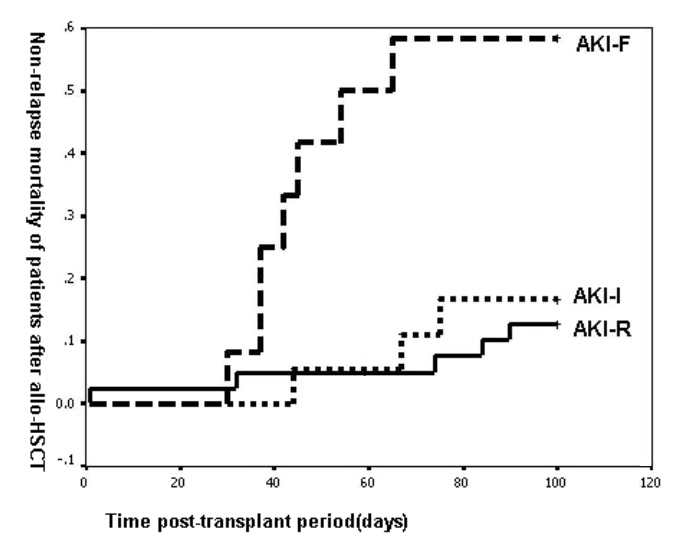
Figure 1
Worsening RIFLE category was associated with the increased mortality of patients in the 100 days post-transplant period.
DOI: https://doi.org/10.4414/smw.2011.13225
Myeloablative allogeneic hematopoietic stem cell transplantation (HSCT) is an effective treatment for haematological malignancies and refractory non-malignant blood diseases. However, several complications after transplantation, including graft versus host disease (GVHD), infections, interstitial pneumonitis and hepatic veno-occlusive disease (HVOD), influence the success of allo-HSCT. Acute kidney injury (AKI) is an important complication after allo-HSCT. Epidemiological studies have previously described the incidence and clinical outcomes associated with AKI after allo-HSCT. Compared to patients without AKI, patients with AKI have a 3 times higher mortality rate. Mortality rates in patients who need dialysis may increase to 80% and above [1]. The causes of AKI after transplantation are still unclear, but many side effects of medicine, including total body irradiation (TBI), infections and nephrotoxicity may contribute to AKI after allo-HSCT [2]. However, inferences from these investigations are often limited due to variation in the definitions used to classify AKI. Lack of a consensus definition is a major problem in AKI research. To address this issue, the Second International Conference of the Acute Dialysis Quality Initiative (ADQI) Group developed a consensus definition for AKI (the RIFLE criteria) based on changes in the patients’ creatinine and/or urine output [3]. RIFLE criteria have since been evaluated in several studies and it has been shown that AKI is associated with a significantly higher mortality rate [4–6].
In order to develop prevention strategies, a better understanding of the causes of AKI after myeloablative allo-HSCT is needed. The purpose of this study was to analyse retrospectively major risk factors for AKI at the time of myeloablative allo-HSCT and evaluate the capacity of the RIFLF criteria to predict mortality in myeloablative allo-HSCT.
The clinical data were analysed retrospectively in 143 adult patients with haematological malignancies who received myeloablative allo-HSCT at the transplant centre of Blood Diseases Hospital, CAMS and PUMC between January 2003 and January 2008. All patients received haematopoietic stem cells from HLA-matched related donors. The clinical data of patients gathered include age, gender, baseline serum creatinine, medical history of chronic kidney disease (CKD), primary diagnosis, vasopressor use, liver function, and a need for mechanical ventilation or renal replacement therapy. Patients with CKD previous to myeloablative allo-HSCT were excluded from the analysis. CKD is defined as the presence of proteinuria or a decreased glomerular filtration rate for three or more months [7]. This study was approved by the Research Ethics Committee of the Institution. Patients were classified according to RIFLE criteria.
All patients received myeloablative conditioning regimens according to their underlying haematological malignancies and disease status. 35 patients received conditioning regimens including total body irradiation (TBI).
All patients received cyclosporine A (CSA) and short-term methotrexate for GVHD prophylaxis. CSA was started as a continuous intravenous infusion at day –1 until oral CSA could be tolerated. The dose of CSA was adjusted according to the serum bilirubin or creatinine concentration to maintain the blood concentration of 200~400 ng/mL. Methotrexate was administered by intravenous infusion on days +1, +3, and +6.
The severity of AKI was classified according to the RIFLE criteria. Using the RIFLE criteria (acronym indicating Risk of renal dysfunction; Injury to the kidney; Failure of kidney function; Loss of kidney function and End-stage kidney disease), AKI was classified into three categories based on severity (Risk, Injury and Failure) and two categories based on clinical outcome (Loss and End-stage kidney disease). The outcome RIFLE categories Loss and End-stage kidney disease were not evaluated in this study. The criteria for classification of AKI-R, AKI-I, and AKI-F are as follows: AKI-R was considered if there was an increase in serum creatinine to more than 1.5-fold of baseline value, or a urinary output lower than 0.5 mL/kg/h for 6h. AKI-I was considered if there was an increase in serum creatinine to more than 2-fold of baseline value or urinary output lower than 0.5 mL/kg/h for 12h. AKI-F was considered if there was an increase in serum creatinine to more than 3-fold baseline value or urinary output lower than 0.3 mL/kg/h for 24h. Because other confounding factors may influence urine output, in the present study patients were assigned to RIFLE categories according to serum creatinine only. The serum creatinine levels used in AKI classification were the peak value during the first 100 days after transplantation.
Blood parameters were measured every two or three days. If an abnormal parameter was present it would be monitored every day until normal.
The baseline serum creatinine was defined as the mean value in serum creatinine level during the two-week period prior to myeloablative allo-HSCT.
Because the high bleeding risk in the early transplant phase precludes percutaneous liver biopsy, clinical criteria for the diagnosis of HVOD were proposed in 1984 and applied to clinical diagnosis now [8]. Clinical diagnosis of HVOD was established according to clinical criteria when two of the following events occurred within 20 days after transplantation: hyperbilirubinaemia, hepatomegaly, right upper quadrant pain of liver origin, and sudden unexplained weight gain (>2% of baseline body weight) due to fluid accumulation.
The mean values of total serum bilirubin level and cyclosporine blood level during the two weeks’ period prior to the development of AKI were evaluated as predictors of increased total bilirubin and cyclosporine. 40 μmol/L was considered a limitation of increased total bilirubin.
In the present study sepsis diagnosis is based on temperature >38°C or <36°C, white blood cell count >10 000/mm3 or <4000/mm3, and positive blood culture for bacteria. Liver toxicity was defined as increased levels of alanine aminotransferase (ALT) >80 u/L or aspartate aminotransferase (AST) >80 u/L. Lung toxicity diagnosis was based on respiratory rate >20 breaths per minute and PaO2 <60 mm Hg.
Patients were monitored during the first 100 days after transplantation for complications including sepsis, cytomegalovirus (CMV) infection, organ toxicity, increased total bilirubin, HVOD, and aGVHD.
Survival of patients at day 100 and day 180 after all-HSCT was also recorded.
Continuous variables were expressed as mean ± standard deviation (SD) and compared by the F test. The categorical variables were expressed as percentages and compared by the Chi-square test or Fisher’s exact test. Multivariate Cox regression analysis was used to examine the relative contributions of parameters on the outcome of AKI and to calculate odds ratios. The Kaplan-Meier method was used to calculate time to death for various classes of AKI. The survival curves were compared using the log-rank test. All p-values were two-tailed, and a value of ≤0.05 was considered statistically significant. SPSS version 10.0 statistical software was used to perform the analysis.
The median age of all patients at transplantation was 36 years with a range of 16–54 years. The baseline characteristics of the 143 patients are shown in table 1.

Figure 1
Worsening RIFLE category was associated with the increased mortality of patients in the 100 days post-transplant period.
Of 143 patients, 70 (48.9%) developed AKI according to the RIFLE criteria during the first 100 days after transplantation. The median time to development of AKI was day +33 (range day +1 to +96). 40 patients (28%) were categorised as AKI-R. 18 patients (12.6%) were categorised as AKI-I, and 12 (8.4%) as AKI-F. No patients with AKI-F needed dialysis. The incidence of AKI defined by RIFLE categories is shown in table 2.
There were no statistical differences in age, gender, baseline serum creatinine, TBI, or primary disease among the various classes of AKI (table 2). Univariate analysis demonstrated that the major factors associated with AKI were HVOD, grade III–IV aGVHD, and increased total bilirubin. Ten of all patients presented HVOD. The incidence of AKI-F in patients with HVOD was significantly higher than in patients without HVOD (p = 0.002). Eleven of all patients developed grade III–IV aGVHD. The incidence of AKI-I and AKI-F in patients with grade III-IV aGVHD was significantly higher than in patients without III-IV aGVHD (P = 0.001). The incidence of AKI-I and AKI-F in sixteen patients with increased total bilirubin was significantly higher than in patients without increased total bilirubin (P <0.001). The characteristics of all patients after allo-HSCT are listed in table 3.
Amphotericin B, sepsis, CMV infection, CSA levels, liver toxicity and lung toxicity were not associated with a significantly increased risk of developing AKI. Moreover, in the present study CSA levels >300 ng/mL were assigned as a factor in order to analyse the relationship between CSA levels >300 ng/mL and AKI. The results showed no difference in CSA levels >300 ng/mL in patients with AKI. In the present study no patient received aminoglycosides or vancomycin.
Multivariate analysis showed that HVOD is an independent risk factor for AKI-F (OR 5.058, 95% CI 1.317–19.424, p = 0.018), and increased total bilirubin is an independent risk factor for AKI-F (OR 5.126, 95% CI 1.403–18.998, P = 0.014). The p-value (0.065) for grade III-IV aGVHD was marginally above 0.05 (table 4).
With the deterioration of renal function, 100-day mortality in patients after allo-HSCT increased significantly (P <0.001, fig. 1). 180-day survival rates of AKI-R, AKI-I and AKI-F patients after transplant were 77.5%, 66.7%, and 41.7% respectively, and differed significantly among groups (p = 0.0095) (table 5).
Further multivariate regression analysis showed that AKI is an independent risk factor for mortality (RR = 6.984, 95%CI 1.227–39.762, p = 0.029) and is associated with a significantly higher risk of death than normal renal function (table 6).
| Table 1: Patient demographics and primary diagnosis for the entire cohort. | |
| Characteristics | Value |
| Age (years) | 36 |
| Gender (M/F) | 88/55 |
| Baseline serum creatinine (μmol/L) | 59.96 ± 12.69 |
| TBI (%) | 24.5 |
| Primary disease diagnosis | |
| Chronic myelogeneous leukaemia | 80 |
| Acute myeloid leukaemia | 38 |
| Acute lymphoblastic leukaemia | 18 |
| Myelodysplastic syndrome | 4 |
| Multiple myeloma | 2 |
| Lymphoma | 1 |
| Table 2: Characteristics of patients stratified by RIFLE category. | |||||
| Characteristics | Non-AKI | Patients with AKI | P | ||
| Class R | Class I | Class F | |||
| RIFLE category | 73 (51%) | 40 (28%) | 18 (12.6%) | 12 (8.4%) | |
| Gender (M/F) | 46/27 | 23/17 | 11/7 | 8/4 | NS |
| Age | 34.5 ± 8.5 | 37.2 ± 8.2 | 35.9 ± 9.6 | 33.6 ± 6.8 | NS |
| Baseline creatinine (μmol/L) | 62.41 ± 13.38 | 57.13 ± 11.63 | 57.44 ± 13.46 | 58.08 ± 7.88 | NS |
| TBI | 19 (26.0%) | 8 (20.0%) | 4 (22.2%) | 4 (33.3%) | NS |
| Chronic myelogenous leukaemia | 42 (57.5%) | 21 (52.5%) | 10 (55.6%) | 7 (58.3%) | NS |
| Acute myeloid leukaemia | 18 (24.7%) | 12 (30.0%) | 4 (22.2%) | 4 (33.3%) | NS |
| Acute lymphoblastic leukaemia | 10 (13.7%) | 5 (12.5%) | 2 (11.1%) | 1 (8.3%) | NS |
| Other disease | 3 (4.1%) | 2 (5.0%) | 2 (11.1%) | – | NS |
| TBI: total body irradiation | |||||
| Table 3: Univariate analysis for risk factors for AKI in patients after allo-HSCT. | |||||
| Characteristics | Patients with normal renal function | Patients with AKI | P | ||
| Class R | Class I | Class F | |||
| Amphotericin B | 3 (4.1%) | 2 (5.0%) | 1 (5.6%) | – | NS |
| CMV infection | 27 (37.0%) | 15 (37.5%) | 3 (16.7%) | 4 (33.3%) | NS |
| Sepsis | 6 (8.2%) | 1 (2.5%) | – | 1 (8.3%) | NS |
| Cyclosporine levels (ng/ml) | 483.20 ± 153.39 | 531.82 ± 147.99 | 429.63 ± 149.30 | 448.87 ± 106.44 | NS |
| Liver toxicity | 62 (84.9%) | 27 (67.5%) | 17 (94.4%) | 12 (100%) | NS |
| Lung toxicity | 8 (11.0%) | 3 (7.5%) | 3 (16.7%) | 3 (25%) | NS |
| HVOD | 4 (5.5%) | 2 (5.0%) | – | 4 (33.3%) | 0.002 |
| III-IV aGVHD | 4 (5.5%) | 1 (2.5%) | 3 (16.7%) | 5 (41.7%) | 0.001 |
| Increased total bilirubin | 2 (2.7%) | 2 (5.0%) | 5 (27.8%) | 7 (58.3%) | 0.000 |
| CMV: cytomegalovirus; HVOD: hepatic veno-occlusive disease; aGVHD: acute graft-versus-host disease. | |||||
| Table 4: Independent risk factors for AKI-F in patients after allo-HSCT. | |||
| Parameter | OR | 95% CI | P |
| HVOD | 5.058 | 1.317–19.424 | 0.018 |
| Increased total bilirubin | 5.126 | 1.403–18.998 | 0.014 |
| III-IV aGVHD | 3.545 | 0.924–13.608 | 0.065 |
| Table 5: Clinical outcomes stratified by RIFLE category. | ||
| 100 days after HSCT | 180 days after HSCT | |
| Survivors | Survivors | |
| Non-AKI | 67 (91.8%) | 57 (78.1%) |
| AKI-R | 35 (87.5%) | 31 (77.5%) |
| AKI-I | 15 (83.3%) | 12 (66.7%) |
| AKI-F | 5 (41.7%) | 5 (41.7%) |
| P | 0.0003 | 0.0095 |
| Table 6: Independent risk factors for death in patients after 100 days allo-HSCT. | |||
| Parameter | OR | 95% CI | P |
| TBI | 10.201 | 1.501–69.334 | 0.018 |
| Sepsis | 6.564 | 0.932–46.229 | 0.059 |
| AKI | 6.984 | 1.227–39.762 | 0.029 |
| HVOD | 3.640 | 0.679–19.505 | 0.131 |
| Increased total bilirubin | 4.346 | 0.739–25.563 | 0.104 |
| III–IV aGVHD | 6.271 | 1.624–24.220 | 0.008 |
AKI occurs with significantly higher frequency after myeloablative allo-HSCT. In the present study renal dysfunction is a common and potentially life-threatening complication after myeloablative allo-HSCT. Several studies reported that the incidence of AKI ranged from 26% to 73% in cohorts of patients receiving myeloablative allo-HSCT [9–13]. RIFLE criteria are a newly developed consensus definition for AKI and have the advantage of early and accurate diagnosis for the stage of renal dysfunction [3]. The present study stratified patients based on the RIFLE criteria to identify the relationship between risk factors and various degrees of AKI and between AKI and survival.
Using the RIFLE criteria, this study showed that 48.9% of allo-HSCT patients have various degrees of AKI, including 40 patients (28%) in AKI-R, 18 (12.6%) in AKI-I and 12 (8.4%) in AKI-F. The median time for the development of AKI after allo-HSCT was 33 days. Factors associated with kidney injury during the first month after allo-HSCT can cause an increased incidence of AKI. Gruss et al. [9] also showed that the first month after HSCT was a critical period for the occurrence of renal dysfunction. Therefore the application of any potential therapeutic agent to prevent kidney injury should last four to five weeks after allo-HSCT. Close monitoring of renal function and elimination of risk factors is beneficial in preventing kidney injury and decreasing the incidence of AKI.
Further analysis showed that the major factors associated with AKI-I and AKI-F after myeloablative allo-HSCT were HVOD, grade III-IV aGVHD and increased total bilirubin. Previous studies have identified HVOD as a risk factor for the development of AKI [9–12, 14]. The signs and symptom of HVOD precede the development of renal dysfunction. It has been assumed that portal hypertension resulting from hepatic sinusoidal injury leads to both decreased renal perfusion and tubular injury, the decreased renal perfusion probably being more significant at the beginning of AKI. This study showed that the presence of HVOD and increased total bilirubin was associated with increased risk of AKI-F. Patients with HVOD or increased total bilirubin had an approximately 5 times higher association with AKI-F. In the present study increased total bilirubin is an independent manifestation and the patients with increased total bilirubin have no HVOD. HVOD and increased total bilirubin were therefore considered to be independent risk factors for AKI-F after allo-HSCT. Patients with HVOD or increased total bilirubin were prone to develop more severe AKI-F rather than AKI-I and AKI-R.
The present study also showed that the incidence of AKI-I and AKI-F in patients with grade III-IV aGVHD was significantly higher than in those without grade III-IV aGVHD. Some research has demonstrated that renal dysfunction was more common in myeloablative allo-HSCT than autologous HSCT, and the difference may be due to GVHD [15]. GVHD may affect renal function through cytokine and immune-related injury [16]. Support for this theory arises from research on mice models which showed severe infiltration of cytotoxic T-cells in their kidneys during GVHD [17].
The nephrotoxicity of CSA is well known. It is not surprising to find a close relationship between increased blood CSA level and AKI [15, 18–19]. The present study found that the blood CSA level of patients with AKI-I was slightly higher than that of patients with other AKI classes, but the difference was not statistically significant. This study failed to show a significant association between blood CSA concentration and various degrees of AKI, which may be due to the strict surveillance of blood CSA concentration and active dosage adjustment.
In the present study different risk factors contributed to different categories of AKI. It reported an association between TBI, CMV infection, sepsis, drug, HVOD and aGVHD and the incidence of AKI [10–11, 13, 15, 20]. However, this study failed to find a relationship between the incidence of AKI and baseline creatinine, TBI, receiving amphotericin B, sepsis, cyclosporine levels, liver toxicity, or lung toxicity.
Previous studies have reported that renal dysfunction after allo-HSCT contributes to patients’ mortality [9–10, 14, 20–23]. The present study showed that patients who had AKI after allo-HSCT had a significantly higher mortality at day 100 as compared to those without AKI. Moreover, according to RIFLE criteria the mortality rates of patients with various degrees of AKI rise as renal dysfunction becomes more severe [24]. The survival curve of three categories of AKI demonstrated that 180-day survival of patients with various categories of AKI decreased gradually as the severity of the AKI category increases, and there was a significant difference in 180-day survival of patients among the three AKI categories. Although AKI is a reversible process, many of these patients die before renal recovery. Some studies argued that patients who developed AKI were at an additional increased risk of death in some way related to renal failure itself [21]. The present study showed that AKI was an independent prognostic factor for mortality after allo-HSCT, and patients with AKI had 6.984 times higher mortality. AKI was not a marker of severe HVOD or aGVHD. HVOD and aGVHD are associated with mortality and AKI worsens the prognosis in patients with HVOD or GVHD. Hence therapies preventing and treating AKI may substantially improve patients’ prognosis after allo-HSCT.
Accurately categorising renal dysfunction of patients after allo-HSCT using RIFLE criteria is of high significance. The RIFLE criteria appear to be sensitive to early changes in kidney function after allo-HSCT and can classify AKI into several categories based on severity. Early diagnosis of AKI-R, taking adequate prophylactic measures, and controlling HVOD, increased total bilirubin and aGVHD may prevent occurrence and development of AKI from AKI-R to AKI-I or even AKI-F. Those effective measures may improve the survival of patients after allo-HSCT and decrease the mortality rate of patients after allo-HSCT. In addition, the RIFLE criteria allow monitoring of the progression of AKI and appear to be a robust means of discriminating clinically relevant outcomes after allo-HSCT. The present study shows a significant increase in mortality during the first 100 days after allo-HSCT, with increasing severity of AKI by RIFLE category. Moreover, this study demonstrated that 6-month survival rates of each AKI category decreased with the increase in AKI severity. Several studies have revealed that AKI during the first 100 days post-transplant was associated with development of CKD [26–27], and a decreasing incidence of AKI after allo-HSCT may reduce the morbidity of CKD following myeloablative allo-HSCT. Thus, measures to lower the frequency and severity of AKI in patients with myeloablative allo-HSCT should be taken in order to lower morbidity and mortality in these patients. Strategies to reduce AKI following allo-HSCT may have a tremendously beneficial and profound impact on this population.
There are some limitations in the present study. First, a relatively small cohort of patients from the transplant centre of Blood Diseases Hospital in China was included in this retrospective study. Second, due to other confounding factors that may influence urine output, such as diuretic therapy, patients were assigned to RIFLE categories according to serum creatinine only. Third, the peak value of the serum creatinine levels during the first 100 days after transplant was used to classify AKI. Dynamic changes in serum creatinine were not analysed.
The RIFLE classification was used to diagnose renal dysfunction of myeloablative allo-HSCT. This study demonstrated that AKI is a very common complication after allo-HSCT. HVOD, increased total bilirubin, and grade III-IV aGVHD were identified as risk factors for AKI categories. AKI was an independent prognostic factor for mortality after allo-HSCT. The 100-day mortality rate of patients after allo-HSCT increased with the increase in severity of AKI categories, and 180-day survival decreased gradually as renal dysfunction became more severe. Preventing progression from AKI-R to AKI-I or even AKI-F would significantly improve patients’ prognosis after allo-HSCT. The RIFLE criteria appear to be an important tool to stratify these patients according to risk of death.
1 Hahn T, Rondeau C, Shaukat A, et al. Acute renal failure requiring dialysis after allogeneic blood and marrow transplantation identifies very poor prognosis patients. Bone Marrow Transplant. 2003;32:405–10.
2 CR Parikh, SG Coca. Acute renal failure in hemotopietic cell transplantation. Kidney Int. 2006;69:430–5.
3 Bellomo R, Ronco C, Kellum JA, et al. the ADQI workgroup: Acute renal failure – definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–R212.
4 Bell M, Liljestam E, Granath F, et al. Optimal follow-up time after continuous renal replacement therapy in actual renal failure patients stratified with the RIFLE criteria. Nephrol Dial Transplant. 2005;20:354–60.
5 Hoste EA, Clermont G, Kersten A, et al. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: A cohort analysis. Crit Care. 2006;10:R73.
6 Kuitunen A, Vento A, Suojaranta-Ylinen R, et al. Acute renal failure after cardiac surgery: Evaluation of the RIFLE classification. Ann Thorac Surg. 2006;81:542–6.
7 Levey AS, Coresh J, Balk E, et al. National Kidney Foundation: National Kidney Foundation practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–47.
8 McDonald GB, Sharma P, Matthews DE, et al. Venocclusive disease of the liver after bone marrow transplantation: diagnosis, incidence, and predisposing factors. Hepatology. 1984;4:116–22.
9 Gruss E, Bernis C, Tomas JF, et al. Acute renal failure in patients following bone marrow transplantation: prevalence, risk factor and outcome. Am J Nephrol. 1995;15:473–9.
10 Parikh CR, McSweeney PA, Korular D, et al. Renal dysfunction in allogeneic hematopoietic cell transplantation. Kidney Int. 2002;62:566–73.
11 Hingorani SR, Guthrie K, Batchelder A, et al. Acute renal failure after myeloablative hematopoietic cell transplant: incidence and risk factor. Kidney Int. 2005;67:272–7.
12 Parikh CR, Schrier RW, Storer B, et al. Comparison of ARF after myeloablative and nonmeyloablative hematopoietic cell transplantation. Am J kidney Dis. 2005;45:502–9.
13 Zager RA, O’Quigley J, Zager BK, et al. Acute renal failure following bone marrow transplantation: A retrospective study of 272 patients. Am J Kidney Dis. 1989;13:210–6.
14 Liu H, Ding JH, Liu BC, et al. Early renal injury after nonmyeoloablative allogeneic peripheral blood stem cell transplantation in patient with chronic myelocytic leukemia. Am J Nephrol. 2007;27:336–41.
15 Caliskan Y, Besisik SK, Sargin D, et al. Early renal injury after myeloablative allogeneic and autologous hematopoietic cell transplantation. Bone Marrow Transplant. 2006;38:141–7.
16 Rao PS. Nephrotic sydrome in patients with peripheral blood stem cell transplant. Am J Kidney Dis. 2005;45:780–5.
17 Panoskaltsis-Mortari A, Price A, Hermanson JR, et al. In vivo imaging of graft-versus-host-disease in mice. Blood. 2004;103:3590–8.
18 Zager RA. Acute renal failure in the setting of bone marrow transplantation. Kidney Int. 1994;46:1443–58.
19 Kist-van Holthe J, Goedvolk CA, Brand R, et al. Prospective study of renal insufficiency after bone marrow transplantation. Pediatr Nephrol. 2002;17:1032–7.
20 H Liu, Y-F Li, BC Liu, et al. A multicenter, retrospective study of acute kidney injury in adult patients with nonmyeloablative hematopoietic SCT. Bone Marrow Transplant. 2010;45:153–8.
21 Parikh CR, McSweeney P, Schrier RW. Acute renal failure independently predicts mortality after myeloablative allogeneic hematopoietic cell transplant. Kidney Int. 2005;67:1999–2005.
22 Lopes JA, Jorge S, Silva S, et al. Prognostic utility of the acute kidney injury network criteria for acute kidney injury in myeloablative haematopoietic cell transplantation. Bone Marrow Transplant. 2007;40:1005–6.
23 Kersting S, Dorp SV, Theobald M, et al. Acute renal failure after nonmyeloablative stem cell transplantation in adults. Biol Blood Marrow Transplant. 2008;14:125–31.
24 JA Lopes, S Jorge, S Goncolves, et al. Contemporary analysis of the influence of acute kidney injury (AKI) after myeloablative hematopoietic cell transplantation on long-term patients’ survival. Bone Marrow Transplant. 2008;42:139–41.
25 Lopes JA, Jorge S, Silva S, et al. Acute renal failure following myeloablative autologous and allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2006;38:707.
26 Weiss AS, Sandmaier BM, Storer B, et al. Chronic kidney disease following nonmyeloablative hematopoietic cell transplantation. Am J Transplant. 2006;6:89–94.
27 Delgado J, Cooper N, Thomson K, et al. The importance of age, fludarabine, and total body irradiation in the incidence and severity of chronic renal failure after allogeneic hematopoietic cell transplantation. Biol Blood Marrow transplant. 2006;12:75–83.
Acknowledgement:We express appreciation here for the support given by all colleagues in the study centre.
Funding / competing conflicts of interests:None of the authors declared competing interests. This work is supported by a Heilongjiang Provincial Youth Science Grant (QC2009C53).