DOI: https://doi.org/10.4414/smw.2011.13236
Dental disease is associated with atherosclerosis [1–2]. Previously we reported that dental status and oral hygiene are independent risk factors for progression of atherosclerosis in the carotid arteries [3]. Inflammation plays a key role in the development of atherosclerosis; inflammatory dental and oral processes may thus exaggerate vascular disease and promote its progression [4, 5]. However, the bearing of dental disease on mortality among a population with known vascular disease has not been fully understood as yet. While the development of coronary heart disease among a large population without known atherosclerotic disease was not associated with dental disease, others observed a significant association [6–8].
We hypothesised that dental disease is associated with mortality in patients with pre-existing atherosclerosis. The aim of the present study was to investigate the impact of dental disease on mortality in patients with atherosclerosis in the carotid arteries.
All consecutivepatients who underwent duplex ultrasound scanning of theextracranial carotid arteries from March 2002 to March 2003 were prospectively enrolled in the Inflammation in Carotid Arteries Risk for Arthrosclerosis Study (ICARAS). Detailed inclusion and exclusion criteria have been reported previously [9]. In brief, patients with prevalent atherosclerotic carotid artery disease, as defined by the presence of non-stenotic plaques or carotid stenosis of any degree, who were clinically asymptomatic at the time of screening, were enrolled. Patients with enhanced intima media thickness but without plaques were not eligible. Patients with a cardiovascular event (myocardial infarction, stroke, coronary revascularisation, peripheral vascular surgery) during the preceding 6 months were excluded. The rationale behind this exclusion criterion was the assumption that acute cardiovascular events might impact specific biomarker levels or other clinical measures and therefore rather reflect the severity of an acute situation than chronic atherosclerotic disease. Patients with active malignant disease were also not included in ICARAS. The study was approved by the local ethics committee and all patients gave their written informed consent.


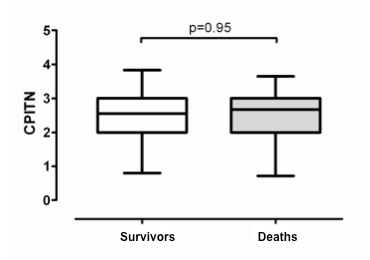
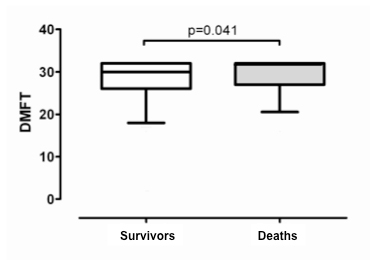
Figure 1
DMFT (fig. 1A), SLI (fig. 1B), and CPITN (fig. 1C) indices in patients who died (n = 107) compared to survivors (n = 304). Box bottom marks the 25th percentile, the line within marks the median, and the box top marks the 75th percentile; the bottom and top of the vertical lines mark the 5th and 95th percentiles respectively.
A total of 1268 patients were included in ICARAS. Of these, a 450-patient random sample, using computer-generated random digits, was identified for inclusion in the dental sub-study. 411 (91%) accepted the invitation to participate and were included. No significant differences in baseline characteristics and demographics were found when comparing the sub-study participants with the entire ICARAS population.
The study endpoint was defined as death from any cause. This was evaluated by screening the national register of death, including screening for the specific cause of death (according to the International Statistical Classification of Diseases and Related Health Problems 10th Revision).
Dental examinations were performed one to four weeks after the initial ultrasound examination by four specifically trained dentists who were blinded to the patients’ clinical and ultrasound data. All patients were investigated by two observers in consensus. Three World Health Organization-approved dental indices were selected to quantify dental disease [10]. We used DMFT (decayed, missing, filled teeth), to evaluate the dental status, SLI (Silness-Löe plaque index) to measure oral hygiene, and CPITN (community periodontal index of treatment needs) as a surrogate marker of periodontal disease. Dental status and the amount of decay in an individual are described by DMFT as a means of expressing caries prevalence numerically. The score is generated by calculating the number of decayed (D), missing (M), and filled (F) teeth (T). DMFT was calculated for 32 teeth.SLIis based on recording both soft debris and mineralised deposits on teeth 12, 16, 24, 36, and 44. Each of the surfaces (buccal,lingual, mesial, and distal) is given a score from 0 (no plaque)to 3 (abundance of soft matter within the gingival pocket and/oron the tooth and gingival margin). The index is obtained by calculating the mean for all investigated teethand surfaces. In edentulous patients SLIwas obtained from the dentures.Assessment of CPITN includes recording of signs of gingival bleeding, supra-or subgingival calculus, and periodontal pockets, subdividedinto shallow (4 to 5 mm) and deep (6 mm or more). We used a standardised lightweight periodontal probe with a 0.5-mm ball tip to probe 10 standardised index teeth which were then classified from 0 (healthy) to 4 (pocket >6 mm). Index teeth were investigatedas recommended; if the index teeth were missing, the nextadjacent teeth were used for evaluations. CPITN for edentulous patients was calculated in a separate category.
Duplex examinations at baseline and during follow-up were performed on an Acuson 128 XP10 with a 7.5-MHZ linear array probe (Acuson) by experienced technical assistants who were supervised by 2 of the authors. All duplex operators were blinded with respect to patients’ clinical data and dental status.Duplex grading of the carotid stenosis was performed as describedpreviously [3]. The validity of our classification ofthe degree of stenosis with respect to angiography was assessedpreviously in our duplex laboratory in an independent cohortincluding 1006 carotid arteries [11]. Assuming angiography as the gold standard, positive predictive values and negative predictivevalues ranged from 70% to 98%. With respect to the absolute degree of stenosis, we recorded excellent inter-observer agreement (kappa,0.83; 95% confidence interval [CI], 0.79 to 0.88).
Pharmacotherapy of patients with evidence of carotid atherosclerosis was prescribed following a standard protocol: Patients received antithrombotic therapy with either acetylsalicylic acid 100 mg or clopidogrel 75 mg once daily. Patients with hyperlipidaemia (LDL cholesterol >130 mg/dL) received inhibitors of the 3-hydroxy-3-methylglutaryl coenzyme A reductase (statins).
Continuous data are presented as the median and the interquartile range (IQR, range from the 25th to the 75th percentile). Discrete data are given as counts and percentages. We used Mann-Whitney U tests and Fisher exact tests for univariate analyses, as appropriate. Separate Cox regressions for the independent variables DMFT, SLI, and CPITN in tertiles were performed. Hazard ratios (HR) and 95% CI are presented. Additionally, estimated effects of increasing continuous measures of DMFT, SLI, and CPITN were calculated. To allow for potential confounding effects, we calculated the risk of death by multivariableCox proportional hazards analysis adjusting for age (years),sex (male/female), body mass index (kg/m2), smoking (in categories), hypertension (yes/no),low-density lipoprotein cholesterol level (mg/dL), glycated haemoglobin A1 level (%), history of myocardial infarction (yes/no), peripheral artery disease (yes/no),history of stroke (yes/no), baseline degree of carotid stenosis(in categories), and statin treatment (yes/no). We assessed the overall model fit using Cox-Snell residuals. Furthermore, we tested the proportional hazard assumption for all covariates using Schoenfeld residuals (overall test) and the scaled Schoenfeld residuals (variable-by-variable testing). P-values below 0.05 are considered statistically significant. Sample size calculation indicated that a sample size of 418 patients allows detection of a difference in mortality of 12% assuming an overall mortality of 25% during follow-up (alpha 0.05, power 80%). To compensate for possible missing data, 450 patients were included. Data analysis was done in SPSS (version 15.0) and SAS (version 9.1).
The median age was 69 years (IQR, 62–76 years) and 271 (66%) were male. Detailed baseline demographics and clinical characteristics are given in table 1. Median DMFT index was 30 (IQR, 26–32). Median numbers of decayed teeth, filled teeth and missing teeth were 0 (IQR, 0 to 2), 7 (IQR, 0 to 13), and 18 (IQR, 11–29), respectively. Median SLI was 0.75 (IQR, 0.38–1.21), and median CPITN was 2.5 (IQR, 2.0–3.0) (excluding edentulous patients) respectively. Overall, 92 patients (22%) were edentulous and wore complete dentures; these patients were included as a separate category in the calculations of CPITN.
During a median of 6.2 years (IQR, 5.8 to 6.6 years), 107 (26%) deaths were recorded. Of these, 74 patients (69.2%) died from cardiovascular causes (47 patients from coronary artery disease, 10 from stroke, 7 from peripheral vascular disease and 10 from other vascular causes). Thirty patients (28%) died of cancer (13 patients died from lung cancer, 3 from pancreatic cancer, 6 from colo-rectal carcinoma, 4 from breast cancer and 4 from other cancer entities). Three patients (2.8%) died from other causes.
| Table 1: Patients’ baseline characteristics and demographics. | |
| n = 411 | |
| Age (years) | 69 (62–76) |
| Males/females | 271 (66%)/140 (34%) |
| Body mass index (kg/m2) | 26.1 (24.2–28.7) |
| Arterial hypertension | 296 (72%) |
| Hyperlipidaemia | 286 (70%) |
| Total cholesterol (mg/dL)a | 205 (176–239) |
| Low-density lipoprotein cholesterol (mg/dL)a | 117 (92–148) |
| High-density lipoprotein cholesterol (mg/dL)a | 51 (43–60) |
| Glycated haemoglobin A1 (%) | 5.9 (5.6–6.4) |
| Family history of atherosclerosis | 239 (58%) |
| History of peripheral artery disease | 172 (42%) |
| History of myocardial infarction | 94 (23%) |
| History of stroke | 53 (13%) |
| Baseline degree of carotid stenosis (right/left) | |
| 0%–29% (plaque only) | 265 (64.5%)/269 (65.5%) |
| 30% or higher | 146 (35.5%)/142 (34.5%) |
| Smoking status | |
| 1–10 cigarettes daily | 35 (9%) |
| 11–20 cigarettes daily | 34 (8% |
| >20 cigarettes daily | 29 (7%) |
| Former smoker | 78 (19%) |
| Statin treatment | 256 (62%) |
| Antihypertensive treatment | 291 (71%) |
| Continuous data are presented as the median and the interquartile range. Discrete data are given as counts and percentages. a Multiply by 0.0259 to convert variables to millimoles per liter. | |
A significant association between dental status and all-cause mortality was observed (fig. 1A). DMFT showed a significant association with all-cause mortality when incorporated as a continuous variable into a proportional hazard model (adjusted HR 1.06 [95% CI, 1.0 to 1.12; p = 0.04]). For increasing tertiles, the adjusted HR for death were 1.19 (95% CI, 0.64–2.19) and 1.57 (95% CI, 0.93–2.64), compared to the lowest tertile respectively, indicating a gradual association between dental status and all-cause mortality (fig. 2).
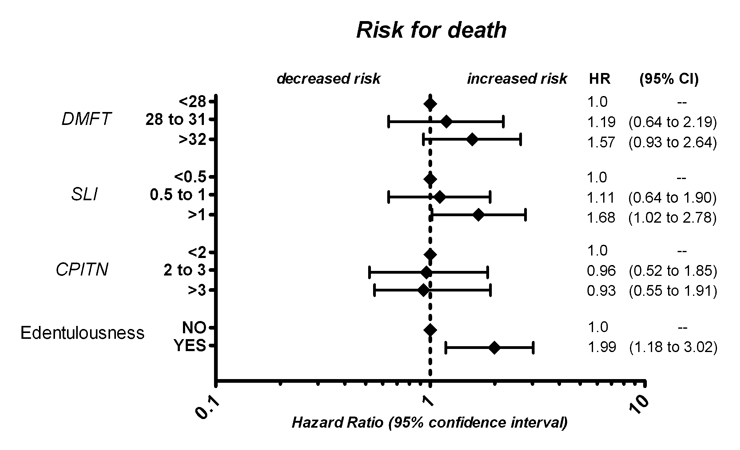
Figure 2
Adjusted risk for overall mortality according to tertiles of DMFT, SLI, CPITN and edentulousness respectively.
A significant association between oral hygiene and all-cause mortality was observed (fig. 1B). For continuous values of SLI, the adjusted HR for death was 1.43 (95% CI, 1.01–2.03; p = 0.04). For increasing tertiles, the adjusted HR for death were 1.11 (95% CI, 0.64–1.90) and 1.68 (95% CI, 1.02–2.78), compared to the lowest tertile respectively (fig. 2), indicating a gradual relationship between oral hygiene and all-cause mortality.
CPITN was not found to be associated with all-cause mortality (fig. 1C). The adjusted HR for death was 1.05 (95% CI, 0.73–1.50; p = 0.81) when CPITN was used as a continuous variable. For increasing tertiles the adjusted HR was 0.96 (95% CI, 0.52–1.85) and 0.93 (95% CI, 0.55–1.91), compared to the lowest tertile respectively (fig. 2).
Patients without any teeth were at increased risk for death of any cause. For edentulous patients, the adjusted HR for death was 1.99 (95%CI, 1.18–3.02; p = 0.008, fig. 2).
Dental disease was found to be a risk factor for death in patients with prevalent carotid atherosclerosis. Teeth status, as evaluated by DMFT and the level of oral hygiene, as evaluated by SLI, were significantly associated with mortality. Edentulousness was consistently strongly associated with mortality. In contrast, CPITN, as a surrogate for periodontal disease, was not found to be an independent risk factor for death.
Adverse socioeconomic circumstances are risk factors for the development of dental disease as well as atherosclerosis [12, 13]. This relation certainly confers a higher risk of adverse cardiovascular outcome and death. However, almost all residents of Austria are covered by national health insurance. In this context, a study from Sweden, a country with excellent health coverage and high educational standards, did not find socioeconomic circumstances associated with cardiovascular disease [14]. Economic aspects thus might be less important among our cohort than individual factors such as education and health consciousness. These latter factors might be displayed by the oral hygiene status. Poor oral hygiene, decayed teeth and edentulousness thus may reflect patients’ reluctant attitude to health care prophylaxis in general.
Another potentially important aspect is the association between periodontal inflammation and atherosclerosis. In particular, chronic microbial infection, including several periodontal pathogens, may play an important role in the development of atherosclerotic disease [15–17]. This has been investigated in numerous publications reporting conflicting data. While several studies suggest a clear association between periodontal infection and mortality, others did not find periodontitis as a risk factor for poor outcome [5, 18–22]. Nor, in this context, did we observe a significant association between periodontal status as measured by the CPITN index and mortality. A possible explanation for these latter findings may be the fact that our population predominantly consisted of elderly subjects with advanced carotid artery disease, as shown by the presence of atherosclerotic plaques or stenosis. Since bacterial infection is thought to be linked to an early initiation of atherosclerotic lesions, measurement of the intima-media thickness may be a better parameter to investigate the association between periodontal infection and early stage atherosclerosis.
The rate of 28% of patients who died of cancer is in line with cancer-death rates in the general population. Since our patients were predominantly elderly subjects (median 70 years), the occurrence of cancer among a certain percentage of our population was not unexpected.
To the best of our knowledge the present study is the first to demonstrate clearly a significant relationship between dental disease, especially tooth loss, and death among a population with asymptomatic atherosclerosis. Our findings are in line with results from a Japanese study investigating dental disease among a predominantly older population [23]. Clinical implications derived from our findings could be as follows: once a dentist diagnoses advanced dental disease or signs of poor oral hygiene, the patient should be referred to an internist for further screening and/or treatment of cardiovascular risk factors.
The following limitations of our study should be mentioned. First, our study population consisted of patients with preexisting atherosclerosis. Hence we were unable to draw conclusions regarding the impact of dental and periodontal disease on mortality in a community based cohort. Second, microbial aspects, which have been shown to be more specific than clinical signs of periodontitis, were not covered in our study. Third, specific limitations of the applied indices needto be mentioned. In populations with a high prevalence of decay, DMFT has been reported to be less suitable,and this might not be relevant for our cohort [24]. The validity of SLImay be limited in milder formsof inflammation and by the need for probing [25]. Finally, severe stages of periodontitis may be underestimatedor even missed (edentulousness) by CPITN [26].
Dental status and oral hygiene were significantly associated with mortality in patients with carotid atherosclerosis regardless of conventional cardiovascular risk factors.
1 Haynes WG, Stanford C. Periodontal disease and atherosclerosis – from dental to arterial plaque. Arterioscler Thromb Vasc Biol. 2003;23(8):1309–11.
2 Tonetti MS. Periodontitis and risk for atherosclerosis: an update on intervention trials. J Clin Periodontol. 2009;36(Suppl 10):15–9.
3 Schillinger T, Kluger W, Exner M, Mlekusch W, Sabeti S, Amighi J, et al. Dental and periodontal status and risk for progression of carotid atherosclerosis: the inflammation and carotid artery risk for atherosclerosis study dental substudy. Stroke. 2006;37(9):2271–6.
4 Libby P, Ridker PM, Hansson GK; Leducq Transatlantic Network on Atherothrombosis. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54(23):2129-38.
5 Niessner A, Graf S, Nikfardjam M, Lehr S, Maurer G, Wojta J, et al. The adaptive immune system and long-term outcome in patients with stable coronary disease. Predictive value of routine laboratory measurements. Thromb Haemost. 2005;93(2):257–60.
6 Hujoel PP, Drangsholt M, Spiekerman C, DeRouen TA. Periodontal disease and coronary heart disease risk. JAMA. 2000; 284(11):1406–10.
7 Mattila KJ, Valle MS, Nieminen MS, Valtonen VV, Hietaniemi KL. Dental infections and coronary atherosclerosis. Atherosclerosis. 1993;103(2):205–11.
8 DeStefano F, Anda RF, Kahn HS, Williamson DF, Russell CM. Dental disease and risk of coronary heart disease and mortality. Br Med J. 1993;306(6879):688–91.
9 Schillinger M, Exner M, Mlekusch W, Sabeti S, Amighi J, Nikowitsch R, et al. Inflammation and Carotid Artery Risk for Atherosclerosis Study (ICARAS). Circulation. 2005;111(17):2203–9.
10 WHO Oral Health Country/Area Profile Programme [WWW document]. URL http://www.whocollab.od.mah.se [accessed on 20 September 2010].
11 Sabeti S, Schillinger M, Mlekusch W, Willfort A, Haumer M, Nachtmann T, et al. Quantification of internal carotid artery stenosis with duplex US: comparative analysis of different flow velocity criteria. Radiology. 2004;232(2):431–9.
12 Borrell LN, Beck JD, Heiss G. Socioeconomic disadvantage and periodontal disease: the Dental Atherosclerosis Risk in Communities study. Am J Public Health. 2006;96(2):332–9.
13 Deans KA, Bezlyak V, Ford I, Batty GD, Burns H, Cavanagh J, et al. Differences in atherosclerosis according to area level socioeconomic deprivation: cross sectional, population based study. BMJ. 2009;339:b4170.
14 Cabrera C, Hakeberg M, Ahlqwist M, Wedel H, Björkelund C, Bengtsson C, et al. Can the relation between tooth loss and chronic disease be explained by socio-economic status? A 24-year follow-up from the population study of women in Gothenburg, Sweden. Eur J Epidemiol. 2005;20(3):229–36.
15 Desvarieux M, Demmer RT, Rundek T, Boden-Albala B, Jacobs DR Jr, Sacco RL, et al. Periodontal microbiota and carotid intima-media thickness: the Oral Infections and Vascular Disease Epidemiology Study (INVEST). Circulation. 2005;111(5):576–82.
16 Desvarieux M, Demmer RT, Rundek T, Boden-Albala B, Jacobs DR Jr, Papapanou PN, et al. Relationship between periodontal disease, tooth loss, and carotid artery plaque: the Oral Infections and Vascular Disease Epidemiology Study (INVEST). Stroke. 2003;34(9):2120–5.
17 Niessner A, Sato K, Chaikof EL, Colmegna I, Goronzy JJ, Weyand CM. Pathogen-sensing plasmacytoid dendritic cells stimulate cytotoxic T-cell function in the atherosclerotic plaque through interferon-alpha. Circulation. 2006;114(23):2482–9.
18 Mattila KJ, Valle MS, Nieminen MS, Valtonen VV, Hietaniemi KL. Dental infections and coronary atherosclerosis. Atherosclerosis 1993;103(2):205–11.
19 Arbes SJ, Slade GD, Beck J. Association between extent of periodontal attachment loss and self-reported history of heart attack: an analysis of NHANES III data. J Dent Res. 1999;78(12):1777–82.
20 Garcia RI, Krall EA, Vokonas PS. Periodontal disease and mortality from all causes in the VA Dental Longitudinal Study. Ann Periodontol. 1998;3(1):339–49.
21 Morrison HI, Ellison LF, Taylor GW. Periodontal disease and risk of fatal coronary heart and cerebrovascular diseases. J Cardiovasc Risk. 1999;6(1):7–11
22 Joshipura KJ, Rimm EB, Douglass CW, Trichopoulos D, Ascherio A, Willett WC. Poor oral health and coronary heart disease. J Dent Res. 1996;75(9):1631–6.
23 Ansai T, Takata Y, Soh I, Awano S, Yoshida A, Sonoki K, et al. Relationship between tooth loss and mortality in 80-year-old Japanese community-dwelling subjects. BMC Public Health. 2010;10:386.
24 Schuler AA, Holst D. Oral status indicators DMFT and FS-T: reflections on index selection. Eur J Oral Sci. 2001;109(3):155–9.
25 Mars RG, Magnusson I. Evaluation of reliability and reproducibility of dental indices. J Clin Periodontol. 1993;20(1):54–8.
26 Holmgren CJ. CPITN – interpretation and limitations. In Dent J. 1994;44(5 Suppl 1):533–46.
Funding / potential competing interests: No financial support and no other potential conflict of interest relevant to this article was reported.