Figure 1
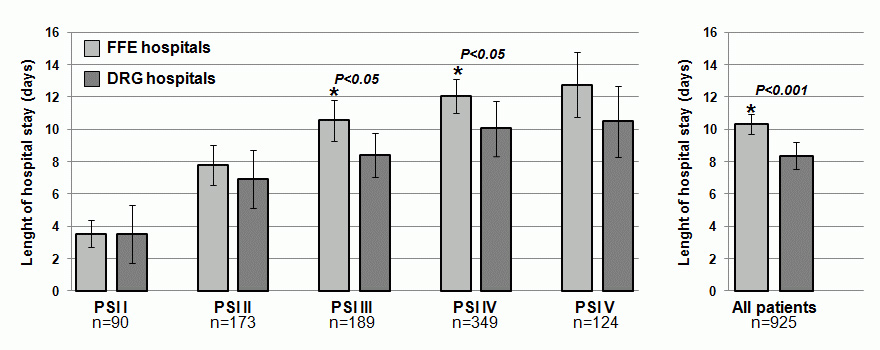
Length of Hospital stay according to severity of pneumonia in hospitals with a FFS system (light gray) or a DRG system (dark gray); asterisk refers to significant difference of FFE compared to DRG hospital.
DOI: https://doi.org/10.4414/smw.2011.13228
In Switzerland, the reimbursement system for inpatient treatment is currently in transition. The Swiss Parliament passed a law dictating that starting in 2012 a Diagnosis Related Groups (DRG) hospital financing system based on the German G-DRG system will replace the currently used fee-for-service system (FFS) nationwide [1, 2]. Thus far, DRG-based hospital reimbursement has not been simultaneously realised in all Swiss cantons: while hospitals in some cantons currently already use DRG based systems based on the 3M’s AP-DRG system for hospital financing [3], others still use the traditional FFS based systems. This situation provides the opportunity to perform a head-to-head comparison between the two reimbursement systems.
While in FFS systems hospitals (and physicians in patients with private insurance) receive reimbursement for each health care service provided and for each additional day of hospital stay, the DRG reimbursement system classifies hospital cases into groups expected to have similar hospital resource use as part of a prospective payment system. Thus, while in the FFS system, hospitals and physicians have no financial benefit when patients are discharged early, the opposite is true in DRG systems. Hence, the DRG system is expected to create an incentive for hospitals and physicians to reduce patient’s length of hospital stay (LOS) and thereby curtail the increasing costs associated with in-hospital patient care [4–7]. Whether the quality of health care within Switzerland will also be affected by this transition is unclear and currently a matter of public debate and concern [8, 9]. Most literature on this topic is based exclusively on claims data and focuses on mortality and readmission rates on a population level only, while patient-centred data (such as individual satisfaction with care and quality of life of patients) are scarce. Busato and von Below[2], for example, used the complete dataset of all hospital discharges in Switzerland (2003–2007) and corresponding claims data to analyse differences of volume and major quality indicators of care between areas with or without DRG-based hospital reimbursement from a population based perspective. They found a (desired) shift to practice-based outpatient care, but higher 90-day rehospitalisation rates of almost 15% in areas with DRG reimbursement. Patient-centred data were not available in this study and whether DRG based systems negatively affected patients’ satisfaction with care and quality of life remains unclear.
The aim of this analysis of community-acquired pneumonia patients from a previous randomised-controlled multicentre trial [10, 11] was to compare LOS and patient outcomes between hospitals with DRG systems and FFS systems in Switzerland.
The aim of this retrospective analysis was to investigate differences in patient care and patient relevant outcomes in FFS hospitals compared to hospitals that have already implemented a SwissDRG reimbursement system in Switzerland.
This is a post-hoc analysis including all 925 patients with a definite diagnosis of community-acquired pneumonia from a previous prospective randomised-controlled multicentre trial [10]. A detailed study protocol has been published previously [11]. In brief, from October 2006 to March 2008, from a total of 1825 potential patients, 1359 patients with a presumptive diagnosis of a lower respiratory tract infection were consecutively enrolled in six different hospitals located in the northern part of Switzerland. The aim of the initial non-inferiority study was to compare length of antibiotic therapy and outcomes in patients with respiratory tract infections managed with a procalcitonin algorithm compared to a control group. The main analysis was an intention-to-treat analysis with imputation of the outcome of one patient with missing follow-up information. Secondary aims were to investigate prognostic markers for blood culture positivity and adverse outcomes [12–18] and preferences of patients and physicians towards inhospital treatment [19, 20]. Baseline characteristics of the six participating hospitals have previously been reported [11, 21]. Two of these hospitals had, due to regulations in the respective canton where they are located, an AP-DRG reimbursement system based on the German G-DRG system [1, 2] in place starting about 5 years before the study period, while the remaining four hospitals received reimbursement on a FFS basis. This AP-DRG system is similar to systems used in other countries, but in this transition period, these hospitals were still protected from financial losses by the cantons.
The study protocol was approved by all of the local ethical committees, and written informed consent was obtained from all participants.
Inclusion criteria were age >18 years and suspected lower respiratory tract infection as principal diagnosis on admission. In accordance with guidelines, lower respiratory tract infection was defined by the presence of at least one respiratory symptom plus at least one finding during auscultation, or one sign of infection [22, 23]. Community-acquired pneumonia was defined as a new infiltrate on chest X-ray [22, 23]. Patients were examined on admission to the emergency department by a resident supervised by a board-certified specialist in internal medicine. The standardised baseline assessment included medical history, clinical examination, lab tests and chest X-ray. For all patients with community-acquired pneumonia, the Pneumonia Severity Index (PSI) was calculated to assess disease severity [24]. The PSI categorises patients into 5 risk classes: those in classes I–III (PSI ≤90 points) designate lower risk patients with an estimated mortality of about 0.3%, 0.6% and 0.9% and those in classes IV–V (PSI >90 points) represent high risk individuals with an estimated mortality of around 10% and 30%, respectively. Within the study, the decision to discharge patients was left to the discretion of the treating physicians without interference of the study team.
Patients were monitored by the study team during their hospital stay and blinded medical students performed structured phone interviews at 30, 180 days and at 18 months after enrolment to assess outcomes [29]. LOS was defined as the time from admission to hospital until discharge or death. To measure changes in mortality we used all-cause mortality within 30 days and 18 months; recurrent infection was defined as radiologically, microbiologically or clinically confirmed recurrence of infection requiring antibiotics as judged by the treating physician (primary care physicians or hospital physician). These outcomes were monitored by an independent Data Safety and Monitoring Board. In case a patient could not be reached, we contacted the patient’s family or the primary care physicians to verify the survival status.
Patients were also asked during the interview on day 30 about on-going discomfort related to the initial infection and about satisfaction with the discharge process including whether timing of discharge was appropriate as part of a secondary study aim [19, 20]. We further assessed quality of life as measured with the EQ-5D instrument [25]. The EQ-5D includes the 15-item tool EQ-5Dself-classifier which assesses the health related quality of life along five dimensions (mobility, self-care, usual activities, pain/discomfort and anxiety/depression) and also allows calculation of a weighted Quality of life index [26]. During the 30 days follow up, 1 patient was lost to follow up. Between 5–10% of patients did prefer not to respond to at least one question about quality of life or satisfaction with care; these patients were excluded from the respective analysis (complete case analysis).
We used descriptive statistics including mean with standard deviation and frequencies to describe the populations, as appropriate. We compared LOS in FFS and DRG hospitals stratified by disease severity using the PSI [24]. We calculated Cox proportional hazard models to assess the association of reimbursement system and time to hospital discharge adjusted for disease severity (PSI), age, gender and important comorbidities (congestive heart failure, chronic renal failure, chronic obstructive pulmonary disease) and also adjusting for clustering of patients within hospitals. We also investigated whether patients’ types of medical insurance (basic or private) have an influence on LOS by inclusion of an interaction term between insurance type and hospital financing system to exclude effect modification. Generalised estimating equation (GEE) method was applied for outcome evaluation between the two reimbursement systems accounting for clustering within hospitals. All models were adjusted for disease severity (PSI), age, gender and comorbidities.
This study included 925 patients with a definite diagnosis of community-acquired pneumonia enrolled in either one of four FFS hospitals (n = 666) or one of two DRG based hospital (n = 259). There was no difference in terms of patient age and gender or previous health history. The patient population in DRG hospitals had higher rates of chronic renal failure (27% vs 20%) and lower rates of congestive heart failure (11% vs 19%) as compared to FFS hospitals. There was no difference in the distribution of severity of disease as assessed with the PSI. Baseline characteristics of the population overall and stratified by hospital financing type are displayed in table 1.

Figure 1
Length of Hospital stay according to severity of pneumonia in hospitals with a FFS system (light gray) or a DRG system (dark gray); asterisk refers to significant difference of FFE compared to DRG hospital.
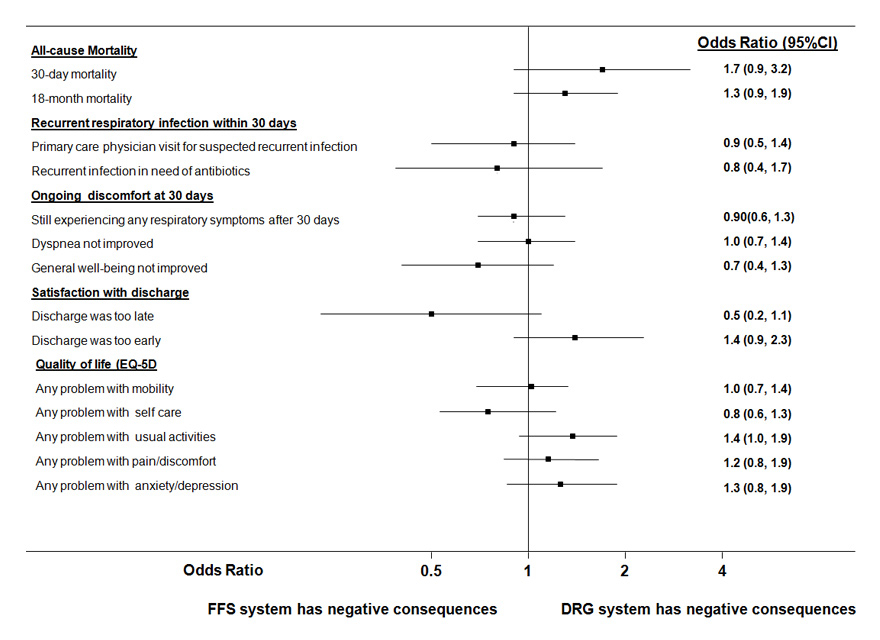
Figure 2
Forest plot showing individual outcomes (separately analysed) according to hospital reimbursement; of note, odds ratios = 0 indicate no effect, while odds ratios >1 indicate a positive association between the exposure variable and hospitals with DRG financing systems; models are adjusted for age, gender, severity (PSI) and comorbidities (congestive heart failure, COPD, chronic renal failure) and account for clustering of patients within hospitals.
Overall, LOS in DRG hospitals was about 20% shorter compared to FFS hospitals (8.4 vs 10.3 days, absolute difference 1.9 days [95%CI 0.8–3.1], p <0.001). As shown in figure 1, this difference was most pronounced in the subgroup of patients with PSI classes III and IV. A significantly shorter LOS in DRG hospitals was also confirmed in multivariate Cox models for time to discharge of patients (hazard ratio 1.20 [95% 1.11–1.32]) adjusted for age, gender, severity (PSI) and comorbidities. Note, hazard ratios higher than 1 indicate an association of the factor with shorter LOS. This result remained robust when restricting to hospital survivors only (data not shown).
We also investigated whether the 19% of patients (n = 174) with private medical insurance had a different LOS than patients with only basic insurance and whether this association was different in FFS and DRG hospitals. We found no association between insurance type and LOS (hazard ratio of private insurance 1.1 (95%CI 0.9–1.2) and no evidence for effect modification by type of hospital financing system (p of the interaction term = 0.27).
Table 2 shows outcomes of patients being treated in FFS hospitals and DRG hospitals. There was no significant difference in 30 days and 18 month mortality rates (adjusted odds ratio 1.7 (95%CI 0.9–3.2) and 1.3 (95%CI 0.9–1.9) and both groups had similar recurrence rates (adjusted odds ratio 0.8 [95% 0.4–1.7]) (fig. 2). Also, a similar proportion of patients in both groups still experienced discomfort related to the initial infection, namely respiratory symptoms, dyspnoea or were generally not feeling well. The majority of patients in both groups were satisfied with the discharge process with no differences between hospitals: 14% and 10% of patients in FFS and DRG hospitals indicated that discharge was too early (adjusted odds ratio 1.4, 95%CI 0.9–2.3). Finally we also compared quality of life after 30 days in both groups using the EQ-5D questionnaire. There was no difference in the weighted quality-of-life index [26] between FFS and DRG hospitals (0.82 vs 0.83, absolute difference –0.007 [95%CI –0.04–0.02], p = 0.64). Also, there was no significant difference in any of the 5 documented quality-of-life domains (mobility, self-care, usual activities, pain/discomfort and anxiety/depression) (see fig. 3).
| Table 1:Baseline characteristics of patients with community-acquired pneumonia according to hospital reimbursement system. | ||||
| All patients (n = 925) | FFS hospitals (n = 666) | DRG hospitals (n = 259) | P | |
| Demographics – Age (years)* – Sex (male) – no. (%) | 68 (±17.8) 544 (59%) | 68 (±17.8) 390 (58%) | 66 (±18.2) 154 (59%) | 0.06 0.80 |
| Co-existing illnesses – Congestive heart failure – Renal failure – COPD – Diabetes | 159 (17%) 206 (22%) 282 (30%) 162 (18%) | 130 (19%) 135 (20%) 211 (32%) 110 (17%) | 29 (11%) 71 (27%) 71 (27%) 52 (20%) | <0.01 0.02 0.21 0.20 |
| Previous history – Former or current smoker – Previous Treatment with AB – History of fever – History of chills | 233 (26%) 236 (26%) 618 (67%) 301 (37% | 164 (25%) 172 (26%) 456 (69%) 214 (37%) | 69 (27%) 64 (25%) 162 (63%) 87 (37%) | 0.63 0.18 0.06 0.96 |
| Clinical findings – Confusion – no. (%) – Respiratory rate (b/min)* – Systolic BP (mm Hg)* – Heart rate (beats/minute)* – Body temperature (C°)* | 74 (9%) 22 (±9) 133 (±19) 96 (±18) 38.1 (±1,1) | 57 (9%) 23 (±9) 134 (±22) 96 (±21) 38.1 (±1,1) | 17 (7%) 21 (±8) 129 (±21) 95 (±20) 37.8 (±1.1) | 0.33 0.06 <0.01 0.38 <0.01 |
| Severity of disease – I, II, III – IV – V | 92 (±36) 452 (49%) 349 (38%) 124 (13%) | 93 (±36) 314 (47%) 126 (39%) 92 (14%) | 90 (±37) 138 (53%) 89 (34%) 32 (12%) | 0.25 0.38 |
| COPD, chronic obstructive pulmonary disease; BP, blood pressure; PSI Pneumonia Severity Index; *expressed as mean (± standard deviation); AB, antibiotics P values refer to t-tests and chi-square tests | ||||
| Table 2:Patient outcomes with regard to hospital type. | ||||
| Outcome | FFS hospital patients | DRG hospital patients | Adjusted* odds ratio (95%CI) | p value |
| All-cause mortality, % (n) | ||||
| 30–day mortality | 4.8% (32/666) | 6.9% (18/259) | 1.7 (0.9, 3.2) | 0.127 |
| 18–month mortality | 21.0% (140/666) | 23.2% (60/259) | 1.3 (0.9, 1.9) | 0.16 |
| Recurrent respiratory infection within 30 days, % (n) | ||||
| Primary care physician visit for suspected recurrent infection | 9% (60/605) | 9% (22/234) | 0.9 (0.5, 1.6) | 0.734 |
| Confirmed recurrent infection in need of antibiotics | 5% (30/666) | 4% (9/259) | 0.8 (0.4, 1.7) | 0.499 |
| Ongoing discomfort at 30 days, % (n) | ||||
| Still experiencing any respiratory symptoms | 40% (248/626) | 39% (92/238) | 0.9 (0.6, 1.3) | 0.634 |
| Dyspnoea not improved | 23% (138/612) | 22% (52/232) | 1.0 (0.7, 1.4) | 0.879 |
| General well-being not improved | 8% (55/614) | 6% (15/233) | 0.7 (0.4, 1.3) | 0.247 |
| Satisfaction with discharge process, % (n) | 0.08 | |||
| Satisfied with discharge process | 83% (483/585) | 83% (184/223) | ||
| Discharge was too late | 7% (42/585) | 4% (8/223) | 0.5 (0.2, 1.1) | 0.096 |
| Discharge was too early | 10% (60/585) | 14% (31/223) | 1.4 (0.9, 2.3) | 0.147 |
| Quality of life (EQ-5D), % (n) | ||||
| Any problems with mobility | 33% (200/613) | 31% (73/235) | 1.0 (0.7, 1.4) | 0.943 |
| Any problems with self care | 20% (124/618) | 16% (38/237) | 0.8 (0.6, 1.3) | 0.439 |
| Any problems with usual activities | 34% (208/617) | 37% (88/237) | 1.4 (1.0, 1.9) | 0.074 |
| Any problems with pain/discomfort | 31% (193/619) | 35% (82/234) | 1.2 (0.8, 1.9) | 0.428 |
| Any problems with anxiety/depression | 16% (96/605) | 19% (44/236) | 1.3 (0.8, 1.9) | 0.29 |
| *models are adjusted for age, gender, severity (PSI) and comorbidities (congestive heart failure, COPD, chronic renal failure) and account for clustering of patients within hospitals | ||||
Within this study focusing on community-acquired pneumonia patients from a previous multicentre trial, we investigated differences in LOS and patient relevant outcome between FFS hospitals and DRG hospitals. We found significant shorter LOS of about 20% in hospitals that used DRG reimbursement system. In the Swiss health care system, one in-hospital day costs roughly 1000–1200 USD; thus the observed reduction in LOS amounts to about 2000 USD per patient from a total patient cost of around 10’000 USD. Although our study was not powered to detect small differences in rare outcomes such as mortality, we found no apparent harm associated with hospitals using DRGs in regard to short and long term mortality, recurrence rate, patient satisfaction with the discharge process and different quality of life measures.
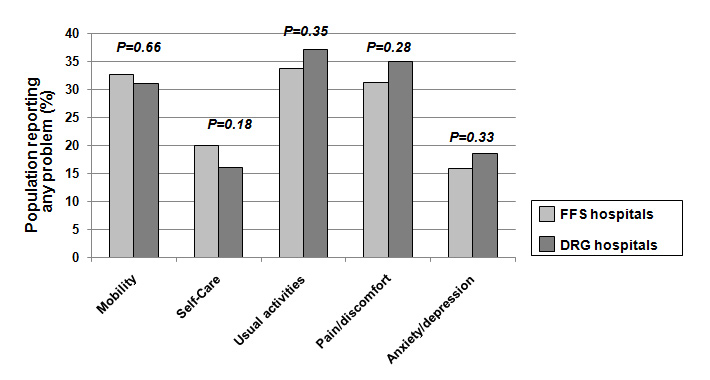
Figure 3
Quality of life according to reimbursement status; for all comparisons there was no statistical significant difference (p <0.05 for all chi-square tests).
The DRG system was developed at Yale University to provide hospitals with incentives to focus more on costs [4, 5]. Hospital Medicare inpatients are classified into groups that are allegedly clinically coherent and homogenous with respect to resource use. The classification is dependent on principal and secondary diagnoses and procedures, age, gender, and discharge status of the patient. Reimbursement is flat-rate and determined by the way patients are classified. Hospitals can create excess revenues by treating the patient more efficiently and economically, or conversely absorb monetary losses. It is argued that hospitals will become more frugal and that physicians will adjust their methods of practice as well.
As a positive example, a Japanese study found that after introduction of DRGs in 2003, women with breast cancer were treated with shorter LOS for surgical therapy and chemotherapy while maintaining the quality of care [27]. Still, whether DRG effectively reduces LOS in the general population without negatively affecting patient outcomes is a matter of controversy [28]. In addition, the influence of DRGs on the quality of care and patient safety is a major public concern. To ultimately answer this question, large randomised-controlled trials allocating hospitals to either reimbursement systems would be desirable, but are hardly feasible. For these reasons, it is important that studies use other trial designs, such as before-after designs, time-series or quasi-experiments, to investigate the effects of DRGs on patient outcomes. Also, qualitative studies grasping views and perceptions on the influence of DRGs on the quality of care are paramount. Large epidemiological studies have used claims data to compare readmission and mortality rates [2]. The strength of these analyses is the large sample size with high statistical power, which allows detection of even small differences in outcomes. Yet, these studies are often hampered by lack of socio-demographic data and disease severity, which is necessary to adjust for confounding. In addition, these studies often do not report patient-specific outcomes, such as quality of life and satisfaction with care. Our analysis of a large scale, well-defined and characterised cohort of patients provides novel insights and is thus noteworthy. Importantly, within our analysis we focused only on patients with community-acquired pneumonia of different severities; further studies are required to validate these findings for other medical and surgical patient populations.
Our data are especially worthy of attention, as they constitute a timely baseline before the nationwide implementation of DRGs. Switzerland is currently in a transition of payment systems from mainly FFS hospitals to a DRG system which will take place in 2012. Importantly, hospitals that are currently using DRGs in Switzerland are still protected from financial losses by the cantons. Therefore, the pressure to optimise patient care in general, and LOS in particular, differs from what is expected to be implemented as of 2012. Hence, the introduction of the SwissDRG may still lead to other consequences based on the issue of rationing at the bedside. It will be important to closely monitor the quality of health care during this transition to prevent negative effects on patients.
This study has some limitations. First, compared to large population based studies, our cohort lacks the statistical power to detect smaller differences in mortality and other infrequent outcomes. This is of particular importance with regard to type II errors (false negative results). Second, the 6 participating hospitals were all relatively large in size and we do not know if our findings unconditionally apply to smaller community hospitals. Also, this study is embedded in the Swiss health care system and may not be valid for other countries/health care systems. Third, although current DRGs use varies regionally within Switzerland (in certain cantons) as opposed to from hospital to hospital, differences in patients’ baseline characteristics may confound our analysis. For this reason, we adjusted the analysis for disease severity using the validated PSI, socio-demographic data and comorbidities. Still, we cannot exclude residual confounding related to baseline differences of patients living in cantons where DRG have been implemented. Fourth, as a secondary analysis of a previous randomised study, selection bias is an issue and specific patient populations (i.e. immune-suppression, patients with dementia) were not included in this study, which may limit the “generalizability" of our results. Thus further research is needed to confirm our findings.
In conclusion, this study focusing on community-acquired pneumonia patients with different severities found a 20% shorter LOS in hospitals with DRG financing compared to FFS hospitals without apparent harmful effects on patient outcomes and quality of life measures. Further studies are required to validate these findings for other medical and surgical patient populations.
We thank Prof. Meredith Rosenthal, Harvard School of Public Health in Boston, USA, for helpful scientific discussions. We are grateful to the Data Safety and Monitoring Board, namely A.P. Perruchoud, S. Harbarth and A. Azzola for continuous supervision of this initial trial and all local physicians, the nursing staff, the patients and their relatives who participated in this study. Especially, we thank the staff of the emergency room, medical clinics and central laboratories of the University Hospital Basel, the Cantonal Hospitals Liestal, Aarau, Luzern and Muensterlingen and the «Buergerspital» Solothurn for their very helpful assistance, patience and technical support. We thank other members of the ProHOSP Study Group for their important help during the study.
The ProHOSP Study group included the following persons: Ursula Schild, RN, Katharina Regez, RN, Rita Bossart, RN, Robert Thomann, MD, Claudine Falconnier, MD, Marcel Wolbers, PHD, Stefanie Neidert, MD, Thomas Fricker, MD, Claudine Blum, MD, Thomas Bregenzer, MD, Claus Hoess, MD, Heiner C. Bucher, MD, Fabian Mueller, Jeannine Haeuptle, Roya Zarbosky, Rico Fiumefreddo, Melanie Wieland, RN, Charly Nusbaumer, MD, Andres Christ, MD, Roland Bingisser, MD, Kristian Schneider, RN, Brigitte Walz, PhD, Verena Briner, MD, Dieter Conen, MD, Andreas Huber, MD, Jody Staehelin, MD, Aarau, Chantal Bruehlhardt, RN, Ruth Luginbuehl, RN, Agnes Muehlemann, PhD, Ineke Lambinon and Max Zueger, MD.
1 Klauss G, Staub L, Widmer M, Busato A. Hospital service areas – a new tool for health care planning in Switzerland. BMC health services research. 2005;5:33.
2 Busato A, von Below G. The implementation of DRG-based hospital reimbursement in Switzerland: A population-based perspective. Health Res Policy Syst. 2010;8:31.
3 Fischer W. Die DRG Familie. CH-9116 Wolfertswil, Switzerland: ZIM-Verlag. 2008.
4 Hervis RM. Impact of DRGs on the medical profession. Clin Lab Sci. 1993;6(3):183–5.
5 Beaty L. Understanding diagnostic related groups (DRGs) and inpatient hospital reimbursement. Gastroenterol Nurs. 2005;28(5):363–8.
6 Rosenthal MB. Beyond pay for performance – emerging models of provider-payment reform. N Engl J Med. 2008;359(12):1197–200.
7 Rosenthal MB. What works in market-oriented health policy? N Engl J Med. 2009;360(21):2157–60.
8 Donaldson C, Magnussen J. DRGs: the road to hospital efficiency. Health Policy. 1992;21(1):47–64.
9 Pretto M, Spirig R, Kaelin R, Muri-John V, Kressig RW, Suhm N. Outcomes of elderly hip fracture patients in the Swiss healthcare system: A survey prior to the implementation of DRGs and prior to the implementation ofa Geriatric Fracture Centre. Swiss Med Wkly. 2010;140:w13086.
10 Schuetz P, Christ-Crain M, Thomann R, Falconnier C, Wolbers M, Widmer I, et al. Effect of procalcitonin-based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. Jama. 2009;302(10):1059–66.
11 Schuetz P, Christ-Crain M, Wolbers M, Schild U, Thomann R, Falconnier C, et al. Procalcitonin guided antibiotic therapy and hospitalization in patients with lower respiratory tract infections: a prospective, multicenter, randomized controlled trial. BMC health services research. 2007;7:102.
12 Albrich WC, Dusemund F, Ruegger K, Christ-Crain M, Zimmerli W, Bregenzer T, et al. Enhancement of CURB65 score with Pro-Adrenomedullin (CURB65-A) for outcome prediction in Respiratory Tract Infections: Derivation of a Clinical Algorithm. BMC infectious diseases. 2011;11:112.
13 Muller F, Christ-Crain M, Bregenzer T, Krause M, Zimmerli W, Mueller B, et al. Procalcitonin levels predict bacteremia in patients with community-acquired pneumonia: a prospective cohort trial. Chest. 2010;138(1):121–9.
14 Schuetz P, Christ-Crain M, Albrich W, Zimmerli W, Mueller B. Guidance of antibiotic therapy with procalcitonin in lower respiratory tract infections: insights into the ProHOSP study. Virulence. 2010;1(2):88–92.
15 Schuetz P, Christ-Crain M, Zimmerli W, Mueller B. Repeated measurements of endothelin-1 precursor peptides predict the outcome in community-acquired pneumonia. Intensive Care Med. 2011;37(6):970-80. Epub 2011 Mar 11.
16 Schuetz P, Suter-Widmer I, Chaudri A, Christ-Crain M, Zimmerli W, Mueller B. Prognostic value of procalcitonin in community-acquired pneumonia. The European respiratory journal: official journal of the European Society for Clinical Respiratory Physiology. 2011;37(2):384–92.
17 Schuetz P, Wolbers M, Christ-Crain M, Thomann R, Falconnier C, Widmer I, et al. Prohormones for prediction of adverse medical outcome in community-acquired pneumonia and lower respiratory tract infections. Critical care (London, England). 2010;14(3):R106.
18 Vazquez M, Jockers K, Christ-Crain M, Zimmerli W, Muller B, Schuetz P. MR-pro-atrial natriuretic peptide (MR-proANP) predicts short- and long-term outcomes in respiratory tract infections: A prospective validation study. Int J Cardiol. 2010 Nov 18.
19 Baehni C, Meier S, Spreiter P, Schild U, Regez K, Bossart R, et al. Which patients with lower respiratory tract infections need inpatient treatment? Perceptions of physicians, nurses, patients and relatives. BMC pulmonary medicine. 2010;10:12.
20 Spreiter P, Meier S, Baehni C, Schild U, Regez K, Bossart R, et al. Steps to Take to Reduce Length of Hospital Stay in Patients With Lower Respiratory Tract Infections: A Prospective Cohort Study. Home Health Care Management & Practice. 2010;10:1–8.
21 Bundesamt für Gesundheit (BAG) EDdI: «Kennzahlen der Schweizer Spitäler 2004». http://www.bagadmin.ch . 2006 01.11.2006.
22 Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27–72.
23 Niederman MS, Mandell LA, Anzueto A, Bass JB, Broughton WA, Campbell GD, et al. Guidelines for the management of adults with community-acquired pneumonia. Diagnosis, assessment of severity, antimicrobial therapy, and prevention. American journal of respiratory and critical care medicine. 2001;163(7):1730–54.
24 Fine MJ, Auble TE, Yealy DM, Hanusa BH, Weissfeld LA, Singer DE, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336(4):243–50.
25 Schrag A, Selai C, Jahanshahi M, Quinn NP: The EQ-5D – a generic quality of life measure-is a useful instrument to measure quality of life in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2000;69(1):67–73.
26 Shaw JW, Johnson JA, Coons SJ. US valuation of the EQ-5D health states: development and testing of the D1 valuation model. Med Care. 2005;43(3):203–20.
27 Kuwabara H, Fushimi K: The impact of a new payment system with case-mix measurement on hospital practices for breast cancer patients in Japan. Health Policy. 2009;92(1):65–72.
28 Gilman BH: Hospital response to DRG refinements: the impact of multiple reimbursement incentives on inpatient length of stay. Health Econ. 2000;9(4):277–94.
29 Guertler C, Wirz B, Christ-Crain M. Zimmerli W, Mueller B, Schuetz P. Inflammatory responses predict long-term mortality risk in community-acquired pneumonia. Eur Respir J. 2011;7(6):1439-46. Epub 2010 Nov 11.
Funding / potential competing interest:The initial trial was supported by grant SNF 3200BO-116177/1 from the Swiss National Science Foundation. Dr. Schuetz was supported by a research grant from the Swiss Foundation for Grants in Biology and Medicine (Schweizerische Stiftung für medizinisch-biologische Stipendien, SSMBS, PASMP3–127684/1). All authors declare that they have no competing interests.