY et al. Determinants of treatment outcome after use of the mandibular advancement device in patients with obstructive sleep apnea. Arch Otolaryngol Head Neck Surg. 2010;136(7):677–81.
53 Liu Y, Lowe AA. Factors related to the efficacy of an adjustable oral appliance for the treatment of obstructive sleep apnea. Chin J Dent Res. 2000;3(3):15–23.
54 Ng AT, Qian J, Cistulli PA. Oropharyngeal collapse predicts treatment response with oral appliance therapy in obstructive sleep apnea. Sleep. 2006;29(5):666–71.
55 Zeng B, Ng AT, Qian J, Petocz P, Darendeliler MA, Cistulli PA. Influence of nasal resistance on oral appliance treatment outcome in obstructive sleep apnea. Sleep. 2008;31(4):543–7.
56 Zeng B, Ng AT, Darendeliler MA, Petocz P, Cistulli PA. Use of flow-volume curves to predict oral appliance treatment outcome in obstructive sleep apnea. Am J Respir Crit Care Med. 2007;175(7):726–30.
57 Bosshard V, Masse JF, Series F. Prediction of oral appliance efficiency in patients with apnoea using phrenic nerve stimulation while awake. Thorax. 2011;66(3):220–5.
58 Chan AS, Lee RW, Srinivasan VK, Darendeliler MA, Cistulli PA. Use of flow-volume curves to predict oral appliance treatment outcome in obstructive sleep apnea: a prospective validation study. Sleep Breath. 2010;
59 Almeida FR, Lowe AA, Otsuka R, Fastlicht S, Farbood M, Tsuiki S. Long-term sequellae of oral appliance therapy in obstructive sleep apnea patients: Part 2. Study-model analysis. Am J Orthod Dentofacial Orthop. 2006;129(2):205–13.
60 Almeida FR, Lowe AA, Sung JO, Tsuiki S, Otsuka R. Long-term sequellae of oral appliance therapy in obstructive sleep apnea patients: Part 1. Cephalometric analysis. Am J Orthod Dentofacial Orthop. 2006;129(2):195–204.
61 Hammond RJ, Gotsopoulos H, Shen G, Petocz P, Cistulli PA, Darendeliler MA. A follow-up study of dental and skeletal changes associated with mandibular advancement splint use in obstructive sleep apnea. Am J Orthod Dentofacial Orthop. 2007;132(6):806–14.
62 Rose EC, Staats R, Virchow C Jr, Jonas IE. Occlusal and skeletal effects of an oral appliance in the treatment of obstructive sleep apnea. Chest. 2002;122(3):871–7.
63 Ueda H, Almeida FR, Lowe AA, Ruse ND. Changes in occlusal contact area during oral appliance therapy assessed on study models. Angle Orthod. 2008;78(5):866–72.
64 Marklund M. Predictors of long-term orthodontic side effects from mandibular advancement devices in patients with snoring and obstructive sleep apnea. Am J Orthod Dentofacial Orthop. 2006;129(2):214–21.
65 Ueda H, Almeida FR, Chen H, Lowe AA. Effect of 2 jaw exercises on occlusal function in patients with obstructive sleep apnea during oral appliance therapy: a randomized controlled trial. Am J Orthod Dentofacial Orthop. 2009;135(4):430 e1-7; discussion -1.
66 Lowe AA, Sjoholm TT, Ryan CF, Fleetham JA, Ferguson KA, Remmers JE. Treatment, airway and compliance effects of a titratable oral appliance. Sleep. 2000;23(Suppl 4):S172–8.
67 Hoekema A, Stegenga B, Wijkstra PJ, van der Hoeven JH, Meinesz AF, de Bont LG. Obstructive sleep apnea therapy. J Dent Res. 2008;87(9):882–7.
68 Ferguson KA, Ono T, Lowe AA, al-Majed S, Love LL, Fleetham JA. A short-term controlled trial of an adjustable oral appliance for the treatment of mild to moderate obstructive sleep apnoea. Thorax. 1997;52(4):362–8.
69 Ferguson KA, Ono T, Lowe AA, Keenan SP, Fleetham JA. A randomized crossover study of an oral appliance vs nasal-continuous positive airway pressure in the treatment of mild-moderate obstructive sleep apnea. Chest. 1996;109(5):1269–75.
70 Clark GT, Blumenfeld I, Yoffe N, Peled E, Lavie P. A crossover study comparing the efficacy of continuous positive airway pressure with anterior mandibular positioning devices on patients with obstructive sleep apnea. Chest. 1996;109(6):1477–83.


