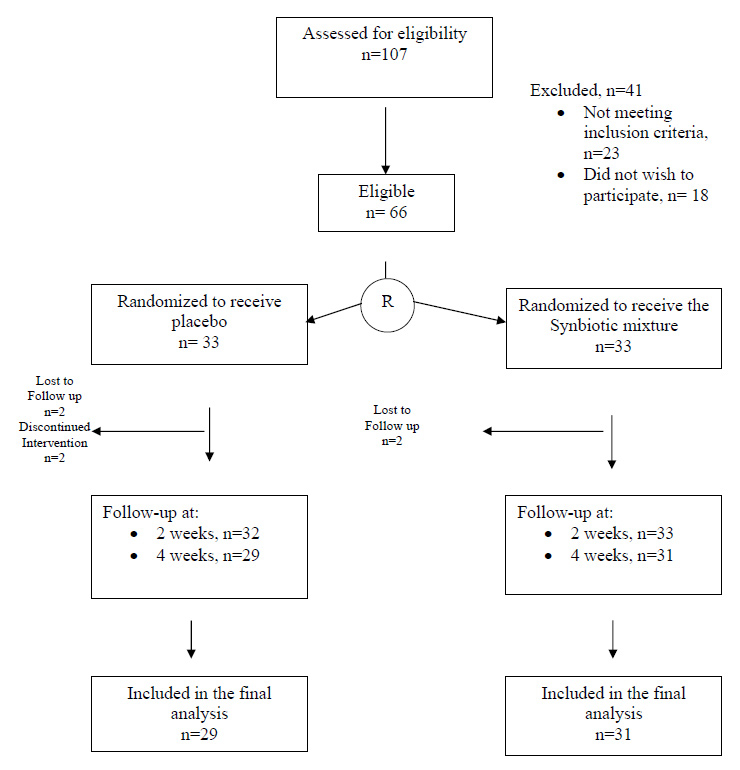
Figure 1
Study flow diagram.
DOI: https://doi.org/10.4414/smw.2011.13239
Functional constipation is a common problem in clinical practice and 2% to 27% of individuals in western countries suffer from constipation [1]. In an Asian population, a prevalence of 14% has been reported for functional constipation [2]. In addition to its adverse effect on quality of life [3], constipation is associated with increased work absenteeism [4] and health care costs [5].
Patients define constipation in different ways, generally as infrequent stools, which would be fewer than three bowel movements per week. Abnormally hard stools, defecation that requires excessive straining, unsuccessful defecation, and incomplete bowel evacuation are other definitions which are commonly used by patients [6]. A broadly accepted and practical definition for constipation is not available [7]. Therefore, to standardise the definition of functional constipation in clinical trials, Rome II, [8] and recently Rome III [9] criteria were introduced.
The large bowel is a pool for many different microorganisms and alteration in the pattern of intestinal bacteria, which is characterised by a decline in the population of necessitate bacteria and enhancement in the number of potentially pathogens microorganism [10], may change large bowel motility and secretary function via changing the metabolic environment of the colon and the amount of physiologically active substances [10, 11]. This hypothesis was raised from investigations that showed alleviation of constipation by administration of probotics [12, 13].
Oral probiotics are defined as “living microorganisms, which upon ingestion in certain numbers exert health benefits beyond inherent basic nutrition” [14]. Prebiotics are “non-digestible food ingredients that beneficially affect the host by selectively stimulating the growth and/or activity of one or a limited number of bacterial species already resident in the colon, and thus attempt to improve host health” [15]. The term synbiotic is used for a food or product that contains both prebiotic and probiotic elements [16].
A few number of randomised controlled trials have evaluated the effect of probiotics on constipation in adults [17–19] and a recent systematic review suggested that there is not sufficient evidence that probiotics can be used in practice for the treatment of constipation [20]. According to this fact and by considering that some authors have described that combination therapy with different strains of probiotics is more effective than single therapy [13], the present study was designed to evaluate the effect of a commercially available product in Iran, which contains a mixture of both probiotic and prebiotic (synbiotic), on functional constipation in young males.
The current study was performed as a 4 week, double-blind, randomised, placebo-controlled trial in young men suffering from functional constipation who had presented to the gastroenterology clinic at the Aja University of Medical Sciences. The Ethical Committee of Human Research at the Aja University of Medical Sciences approved the protocol of this study and all the patients signed an informed consent before enrolling in the study.
From June 2008 to March 2009, all adult men (age >18 years) suffering from functional constipation were included in the study. Subjects only suffered from constipation and were otherwise healthy. Functional constipation was defined according to the Rome III criteria as having at least two or more of the following, during 25% of defecation times: 1, straining; 2, lumpy or hard stools; 3, sensation of incomplete evacuation; 4, sensation of anorectal obstruction/blockage; 5, manual manoeuvres to facilitate defecation; and 6, fewer than three defecations per week. These criteria should have been fulfilled for the 3 previous months with symptom onset at least 6 months prior to diagnosis. In addition, loose stools should not have been present without the use of laxatives and there should be insufficient criteria for the diagnosis of irritable bowel syndrome (IBS) [21]. Patients with alarming symptoms (fever, recent weight loss, lymphadenopathy, and anaemia), those with coexisting systemic disorders including diabetes mellitus, multiple sclerosis, myocardial infarction, patients with a history of gastrointestinal surgery, and those with a diagnosis of constipation dominant IBS were excluded. A computer-generated sequence with a block size of 4 patients was employed to assign the participants to either of the groups (synbiotic mixture group and placebo group). The patients were assigned consecutive numbers based on the order of enrolment in the study. Patients were randomly allocated to receive the synbiotic mixture (Protexin, London, England) containing 108 colony forming units (CFU) of Bifidobacterium, Lactobacillus, Streptococcus species and Fructooligosaccharide (FOS), or a placebo, twice daily after breakfast and after dinner for 4 weeks. Table 1 demonstrates the precise microbial content of the synbiotic mixture. The placebo was a combination of Mg-stearate and maltodextrines. The synbiotic mixture or placebo was given as a capsule with the same shape and colour, and the packaging of both the synbiotic and placebo was identical. The arrangement for pre-prescription evaluation sessions were made with the patients and the assigned numbers were sent to a research assistant whose only role in this study was the preparation of the drugs and placebo. He was the only one who had access to the randomisation list, according to the numbers that he received each time and were delivered in numbered envelops to the clinic one hour before prescription. All participants were asked to continue their routine diet and physical activity during treatment period. Patients were followed up 2 and 4 weeks later.
| Table 1: Microbial content of the synbiotic mixture (protexin capsule). In addition to probiotics, it also contains Fructooligosaccharide (prebiotic). |
| Contents |
| Microbial Content |
| Lactobacillus casei NCIMB1 30185 |
| Lactobacillus rhamnosus NCIMB 30188 |
| Streptococcus thermophilus NCIMB 30189 |
| Bifidobacterium breve NCIMB 30180 |
| Lactobacillus acidophilus NCIMB 30184 |
| Bifidobacterium longum NCIMB 30182 |
| Lactobacillus bulgaricus NCIMB 30186 |
The primary outcome of the present study was increasing stool frequency at week 2.
The “Patient assessment of constipation symptoms questionnaire” (PAC-SYM) and the Bristol stool form scale were filled in at baseline, 2 and 4 weeks later. The PAC-SYM is a validated questionnaire with 12 items that had been designed to assess the effect of constipation treatment over the time. The questionnaire includes three domains of abdominal symptoms including 4 items, rectal symptoms with 3 items, and stool symptoms with 5 items. Items are rated on a 5-point Likert scale (0–4). Responses are scored from 0 (absence of symptom) to 4 (very severe symptoms). The abdominal, rectal and stool domain scores are the mean scores of each domain. The overall score is the mean of all 12 items [22].
The stool form and consistency was evaluated using the Bristol stool form scale, which classifies stool form in seven-group including: 1, nuts-like; 2, lumpy, sausage; 3, sausage with cracks; 4, smooth snake; 5, soft blobs; 6, fluffy pieces; 7, watery that appear upon defecation. The Bristol stool form scale has been previously employed in Iranian patients [23].
Changes in appetite, use of laxatives and manual manoeuvres, and perceived effectiveness of treatment at week 4 were other secondary outcomes. Change in appetite was measured as an increase, no change or decrease. Changes in laxative consumption and manual manoeuvre use were assessed by a four-point scale as increase, no change, decrease, and no usage during the last 4 weeks and study period. The effectiveness of treatment (improvement of symptoms) was assessed using a 1 to 4 Likert scale as very effective, effective, partially effective and not effective.
Patients were also followed up regarding the development of probable adverse effects of synbiotics administration as previously described [24].
Sample size was calculated using STATA software version 8 (Stata Corporation, College Station, Texas) based on increasing stool frequency per week which defined the primary outcome in the current study. In a randomised controlled trial, Yang et al. observed a stool frequency of 2.4 ± 0.9 in the control group and 3.5 ± 1.5 in the test group, after consumption of the studied product [19]. Using these estimates, we included 25 patients in each group to achieve statistical power of 0.80 with a type I error of 0.05 for comparison of stool frequency between the two groups at 2 weeks after synbiotic administration. Assuming that 30% of patients would provide insufficient data due to either non-compliance or loss to follow up, we planned to enrol 33 patients in each group.
Data were analysed using SPSS 11.5 (SPSS Inc, Illinois, USA). Chi square analysis was performed to compare qualitative data between the placebo and synbiotic groups. A repeated measurement analysis was used to compare the primary and secondary outcome measures between the placebo and synbiotic mixture groups. p-values <0.05 were considered to be statistically significant.
A total of 107 volunteers were assessed for eligibility and 66 patients who met the inclusion criteria were equally allocated to receive either the synbiotic mixture or placebo. Six patients failed to complete the study and were excluded from the final analysis (fig. 1). The baseline characteristics of the patients in the two groups are demonstrated in table 2.

Figure 1
Study flow diagram.
At baseline evaluation, there was no significant difference between the mean stool frequency in the synbiotic and placebo groups [mean difference of 0.11 times (95% CI: –0.31–0.55), p = 0.58]. However, mean stool frequency increased significantly in the synbiotic group compared with the placebo group [at 2 and 4 weeks, mean differences of 1.32 times (95% CI: 0.21–2.43) and 1.58 times (95% CI: 0.18–2.99) respectively, p = 0.02]. The details are shown in Table 3.
There was no significant difference between the synbiotic and placebo groups regarding the baseline Bristol stool form score [mean difference of 0.10 (95% CI: –0.18–0.39), p = 0.47]. However, a significant difference (p = 0.006) was found at weeks 2 [mean difference of 0.83 (95% CI: 0.20–1.45)] and 4 [mean difference of 0.91 (95% CI: 0.32–1.51)].
As table 4 demonstrates, among all PAC-SYM items, a significant difference was only detected in “stomach cramps” and “bowel movements too small” between the two groups.
Respectively, 45.2% and 51.6% of patients in the synbiotic group reported an increase or no change in their appetite during the treatment. In the placebo group, 89.6% of patients reported an increase or no change in their appetite (44.8% for each). There was no significant difference between the synbiotic and placebo groups regarding alteration in appetite (p = 0.66). The same situation was found regarding laxative use (p = 0.82) and performing manual manoeuvres (p = 0.45). Table 5 demonstrates the details.
At week 4, the percentage of patients who stated that the treatment was effective or “partially effective” was significantly higher in the synbiotic group compared to the placebo one (77.4% versus 44.8%, p = 0.037, fig. 2).
No adverse effects were reported from either group during the study period.
| Table 2: Baseline characteristics of patients in the synbiotic mixture and placebo groups. All data were expressed as mean ± standard deviation. | ||
| Synbiotic group (n = 31) | Placebo group (n = 29) | |
| Age (year) | 23 ± 4 | 22.62 ± 3.96 |
| Duration of constipation (month) | 17.35 ± 20.47 | 14.03 ± 11.05 |
| Stool frequency per week | 2.29 ± 0.78 | 2.17 ± 0.88 |
| Stool form (Bristol) | 1.96 ± 0.60 | 1.86 ± 0.51 |
| Abdominal symptoms score | 1.42 ± 0.71 | 1.21 ± 0.61 |
| Rectal Symptoms score | 1.18 ± 0.72 | 0.90 ± 0.69 |
| Stool symptoms score | 2.01 ± 0.63 | 1.78 ± 0.52 |
| Overall PAC-SYM Score | 1.61 ± 0.49 | 1.37 ± 0.46 |
| PAC-SYM: Patient Assessment of Constipation Symptoms. | ||
| Table 3: Comparison of stool frequency per week between synbiotic and placebo groups. Data were expressed as mean ± standard deviation. | ||||
| Synbiotic (n = 31) | Placebo (n = 29) | Mean difference (95% CI) | p-value | |
| Baseline | 2.29 ± 0.78 | 2.17 ± 0.88 | 0.11 (–0.31–0.55) | 0.02 |
| Week 2 | 4.81 ± 2.45 | 3.48 ± 1.74 | 1.32 (0.21–2.43) | |
| Week 4 | 5.45 ± 2.91 | 3.86 ± 2.47 | 1.58 (0.18–2.99) | |
| CI: confidence interval | ||||
| Table 4: Comparison of “Patient assessment of constipation symptoms questionnaire” (PAC-SYM) values at baseline, weeks 2 and 4 in the synbiotic and placebo groups. Data were expressed as mean ± standard deviation. | |||||
| Synbiotic group (n = 31) | Placebo group (n = 29) | Difference of mean (95% CI) | p-Value (repeated measurement) | ||
| Abdominal discomfort | Baseline | 1.39 ± 0.88 | 1.14 ± 0.87 | 0.25 (-0.20-0.70) | 0.74 |
| Week 2 | 0.68 ± 0.83 | 0.83 ± 0.75 | -0.15 (-0.56-0.26) | ||
| Week 4 | 0.52 ± 0.72 | 0.79 ± 0.77 | -0.27(-0.66-0.11) | ||
| Abdominal pain | Baseline | 1.13 ± 1.12 | 0.9 ± 0.77 | 0.23 (-0.26-0.72) | 0.43 |
| Week 2 | 0.74 ± 0.89 | 0.55±0.63 | 0.19 (-0.21-0.59) | ||
| Week 4 | 0.58 ± 0.80 | 0.55 ± 0.63 | 0.03 (-0.34-0.40) | ||
| Bloating | Baseline | 2.06 ± 0.96 | 1.59 ± 1.38 | 0.47 (-0.14-1.09) | 0.77 |
| Week 2 | 0.84 ± 0.89 | 1 ± 1.10 | -0.16 (-0.67-0.35) | ||
| Week 4 | 0.29 ± 0.52 | 0.79 ± 0.86 | -0.50 (-0.86- -0.13) | ||
| Stomach cramps | Baseline | 1.13 ± 1.06 | 1.24 ± 1.02 | -0.11 (-0.65-0.42) | 0.02 |
| Week 2 | 0.48 ± 0.62 | 1.10 ± 1.01 | -0.62(-1.05- -0.18) | ||
| Week 4 | 0.23 ± 0.42 | 0.86 ± 0.95 | -0.63(-1.02- -0.24) | ||
| Painful bowel movement | Baseline | 1.68 ± 1.08 | 1.31 ± 0.97 | 0.37 (-0.16-0.89) | 0.81 |
| Week 2 | 0.94 ± 0.96 | 0.93 ± 0.84 | 0.01 (-0.46-0.47) | ||
| Week 4 | 0.68 ± 0.97 | 0.90 ± 0.81 | -0.22 (-0.68-0.24) | ||
| Rectal burning | Baseline | 1.58 ± 1.23 | 1.03 ± 1.08 | 0.55(-0.05-1.14) | 0.65 |
| Week 2 | 0.48 ± 0.67 | 0.59 ± 1.01 | -0.10(-0.54-0.34) | ||
| Week 4 | 0.35 ± 0.60 | 0.52 ± 0.91 | -0.17(-0.56- 0.23) | ||
| Rectal bleeding or tearing | Baseline | 0.29 ± 0.69 | 0.34 ± 0.55 | -0.05 (-0.38-0.27) | 0.60 |
| Week 2 | 0.16 ± 0.58 | 0.28 ± 0.45 | -0.12 (-0.38-0.15) | ||
| Week 4 | 0.16 ± 0.63 | 0.21 ± 0.41 | -0.05 (-0.32-0.23) | ||
| Incomplete bowel movement | Baseline | 2.29 ± 0.97 | 1.86 ± 0.91 | 0.43 (-0.06-0.91) | 0.56 |
| Week 2 | 1.26 ± 0.99 | 1.14 ± 1.09 | 0.12 (-0.42-0.66) | ||
| Week 4 | 0.90 ± 0.87 | 1.07 ± 0.96 | -0.17 (-0.63-0.30) | ||
| Bowel movements too hard | Baseline | 1.81 ± 0.98 | 1.48 ± 0.95 | 0.33 (-0.17-0.82) | 0.84 |
| Week 2 | 0.87 ± 0.92 | 1.07 ± 0.92 | -0.20 (-0.67-0.27) | ||
| Week 4 | 0.61 ± 0.84 | 0.86 ± 0.83 | -0.25 (-0.68-0.18) | ||
| Bowel movements too small | Baseline | 1.35 ± 1.28 | 1.45 ± 1.05 | -0.10 (-0.70-0.51) | 0.03 |
| Week 2 | 0.55 ± 0.85 | 1.21 ± 1.04 | -0.66(-1.15- -0.16) | ||
| Week 4 | 0.45 ± 0.81 | 1.21 ± 1.08 | -0.76 (-1.24- -0.26) | ||
| Straining or squeezing | Baseline | 2.71 ± 0.90 | 2.38 ± 0.90 | 0.33(-0.13-0.79) | 0.71 |
| Week 2 | 1.19 ± 1.01 | 1.45 ± 0.94 | 0.26 (-0.76-0.25) | ||
| Week 4 | 0.97 ± 1.16 | 1.28 ± 0.92 | -0.31(-0.85-0.23) | ||
| False alarm | Baseline | 1.94 ± 0.96 | 1.76 ± 1.35 | 0.18 (-0.42-0.78) | 0.81 |
| Week 2 | 0.87 ± 0.88 | 1 ± 1.13 | 0.13 (-0.65-0.39) | ||
| Week 4 | 0.55 ± 0.67 | 0.76 ± 1.05 | -0.21 (-0.66-0.24) | ||
| Abdominal symptoms | Baseline | 1.42 ± 0.71 | 1.22 ± 0.61 | 0.20 (-0.13-0.55) | 0.40 |
| Week 2 | 0.68 ± 0.54 | 0.87 ± 0.52 | -0.19 (-0.46-0.93) | ||
| Week 4 | 0.40 ± 0.43 | 0.75 ± 0.59 | -0.35(-0.61- -0.08) | ||
| Rectal symptoms | Baseline | 1.18 ± 0.72 | 0.90 ± 0.69 | 0.28 (-0.08-0.65) | 0.86 |
| Week 2 | 0.52 ± 0.60 | 0.59 ± 0.60 | -0.07(-0.38-0.24) | ||
| Week 4 | 0.39 ± 0.52 | 0.54 ± 0.68 | -0.15 (-0.45- 0.16) | ||
| Stool symptoms | Baseline | 2.02 ± 0.63 | 1.79 ± 0.52 | 0.23 (-0.06-0.53) | 0.44 |
| Week 2 | 0.94 ± 0.64 | 1.17 ± 0.65 | -0.23 (-0.55- -0.11) | ||
| Week 4 | 0.69 ± 0.66 | 1.03 ± 0.63 | -0.34 (-0.67- -0.00) | ||
| Overall PAC-SYM | Baseline | 1.61 ± 0.49 | 1.37 ± 0.46 | 0.24(-0.01-0.48) | 0.50 |
| Week 2 | 0.75 ± 0.52 | 0.92 ± 0.47 | -0.17(-0.43-0.8) | ||
| Week 4 | 0.52 ± 0.51 | 0.81 ± 0.48 | -0.29 (-0.55- -0.03) | ||
| Table 5: Comparison of using laxative and manual manoeuvres in the synbiotic and placebo groups. | ||||
| Synbiotic N (%) | Placebo N (%) | p-value | ||
| Laxative use | Increase | 0 (0) | 1 (3.4) | 0.82 |
| No change | 3 (9.7) | 4 (13.8) | ||
| Decrease | 2 (6.5) | 2 (6.9) | ||
| Not use | 26 (83.9) | 22 (75.9) | ||
| Manual manoeuvres | Increase | 0 (0) | 1 (3.4) | 0.45 |
| No change | 4 (12.9) | 7 (24.1) | ||
| Decrease | 2 (6.5) | 1 (3.4) | ||
| Not use | 25 (80.6) | 20 (69.0) | ||
The results of the present study demonstrated that administration of this commercially available mixture of pro- and prebiotics (synbiotic) with the mentioned dosage for 4 weeks in young men suffering from mild to moderate constipation could modify the clinical picture. To the best of our knowledge, the present study was the first one that evaluated this synbiotic mixture (a combination of four lactobacillus bacteria and two bifidobacteria strains, S. thermophilus and FOS) in functional constipation.
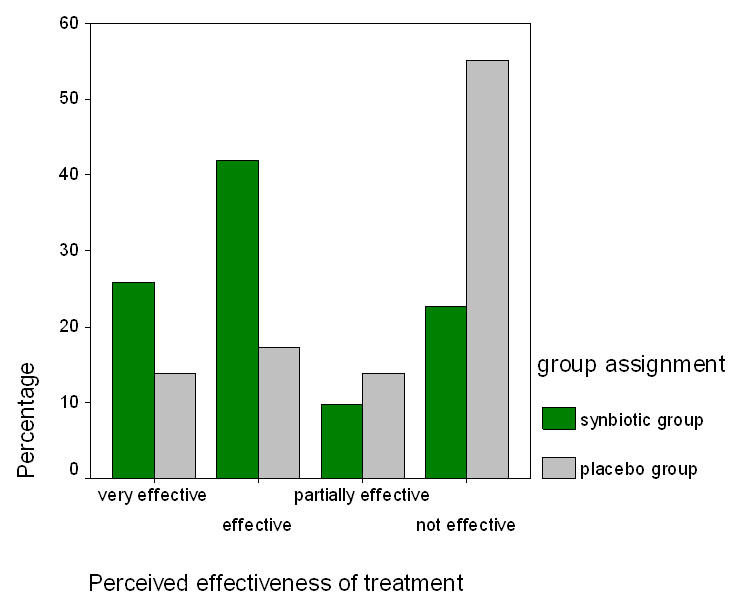
Figure 2
The perceived effectiveness of treatment in the probiotic and placebo groups after 4 weeks of treatment is shown. A total of 77.4% of the synbiotic group and 44.8% of the placebo group regarded the treatment as “effective” or “partially effective” which was significantly different (p = 0.03).
The colon is a pool of a large population of intestinal bacteria. An alteration in normal patterns may result in changing bowel movement and constipation [10, 11]. The idea of using probiotics for treatment of constipation formed from reports of dysbiosis in the intestinal bacteria of patients with chronic constipation [13]. The hypothesis that colonic bacteria affect colonic motility [25] was supported by a previous study demonstrating that the administration of oral vancomycin increased stool frequency in patients with chronic constipation [26]. Colonisation of probiotics such as lactobacillus and bifidobacter, produce lactic acid, acetic acid, and short chain fatty acids (SCFA) stimulating intestinal motor activity. The probable mechanisms of how SCFA exert their stimulatory effects on motor activities of colon is not clear, however, it has been suggested that SCFA may directly interact with intrinsic (enteric) and extrinsic nerves [27].
In the present study, we found a statistically significant improvement in stool frequency and stool consistency, however, our results failed to show a statistically significant improvement in most of the PAC-SYM items. There were not statistically significant differences in laxative and manual manoeuvre usage between the two groups, however, most of our patients had mild to moderate constipation and did not use these modalities frequently. Therefore, the absence of an improvement in these items may be due to infrequent usage of these modalities initially due to mild to moderate constipation symptoms.
The efficacy of probiotics for treatment of functional constipation has been reported in both paediatrics [28, 29] and adults [17–19], however, more evidence is still required for the routine prescription of probiotics for the treatment of functional and chronic constipation [20]. In a randomised clinical trial, Koebnick et al. showed that the probiotic strain L. casei Shirota improved stool frequency consistency, and constipation symptoms [17]. Amenta and colleagues completed a before-after study of the effects of a synbiotic preparation containing B. longum W11 and FOS combined with moderate physical activity on a group of 297 subjects experiencing constipation associated with a weight loss programme [30]. They reported improved constipation in subjects who consumed at least 85% of the prescribed synbiotic preparation. In elderly patients with constipation, two different strains of L. rhamnosus and Propionibacterium freudenreichi resulted in a small but significant increase in stool frequency whereas using a single strain did not affect defecation frequency [13].
The effectiveness of L. casei rhamnosus in improving functional constipation in children under 10 years of age has been shown by a randomised clinical trial [28]. Another study in children showed that lactobacillus GG was not as effective as an adjunct therapy with lactulose in the treatment of constipation [29]. Moreover, Bekkali et al. reported that a probiotic mixture of B. bifidum, B. infantis, B. longum, L. casei, L. plantarum, and L. rhamnosus combined with a toilet training programme increased stool frequency and decreased faecal incontinence episodes in children [31]. In spite of this evidence, comparing these studies with the current study is difficult due to the different studied samples, different methods, and variation in type, dose and duration of prescribed probiotics. Most of the studies, which evaluated effectiveness of probiotics for treatment of functional constipation like the current study, followed up the subjects for a short-term period (2–12 weeks) [19, 29]. Further studies with longer follow up periods are required to evaluate the effectiveness of probiotics for the treatment of functional constipation.
The probiotics were well tolerated, and no adverse effects associated with consumption of these supplements have been reported in any of the trials [20]. Infection and sepsis are the most serious probable complications of probiotic administration, particularly in immuno-compromised patients [24]. No side effects due to the synbiotic mixture were found in our study, which is in accordance with other studies investigating the safety of probiotics [14, 32].
This study has several limitations that the readers should keep in their mind. The study was only performed on young males and this is the main limitation of this study. However, there are a few studies that evaluated the effects of probiotics on constipation only in one sex [19]. Another concern was related to the small number of the studied patients. The numbers of people completing the trial, and whose data have been analysed are too small to draw any firm conclusions about the benefits, or otherwise, of these types of preparations. Moreover, our results showed an efficacy of just this specific commercial mixture in improving stool frequency and consistency in patients with functional constipation. As commercial supplements may each contain different individual amounts of pre and probiotics, the same results might not be achieved with another supplement.
In conclusion, our study showed that this commercially available mixture of pre and probiotics (synbiotic) is safe and effective in improving stool frequency and consistency. However, it failed to find a significant difference between the two groups regarding most of the PAC-SYM items and its overall score. Further studies with longer follow up periods and larger samples including females and elderly people are required to confirm the efficacy of this mixture in improving functional and chronic constipation.
1 Higgins PD, Johanson JF. Epidemiology of constipation in North America: a systematic review. Am J Gastroenterol. 2004;99(4):750–9.
2 Cheng C, Chan AO, Hui WM, Lam SK. Coping strategies, illness perception, anxiety and depression of patients with idiopathic constipation: a population-based study. Aliment Pharmacol Ther. 2003;18(3):319–26.
3 Koloski NA, Talley NJ, Boyce PM. The impact of functional gastrointestinal disorders on quality of life. Am J Gastroenterol. 2000;95(1):67–71.
4 Drossman DA, Li Z, Andruzzi E, Temple RD, Talley NJ, Thompson WG, et al. U.S. householder survey of functional gastrointestinal disorders. Prevalence, sociodemography, and health impact. Dig Dis Sci. 1993;38(9):1569–80.
5 Dennison C, Prasad M, Lloyd A, Bhattacharyya SK, Dhawan R, Coyne K. The health-related quality of life and economic burden of constipation. Pharmacoeconomics. 2005;23(5):461–76.
6 Lembo A, Camilleri M. Chronic constipation. N Engl J Med. 2003;349(14):1360–8.
7 Johanson JF, Kralstein J. Chronic constipation: a survey of the patient perspective. Aliment Pharmacol Ther. 2007;25(5):599–608.
8 Thompson WG, Longstreth GF, Drossman DA, Heaton KW, Irvine EJ, Muller-Lissner SA. Functional bowel disorders and functional abdominal pain. Gut. 1999;45(Suppl 2):II43–7.
9 Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130(5):1480–91.
10 Khalif IL, Quigley EM, Konovitch EA, Maximova ID. Alterations in the colonic flora and intestinal permeability and evidence of immune activation in chronic constipation. Dig Liver Dis. 2005;37(11):838–49.
11 Borriello SP. Bacteria and gastrointestinal secretion and motility. Scand J Gastroenterol Suppl. 1984;93:115–21.
12 Nakamura T, Nishida S, Mizutani M, Iino H. Effects of yogurt supplemented with brewer’s yeast cell wall on constipation and intestinal microflora in rats. J Nutr Sci Vitaminol (Tokyo). 2001;47(6):367–72.
13 Ouwehand AC, Lagstrom H, Suomalainen T, Salminen S. Effect of probiotics on constipation, fecal azoreductase activity and fecal mucin content in the elderly. Ann Nutr Metab. 2002;46(3-4):159–62.
14 Guarner F, Schaafsma GJ. Probiotics. Int J Food Microbiol. 1998;39(3):237–8.
15 Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125(6):1401–12.
16 Madsen K. Probiotics in critically ill patients. J Clin Gastroenterol. 2008;42(Suppl 3 Pt 1):S116–8.
17 Koebnick C, Wagner I, Leitzmann P, Stern U, Zunft HJ. Probiotic beverage containing Lactobacillus casei Shirota improves gastrointestinal symptoms in patients with chronic constipation. Can J Gastroenterol. 2003;17(11):655–9.
18 Mollenbrink M, Bruckschen E. Treatment of chronic constipation with physiologic Escherichia coli bacteria. Results of a clinical study of the effectiveness and tolerance of microbiological therapy with the E. coli Nissle 1917 strain (Mutaflor). Med Klin (Munich). 1994;89(11):587–93.
19 Yang YX, He M, Hu G, Wei J, Pages P, Yang XH, et al. Effect of a fermented milk containing Bifidobacterium lactis DN-173010 on Chinese constipated women. World J Gastroenterol. 2008;14(40):6237–43.
20 Chmielewska A, Szajewska H. Systematic review of randomised controlled trials: probiotics for functional constipation. World J Gastroenterol. 2010;16(1):69–75.
21 Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology. 2006;130(5):1377–90.
22 Frank L, Kleinman L, Farup C, Taylor L, Miner P, Jr. Psychometric validation of a constipation symptom assessment questionnaire. Scand J Gastroenterol. 1999;34(9):870–7.
23 Adibi P, Behzad E, Pirzadeh S, Mohseni M. Bowel habit reference values and abnormalities in young Iranian healthy adults. Dig Dis Sci. 2007;52(8):1810–3.
24 Boyle RJ, Robins-Browne RM, Tang ML. Probiotic use in clinical practice: what are the risks? Am J Clin Nutr. 2006;83(6):1256–64; quiz 446–7.
25 Picard C, Fioramonti J, Francois A, Robinson T, Neant F, Matuchansky C. Review article: bifidobacteria as probiotic agents – physiological effects and clinical benefits. Aliment Pharmacol Ther. 2005;22(6):495–512.
26 Celik AF, Tomlin J, Read NW. The effect of oral vancomycin on chronic idiopathic constipation. Aliment Pharmacol Ther. 1995;9(1):63–8.
27 Yajima T. Contractile effect of short-chain fatty acids on the isolated colon of the rat. J Physiol. 1985;368:667–78.
28 Bu LN, Chang MH, Ni YH, Chen HL, Cheng CC. Lactobacillus casei rhamnosus Lcr35 in children with chronic constipation. Pediatr Int. 2007;49(4):485–90.
29 Banaszkiewicz A, Szajewska H. Ineffectiveness of Lactobacillus GG as an adjunct to lactulose for the treatment of constipation in children: a double-blind, placebo-controlled randomized trial. J Pediatr. 2005;146(3):364–9.
30 Amenta M, Cascio MT, Di Fiore P, Venturini I. Diet and chronic constipation. Benefits of oral supplementation with symbiotic zir fos (Bifidobacterium longum W11 + FOS Actilight). Acta Biomed. 2006;77(3):157–62.
31 Bekkali NL, Bongers ME, Van den Berg MM, Liem O, Benninga MA. The role of a probiotics mixture in the treatment of childhood constipation: a pilot study. Nutr J. 2007;6:17.
32 Del Piano M, Morelli L, Strozzi GP, Allesina S, Barba M, Deidda F, et al. Probiotics: from research to consumer. Dig Liver Dis. 2006;38(Suppl 2):S248–55.
The authors would like to thank Dr. Alireza Khoshdel for his support and assistance with epidemiologic aspects of study. We also thank the Iranian Intellectual Elite Foundation for their support in this study.
Funding / potential competing interests: This study was fully funded by the Aja University of Medical Sciences, Research Committee, Grant number: 10514300.
Registration ID in IRCT: IRCT138802151873N1