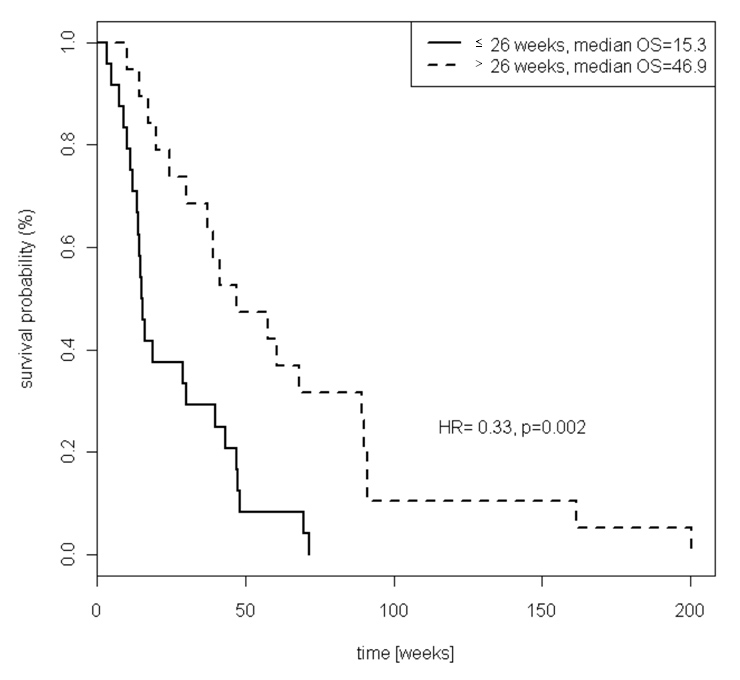
Figure 1
Kaplan-Meier estimates of overall survival by progression free survival at 1st line chemotherapy, less than or equal to 26 weeks versus greater than 26 weeks.
DOI: https://doi.org/10.4414/smw.2011.13249
This study confirms, in an unselected Caucasian population, that clinical parameters predict which patients with metastatic gastric cancer live longer when given second-line chemotherapy.
Gastric cancer is a frequent malignancy with a 2008 worldwide estimated incidence of 990’000 cases, representing 7.8% of all cancers [1]. While many factors have been shown to contribute to gastric carcinogenesis, it is the complex interaction among different aetiological factors leading to both genetic and epigenetic alterations of proto-oncogenes and tumour-suppressor genes, which underlies the pathogenesis of gastric cancer [2, 3]. Unfortunately, gastric cancer is often diagnosed at an advanced stage with approximately half of patients presenting an unresectable locally advanced or metastatic disease. Nearly half of these patients respond to chemotherapy triplets containing cisplatin, 5-Fluorouracil (5-FU) and anthracyclines or taxanes [4, 5]. Unfortunately, median survival remains under twelve months even with the most active combinations [6]. In the remaining potentially curable patients, peri-operative chemotherapy (which is the standard of care in most European countries) significantly improves survival. However, ultimately, more than 60% of the patients will have tumour recurrence and then proceed to palliative chemotherapy [7]. Nearly all of those patients will eventually suffer disease progression after first-line treatment.
Presently, there is no adequately powered randomised-controlled trial showing a benefit from second-line chemotherapy in advanced gastric cancer compared with best supportive care alone. However, many patients with metastatic gastric cancer are in good condition after first-line chemotherapy and are offered further treatment based on promising phase II trials (table 1) and a small underpowered phase III trial involving 40 patients [8]. The latter study showed a significant benefit of second-line chemotherapy compared to best supportive care (overall survival (OS) of 4.1 months vs. 2.4 months; p= 0.0027). No subset analysis to identify patients likely to benefit from second-line chemotherapy could be performed because of the limited sample size. In fact, there are currently no validated prognostic factors to select patients who will most likely benefit from second-line chemotherapy.
A major limitation of most trials which have evaluated second-line chemotherapy in patients with gastric cancer is the inclusion of patients with locally advanced non-operable gastric cancer and patients with metastatic gastric cancer, making the results difficult to interpret. Furthermore, most studies have included Asian patients, treated with first-line chemotherapy agents that are not commonly used in Europe or in the United States (e.g. S-1). Therefore, the current study looked exclusively at Caucasian patients with metastatic gastric cancer treated with second-line chemotherapy at the Geneva University Hospital to see if we could establish prognostic factors for survival. In the absence of an adequately powered placebo-controlled phase III study, these factors might be useful in selecting patients most likely to benefit from further treatment.
| Table 1: Published phase II studies evaluating second-line chemotherapy in advanced gastric cancer. | ||||||||
| Number of patients | First line regimen | Median TTP or PFS at first line | Second line regimen | Tumour Response evaluation | Median TTP or PFS at second line | Median OS | Cumulative median OS | References |
| 33 Japanese patients with LAGC or MGC | S-1 alone | TTP 5.6 months | Paclitaxel | OR 24% DC 57% | TTP 4.2 months | 8 months | 15 months | [17] |
| 45 Japanese patients with LAGC or MGC | Fluoropyrimidine-based (mainly S-1 alone) | – | Paclitaxel | OR 16% DC 48% | PFS 2.6 months | 7.8 months | – | [18] |
| 40 Japanese patients with LAGC or MGC | Fluoropyrimidine-based (mainly S-1 alone) | – | Paclitaxel | OR 17% DC 70% | PFS 111 days | 254 days | – | [19] |
| 26 Caucasian patients with MGC | 5-FU/cisplatin or 5FU/epirubicin | PFS 4.8 months | Paclitaxel + capecitabine | OR 35% DC 42% | PFS 4.5 months | 7.5 months | 15.5 months | [20] |
| 154 Korean patients LAGC or MGC | Fluoropyridmines/platinum | – | Docetaxel | OR 14% DC 43% | TTP 2.6 months | 7.2 months | – | [16] |
| 28 Caucasian patients with MGC | Not specified | – | Docetaxel + capecitabine | OR 29% DC 65% | TTP 4 months | 6 months | – | [21] |
| 30 Japanese patients with LAGC or MGC | S-1 alone or 5-FU/cisplatin | – | Docetaxel + cisplatin | OR 27% DC 63% | TTP 4.5 months | 6 months | 13 months | [22] |
| 32 Caucasian patients with LAGC or MGC | Multiple regimen | – | Docetaxel + cisplatin | OR 16% DC 41% | TTP 5 months | 6 months | 12 months | [23] |
| 38 Caucasian patients with LAGC or MGC | Epirubicin, cisplatin/5-FU or Cisplatin/5-FU | TTP 7.7 months | Docetaxel + oxaliplatin | OR 11% DC 47% | PFS 4.0 months | 8.1 months | - | [24] |
| 64 Korean patients with locally advanced or metastatic GC | Multiple regimen | – | Irinotecan + 5-FU | OR 21% DC 46% | TTP 2.5 months | 7.6 months | – | [25] |
| 51 Korean patients with LAGC or MGC | Platinum-based | – | Irinotecan + 5-FU | OR 18% DC 47% | PFS 3.2 months | 9.1 months | – | [26] |
| 46 Chinese patients with LAGC or MGC | 5-FU/cisplatin or 5-FU/oxaliplatin | – | Irinotecan + capecitabine | OR 27% DC 70% | TTP 4.1 months | 7.6 months | – | [27] |
| 38 Caucasian patients with LAGC or MGC | Multiple regimen | – | Irinotecan + mitomycin | OR 32% DC 53% | TTP 4 months | 8 months | – | [28] |
| Abbreviations: LAGC, locally advanced gastric cancer; MGC, metastatic gastric cancer; TTP, time to progression; PFS, progression free survival; OS, overall survival; 5-FU, 5-fluorouracil; OR, overall response; DC, disease control. | ||||||||
The current study retrospectively reviewed all adult Caucasian patients with metastatic gastric or gastro-oesophageal junction (GOJ) adenocarcinoma who were treated at the Geneva University Hospital, between January 1994 and June 2008. Patients with unresectable locally advanced disease or squamous histology were excluded. We reviewed each medical record and collected demographic and clinico-pathologic characteristics including, age, sex, histological classification according to Lauren [9], localisation of the primary tumour, localisation of metastatic lesions, performance status according to Eastern Cooperative Oncology Group (ECOG), progression-free survival (PFS) at first and second-line chemotherapy as well as OS. PFS for each line of treatment was measured from the start of chemotherapy until disease progression, death or the start of another oncologic treatment (other regimen of chemotherapy or radiotherapy). OS was measured from the start of second line chemotherapy until death. We also looked at the regimen of chemotherapy used at first and second-line. The research ethics committee of our institution approved this study.
As many patients had only peritoneal carcinomatosis with no measurable radiological lesions, we used an indirect assessment of disease control defined as 12 weeks under chemotherapy without radiological or clinical progression.
Factors included in the univariate and multivariate analysis were as follows: age, location of primary tumour, Lauren classification, previous gastrectomy, number of organs involved with the tumour, haemoglobin (Hb), carcinoma embryonic antigen (CEA), performance status and PFS at first line chemotherapy. Age was used as a continuous variable. Number of involved organs, Hb, CEA and performance status were dichotomised according to their median value. PFS at first-line chemotherapy was dichotomised as less than or equal to 26 weeks versus greater than 26 weeks.
The primary endpoint of the study was OS. Predictors were chosen based solely on theory without model selection. Prognostic values of the tumour characteristics, patient’s characteristics at the start of second-line chemotherapy, previous surgery and sensitivity to previous chemotherapy were analysed by Cox univariate and multivariate regression. The proportional hazards assumption was verified graphically. p values <0.05 were considered statistically significant. Survival curves were assessed by Kaplan-Meier estimates and represented graphically.
During the study period, 65 patients with metastatic gastric cancer were treated at our institution up to their death. Of these, 43 received second-line chemotherapy. Their demographic and clinical characteristics are shown in table 2. Treatment regimens and outcomes for first and second-line chemotherapy are shown in table 3.
Univariate analysis showed that three variables were significantly associated with OS: PFS at first-line chemotherapy of more than 26 weeks (hazard ratio (HR) = 0.33, confidence interval (CI) 95% 0.16–0.65, p = 0.002), previous curative surgery (HR = 0.51, CI 95% 0.27–0.96, p = 0.04) and CEA >6.5 μg/l (HR = 1.97, CI 95% 1.06–3.65, p = 0.02) (table 4). In a multivariate model that included all three variables, none remained significantly associated with OS. There was no evident superior second-line chemotherapy regimen (data not shown).
| Table 2: Patient demographics and clinical characteristics (n = 43). | ||
| Characteristics | Number of patients (%) | |
| Sex | Men | 33 (76.7) |
| Women | 10 (23.3) | |
| Age(median, range) | 55 (28–79) | |
| Performance status at second line | ECOG 0 | 18 (41.9) |
| ECOG 1 | 19 (44.2) | |
| ECOG 2 | 5 (11.6) | |
| ECOG 3 | 1 (2.3) | |
| Primary tumour | GI junction tumour | 11 (25.6) |
| Stomach tumour | 32 (74.4) | |
| Histology | Diffuse type | 17 (39.5) |
| Intestinal type | 26 (60.5) | |
| Number of organs involved at 2nd line | 1 | 18 (41.9) |
| 2 | 11 (25.6) | |
| 3 and more | 14 (32.5) | |
| Haemoglobin(median, range) | 11.4 g/dl (8.7–13.8) | |
| CEA (median, range) | 6.5 μg/l (0.5–823) | |
| Previous Surgery | Curative | 16 (37.2) |
| Palliative | 9 (20.9) | |
| None | 18 (41.9) | |
| Previous peri-operative radiotherapy | Neo-adjuvant | 2 (4.6) |
| Adjuvant | 3 (7) | |
| None | 38 (88.4) | |
| Previous peri-operative chemotherapy | Neo-adjuvant | 5 (11.6) |
| Adjuvant | 3 (7) | |
| None | 35 (81.4) | |
| Abbreviations: CEA, carcinoembryonic antigen; ECOG, eastern cooperative oncology group; GI, gastro-intestinal. | ||
| Table 3: Clinical outcomes and treatment regimens for first and second line chemotherapy (n = 43). | ||
| Clinical outcomes | Number of patients (%) | |
| Chemotherapy at 1st line | Docetaxel-cisplatin | 24 (55.9) |
| Irinotecan-based | 9 (20.9) | |
| Anthracycline-based | 5 (11.6) | |
| Other | 5 (11.6) | |
| Disease control under treatment at 1st line | 12 or more weeks | 27 (62.8) |
| <12 weeks | 16 (37.2) | |
| Median PFS at 1st line | 23.9 weeks | |
| Chemotherapy at 2nd line | Irinotecan based | 24 (55.8) |
| Docetaxel-cisplatin-based | 4 (9.3) | |
| Anthracycline-based | 4 (9.3) | |
| Fluoropyrimidines | 4 (9.3) | |
| Oxaliplatin-based | 3 (7) | |
| Other | 4 (9.3) | |
| Disease control under treatment at 2nd line | 12 or more weeks | 22 (51.2) |
| <12 weeks | 21 (48.8) | |
| Median PFS at 2nd line | 13.9 weeks | |
| Median OS (from start of 2nd line therapy) | 30.1 weeks | |
| Median cumulative OS (from start of 1st line therapy) | 59.6 weeks | |
| Abbreviations: OS, overall survival; PFS, progression-free survival. | ||
| Table 4: Univariate analysis (Cox regression) to test association between patient characteristics and overall survival (n = 43). | |||||
| Variables | Modalities | n (%) | Median OS [weeks] | Univariate HR (95% CI) | p value |
| Age [Continuous] | – | 43 | – | 1.01 (0.98–1.03) | 0.52 |
| Performance status | ECOG 0 | 18 (41.9) | 42.4 | 1 | |
| ECOG ≥1 | 25 (58.1) | 17.3 | 1.57 (0.84–2.93) | 0.16 | |
| Localisation of primary tumour | Stomach | 32 (74.4) | 33.6 | 1 | |
| GI junction | 11 (25.6) | 29.0 | 1.74 (0.85–3.58) | 0.13 | |
| Histology | Intestinal type | 26 (60.5) | 22.2 | 1 | |
| Diffuse type | 17 (39.5) | 41.3 | 0.91 (0.48–1.71) | 0.76 | |
| Nb of organs involved | 1 | 18 (41.9) | 42.0 | 1 | |
| ≥2 | 25 (58.1) | 29.0 | 1.50 (0.80–2.80) | 0.21 | |
| Haemoglobin | <11.4 g/dl | 22 (51.2) | 27.9 | 1 | |
| ≥11.4 g/dl | 21 (48.8) | 30.1 | 1.05 (0.56–1.94) | 0.89 | |
| CEA | <6.5 μg/l | 22 (51.2) | 44.1 | 1 | |
| ≥6.5 μg/l | 21 (48.8) | 15.3 | 1.97 (1.06–3.65) | 0.03 | |
| Previous Surgery | No or palliative | 27 (62.8) | 19.9 | 1 | |
| Curative | 16 (37.2) | 43.7 | 0.51 (0.27–0.96) | 0.04 | |
| PFS at 1st line | ≤26 weeks | 24 (55.8) | 15.3 | 1 | |
| >26 weeks | 19 (44.2) | 46.9 | 0.33 (0.16–0.65) | 0.002 | |
| Abbreviations: CEA, carcinoembryonic antigen; HR, hazard ratio; Nb, number; OS, overall survival; PFS, progression-free survival. | |||||
There is a lack of reliable data demonstrating a benefit of systemic treatment after failure of first-line chemotherapy in metastatic gastric cancer. As a consequence, second-line chemotherapy is left to the subjective choice of the clinician. Survival predictors might help clinicians to select patients who will most likely benefit from second-line chemotherapy and spare toxicity to the others. The current analysis, based on 43 Caucasian patients, identified three survival predictors; PFS at first-line chemotherapy of more than 26 weeks (6 months), previous curative surgery and CEA ≤6.5 μg/l predicted longer OS. We were, however, unable to confirm their independent prognostic value, most likely because of our limited sample size. The data should be interpreted carefully because we cannot exclude a potential overlap with confounding factors. For example, curative surgery did not remain significant in the multivariate model, which raises the possibility that it is a surrogate for low tumour burden or good performance status as patients with these characteristics are generally operated on.

Figure 1
Kaplan-Meier estimates of overall survival by progression free survival at 1st line chemotherapy, less than or equal to 26 weeks versus greater than 26 weeks.
PFS at first-line chemotherapy of more than 6 months was the strongest prognostic factor in our study. Patients who had not progressed at 6 months under first-line chemotherapy had a median survival of 46.9 months versus 15.3 months (p = 0.002) for those who had tumour progression (fig. 1). Similarly, Catalano et al. found, in a retrospective analysis of 175 Caucasian patients with advanced gastric cancer treated with second-line chemotherapy at three oncology departments, that time to progression (TTP) at first-line chemotherapy >6 months was the strongest prognostic factor [10]. A previous retrospective analysis also identified a progression-free interval of up to 7 months at first-line chemotherapy as the most suitable criterion to distinguish between patients with a poor and good prognosis with second-line chemotherapy [11].
To date, four large retrospective studies have evaluated the prognostic value of multiple clinical parameters in patients with advanced gastric cancer treated with second-line chemotherapy [10, 12–14]. Unfortunately, the diversity of the studied clinical factors as well as the inconsistent cut-offs does not allow for making definitive conclusion (table 5). Nonetheless, it is important to highlight that a consistent association with OS appears across these studies. All four studies found that patients with poor performance status (ECOG ≥2) had worse OS. Furthermore, longer TTP/PFS at first-line chemotherapy was a good prognostic clinical factor in all three studies that evaluated this clinical parameter. Ji et al. accessed treatment-free interval instead of TTP or PFS, and did not find a significant association with outcome [13]. As it is very common for advanced gastric cancer patients with stable disease under first-line chemotherapy to remain under chemotherapy until progression, treatment-free interval is probably not a good clinical parameter in this setting. Our data further supports longer TTP or PFS as a good prognostic marker in Caucasian patients with metastatic gastric cancer treated with second-line chemotherapy.
We did not find a prognostic role for performance status, comparing ECOG score ≥1 vs. 0. However, it should be emphasised that our study population had an excellent performance status with more than 85% of patients having an ECOG of 0 or 1. This reflects our common practice to only offer second-line chemotherapy to patients with a good performance status.
To our knowledge, this is the first analysis that specifically addressed the prognostic role of curative surgery in a second line setting. Previous gastrectomy (either curative or palliative) was identified as a favourable prognostic factor for overall survival in a multivariate analysis of 1455 patients with metastatic gastric cancer who received first-line chemotherapy [15]. In the second line setting, only one [12] of the published series [10, 13, 16] looking for a relationship between former surgery and success of systemic treatment found a significant association with survival. However, none differentiated curative and palliative surgery. An over-representation of palliative gastric surgery, which by definition is performed on patients with much more advanced disease, might explain the lack of gastrectomy’s prognostic value in the above-mentioned studies. Altogether, our data support the rigorous selection of patients that are treated with first-line surgery.
In the current analysis, median OS from the start of second-line chemotherapy to death was 6.9 months with a median PFS of 3.2 months. The majority of our study population (55.9%) had received a combination of docetaxel and cisplatin as initial chemotherapy. After first-line progression, the majority of our patients (55.8%) were treated with irinotecan and 5-FU. Disease control by second-line treatment was observed in 22 (51.2%) patients. None of the second-line regimens used was superior to the others. These data are in line with previous phase II studies looking at second-line irinotecan and 5-FU activity (table 1). In the retrospective study of Catalano et al., the most frequent second-line regimen was also a 5FU and irinotecan combination (29% of 175 patients). The median survival was 6.1 months with 49.7% of patients achieving at least a stable disease [10].
In conclusion, in absence of a demonstrated survival benefit of second-line chemotherapy in advanced gastric cancer, it may be appropriate to propose such treatments only to patients who have a long enough expected survival time. We believe that there is good evidence to consider sensitivity to previous chemotherapy and good performance status (ECOG 0 or 1) as simple tools to select patients eligible for-second line treatment. As only the median TTP or PFS at first-line chemotherapy was evaluated to date, we propose to consider a contemporary value of 6 months for Caucasian patients [7]. Patients included in randomised studies evaluating second-line treatments should be stratified according to these two prognostic factors. Tumour burden and previous curative gastrectomy may also have prognostic value in a second-line setting. These prognostic clinical parameters should be validated prospectively.
| Table 5: Published adverse prognostic clinical factors in patients with advanced gastric cancer treated with second line chemotherapy. | |||||
| 1st author | Catalano [10] | Kanagavel [14] | Hashimoto [12] | Ji [13] | Current study |
| Patients | 175 LAGC or MGC | 126 MGC | 466 LAGC or MGC | 725 AGC | 43 MGC |
| Haemoglobin | ≤11.5 g/l | <10 g/l | n.s | <9.8 g/l | n.s |
| CEA | CEA >50 μg/l | n.a | n.a | n.a | CEA ≥6.5 μg/l§ |
| Number of metastatic sites | ≥3 metastatic site | n.s | s.u | ns | n.s |
| Performance status | ECOG ≥2 | ECOG ≥2 | ECOG ≥2 | ECOG ≥2 | n.s |
| TTP1 or PFS1 | TTP1 ≤6 months | TTP1 <5 months | PFS1 <4 months | n.a† | PFS1 ≤6 months§ |
| CRP | n.a | n.a | CRP >1 mg/dl | n.a | n.a |
| Bone/peritoneal metastasis | s.u‡ | n.s‡ | Bone or peritoneal metastasis | n.s* | n.a |
| Liver metastasis | n.s | n.s | Liver metastasis | n.s | n.a |
| Albumin | n.s | n.a | Albumin <3.5 mg/dl | n.s | n.a |
| Surgery | n.s | n.a | No gastrectomy | n.s | No gastrectomy or palliative§ |
| Ascites | n.a | n.s | n.a | Ascites | n.a |
| Abbreviations: AGC, advanced gastric cancer; CEA, carcinoembryonic antigen; CRP, C-reactive protein; ECOG, eastern cooperative oncology group, n.a, not available; n.s, not significant; PFS1, progression-free survival at 1st line treatment; s.u, significant in univariate analysis; TTP1, time to progression at 1st line treatment; * Bone metastasis alone; † treatment-free interval and response to 1st line therapy were not significant; ‡ peritoneal metastasis alone; § significant in univariate analysis. | |||||
1 Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–917.
2 Wu WK, Lee CW, Cho CH, Fan D, Wu K, Yu J, et al. MicroRNA dysregulation in gastric cancer: a new player enters the game. Oncogene. 2010;29(43):5761–71.
3 Jenal M, Britschgi C, Fey MF, Tschan MP. Inactivation of the hypermethylated in cancer 1 tumour suppressor--not just a question of promoter hypermethylation? Swiss Med Wkly. 2010;140:w13106.
4 Webb A, Cunningham D, Scarffe JH, Harper P, Norman A, Joffe JK, et al. Randomized trial comparing epirubicin, cisplatin, and fluorouracil versus fluorouracil, doxorubicin, and methotrexate in advanced esophagogastric cancer. J Clin Oncol. 1997;15(1):261–7.
5 Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: A report of the V325 study group. J Clin Oncol. 2006;24(31):4991–7.
6 Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358(1):36–46.
7 Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11–20.
8 Thuss-Patience PC, Kretzschmar A, Deist T, Hinke A, Bichev D, Lebedinzew B, et al. Irinotecan versus best supportive care (BSC) as second-line therapy in gastric cancer: A randomized phase III study of the arbeitsgemeinschaft internistische onkologie (AIO). J Clin Oncol. 2009;27:15s, (suppl; abstr 4540) .
9 Lauren P. The two histological main types of gastric carcinoma: Diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49.
10 Catalano V, Graziano F, Santini D, D’Emidio S, Baldelli AM, Rossi D, et al. Second-Line chemotherapy for patients with advanced gastric cancer: Who may benefit? Br J Cancer. 2008;99(9):1402–7.
11 Stahl M, Müller C, Köster W, Wilke H. Second-Line chemotherapy of advanced disseminated gastric cancer after cisplatin, infusional 5-fluorouracil, folinic acid (PLF): Benefit dependent on progression-free interval after first-line therapy. Onkologie. 2005;28(10):499–502.
12 Hashimoto K, Takashima A, Nagashima K, Okazaki SS, Nakajima TE, Kato K, et al. Progression-Free survival in first-line chemotherapy is a prognostic factor in second-line chemotherapy in patients with advanced gastric cancer. J Cancer Res Clin Oncol. 2010;36(7):1059–64.
13 Ji SH, Lim do H, Yi SY, Kim HS, Jun HJ, Kim KH, et al. A retrospective analysis of second-line chemotherapy in patients with advanced gastric cancer. BMC Cancer. 2009;9:110.
14 Kanagavel D, Pokataev IA, Fedyanin MY, Tryakin AA, Bazin IS, Narimanov MN, et al. A prognostic model in patients treated for metastatic gastric cancer with second-line chemotherapy. Ann Oncol. 2010;21(9):1779–85.
15 Lee J, Lim T, Uhm JE, Park KW, Park SH, Lee SC, et al. Prognostic model to predict survival following first-line chemotherapy in patients with metastatic gastric adenocarcinoma. Ann Oncol. 2007;18(5):886–91.
16 Jo JC, Lee JL, Ryu MH, Sym SJ, Lee SS, Chang HM, et al. Docetaxel monotherapy as a second-line treatment after failure of fluoropyrimidine and platinum in advanced gastric cancer: Experience of 154 patients with prognostic factor analysis. Jpn J Clin Oncol. 2007;37(12):936–41.
17 Matsuda G, Kunisaki C, Makino H, Fukahori M, Kimura J, Sato T, et al. Phase II study of weekly paclitaxel as a second-line treatment for s-1-refractory advanced gastric cancer. Anticancer Res. 2009;29(7):2863–7.
18 Kodera Y, Ito S, Mochizuki Y, Fujitake S, Koshikawa K, Kanyama Y, et al. A phase II study of weekly paclitaxel as second-line chemotherapy for advanced gastric cancer (CCOG0302 study). Anticancer Res. 2007;27(4C):2667–71.
19 Koizumi W, Akiya T, Sato A, Yamaguchi K, Sakuyama T, Nakayama N, et al. Second-Line chemotherapy with biweekly paclitaxel after failure of fluoropyrimidine-based treatment in patients with advanced or recurrent gastric cancer: A report from the gastrointestinal oncology group of the tokyo cooperative oncology group, TCOG GC-0501 trial. Jpn J Clin Oncol. 2009;39(11):713–9.
20 Baize N, Abakar-Mahamat A, Mounier N, Berthier F, Caroli-Bosc FX. Phase II study of paclitaxel combined with capecitabine as second-line treatment for advanced gastric carcinoma after failure of cisplatin-based regimens. Cancer Chemother Pharmacol. 2009;64(3):549–55.
21 Rosati G, Bilancia D, Germano D, Dinota A, Romano R, Reggiardo G, et al. Reduced dose intensity of docetaxel plus capecitabine as second-line palliative chemotherapy in patients with metastatic gastric cancer: A phase II study. Ann Oncol 2007;18(Suppl 6):vi128–32.
22 Kunisaki C, Imada T, Yamada R, Hatori S, Ono H, Otsuka Y, et al. Phase II study of docetaxel plus cisplatin as a second-line combined therapy in patients with advanced gastric carcinoma. Anticancer Res. 2005;25(4):2973–7.
23 Polyzos A, Tsavaris N, Kosmas C, Polyzos K, Giannopoulos A, Felekouras E, et al. Subsets of patients with advanced gastric cancer responding to second-line chemotherapy with docetaxel-cisplatin. Anticancer Res. 2006;26(5B):3749–53.
24 Barone C, Basso M, Schinzari G, Pozzo C, Trigila N, D’Argento E, et al. Docetaxel and oxaliplatin combination in second-line treatment of patients with advanced gastric cancer. Gastric Cancer. 2007;10(2):104–11.
25 Kim ST, Kang WK, Kang JH, Park KW, Lee J, Lee SH, et al. Salvage chemotherapy with irinotecan, 5-fluorouracil and leucovorin for taxane- and cisplatin-refractory, metastatic gastric cancer. Br J Cancer. 2005;92(10):1850–4.
26 Seo MD, Lee KW, Lim JH, Yi HG, Kim DY, Oh DY, et al. Irinotecan combined with 5-fluorouracil and leucovorin as second-line chemotherapy for metastatic or relapsed gastric cancer. Jpn J Clin Oncol. 2008;38(9):589–95.
27 Sun Q, Hang M, Xu W, Mao W, Hang X, Li M, Zhang J. Irinotecan plus capecitabine as a second-line treatment after failure of 5-fluorouracil and platinum in patients with advanced gastric cancer. Jpn J Clin Oncol. 2009;39(12):791–6.
28 Giuliani F, Molica S, Maiello E, Battaglia C, Gebbia V, Di Bisceglie M, et al. Irinotecan (CPT-11) and mitomycin-c (MMC) as second-line therapy in advanced gastric cancer: A phase II study of the gruppo oncologico dell' italia meridionale (prot. 2106). Am J Clin Oncol. 2005;28(6):581–5.
Funding / potential competing interests: No financial support and no other potential conflict of interest relevant to this article were reported.