Optimised patient transfer using an innovative multidisciplinary assessment in Kanton Aargau (OPTIMA I) – an observational survey in lower respiratory tract infections
DOI: https://doi.org/10.4414/smw.2011.13237
K
Rüegger, F
Dusemund, R
Bossart, K
Regez, U
Schild, A
Conca, T
Sigrist, B
Reutlinger
Summary
BACKGROUND:Current medical scores have limited efficiency and safety profiles to enable assignment to the most appropriate treatment site in patients with lower respiratory tract infections (LRTIs). We describe our current triage practice and assess the potential of a combination of CURB65 with proadrenomedullin (ProADM) levels for triage decisions.
METHODS:Consecutive patients with LRTIs presenting to our emergency department were prospectively followed and retrospectively classified according to CURB65 and ProADM levels (CURB65-A). Low medical risk patients were further subgrouped according to biopsychosocial and functional risks. We compared the proportion of patients virtually allocated to triage sites with actual triage decisions and assessed the added impact of ProADM in a subgroup.
RESULTS:Overall, 93% of 146 patients were hospitalised. Among the 138 patients with available CURB65-A, 17.4% had a low medical risk indicating possible treatment in an outpatient or non-acute medical setting; 34.1% had an intermediate medical risk (short-hospitalisation); and 48.6% had a high medical risk (hospitalisation). Fewer patients were in a low CURB65-A class (I) than a low CURB65 class (0,1) (17.4% vs. 46.3%, p <0.001). Mean length of hospitalisation was 9.8 days including 3.6 days after reaching medical stability. In 60.3% of patients, hospitalisation was prolonged after medical stability mainly for medical reasons.
CONCLUSIONS:Current rates of hospitalisation are high in patients with LRTI and length of stay frequently extended beyond time of medical stabilization. The lower proportion of patients reclassified as low risk by adding ProADM to the CURB65 score might improve confidence in the triage algorithm.
Trial registration ISRCTN76703125
Introduction
Community-acquired lower respiratory tract infections (LRTIs) are the most prevalent, the most frequently fatal and the most cost-intensive infectious diseases in western countries [1–3]. The initial site of care decision is arguably the single most important clinical decision made by physicians during the entire course of this illness. It has a direct influence on the intensity of laboratory testing, microbiological evaluation, antibiotic therapy and cost of treatment [4]. The estimated average cost of inpatient care for community-acquired pneumonia (CAP) in the USA is 8–20 times higher than for outpatient management [1].
Accordingly, risk scoring systems have been propagated to assign patients to different risk categories with respect to important outcomes such as mortality or intensive care unit admission [5]. Prognostic severity scores such as the 20-item pneumonia severity score (PSI) [6] and the 5-item CURB65 (measuring new onset confusion, urea levels, respiratory rate, blood pressure and age) [7] provide a risk assessment in CAP patients based on medical criteria alone to aid in the triage decision. Low scores identify patients at low risk of death, thereby suggesting the safety of outpatient treatment. However, implementation into clinical routine has been hampered by their complexity (PSI), only moderate sensitivity and specificity for complications and particularly by their neglect of comorbid conditions, biopsychosocial and organisational factors [8]. Thus, these scores alone do not allow stratification into the most appropriate environment. Poor user confidence in these scores finally leads to hospitalisation of low risk patients with LRTI [9]. Importantly, fear of a complicated medical course by health care workers as well as by patients and relatives is a major driver for hospitalisation independent of clinical morbidity and expected mortality risk as shown during the ProHOSP trial [10]. This is independent of severity of disease levels indicated by clinical risk scores, and does not correlate with the compliance with procalcitonin-guided antibiotic stewardship [11]. Many patients primarily require nursing care and psychological assistance due to general frailty. The acute LRTI may only serve as the trigger for hospital admission.
Unnecessary hospitalisations are associated with adverse events such as nosocomial infections, iatrogenic worsening of frailty and dependence often leading to the requirement of long-term assistance. Therefore, a more precise, individualised and timely assessment and information about expected medical risks is pivotal for adequate triage decisions.
Biomarkers are measurable, quantifiable, objective and dynamic assessment tools for medical risk. Prognostic biomarkers such as urea, plasma lipids, cortisol, proadrenomedullin (ProADM) and pro-Endothelin 1 (Pro-ET1) correlate with CAP severity and predict mortality [12–15], with ProADM consistently being a superior prognostic biomarker [12, 16, 17]. The addition of ProADM significantly improved the prognostic value of clinical scores, while addition of other biomarkers did not lead to any further improvement [17]. ProADM, a prohormone of adrenomedullin and hormokine, is a member of the calcitonin peptide superfamily [18], is a strong vasodilator with immunomodulatory and bactericidal properties and is upregulated ubiquitously in systemic inflammatory conditions [19]. We recently demonstrated that the combination of the CURB65 with admission ProADM-levels resulted in a novel three level risk score (CURB65-A) with a high prognostic potential for adverse events and mortality in patients with LRTIs [20].
To assess the biopsychosocial and functional risk of patients, two predicting scores for post-acute, functional and frailty scores of varying complexity were tested. The self care index (SPI = “Selbstpflegeindex”) is a simple and commonly used two-level nursing tool to assess functional dependence in activities of daily life and a potential post-acute care deficit [21]. As a two-level risk assessment, the post-acute care discharge score (PACD) facilitates discharge planning indicating a risk for discharge to a post-acute care facility [22].
Herein, we describe a prospective observational quality control survey of our current triage process of patients with acute LRTIs. We aimed to identify in a descriptive manner the proportions of patients who would best be cared for at different levels of care based on an interdisciplinary risk assessment using clinical and biopsychosocial and functional scores and patient preferences with and without the addition of the biomarker ProADM. This pragmatic feasibility survey was performed as a validation study using previously developed triage algorithms in an independent prospective cohort in preparation for a future interventional clinical trial.
Methods
Subjects and study design
This was a prospective observational quality control survey with a pre-specified study hypothesis to evaluate the current triage practice for LRTI at the Kantonsspital Aarau, Switzerland. Between November 2009 and April 2010, consecutive adults admitted to the ED with LRTIs were prospectively monitored. All patients were included and there were no exclusion criteria. Diagnostic and therapeutic management decisions were taken by the treating physician without influence by the study team. The CURB65 score [7] was calculated for all patients with CAP on admission with the use of a password-secured website. This website displayed current guidelines for management of LRTI, including the use of a previously established algorithm for procalcitonin-guided antibiotic therapy [11, 12, 23–25]. CURB65 scores were only part of the clinical routine for patients with CAP. For all non-CAP LRTI patients with sufficient data on the five individual CURB65 items CURB65 scores were calculated retrospectively by the study team and were not made available to the treating physicians. We recently reported that the CURB65 score provided similar outcome prediction in patients with non-CAP LRTIs as in CAP [20].
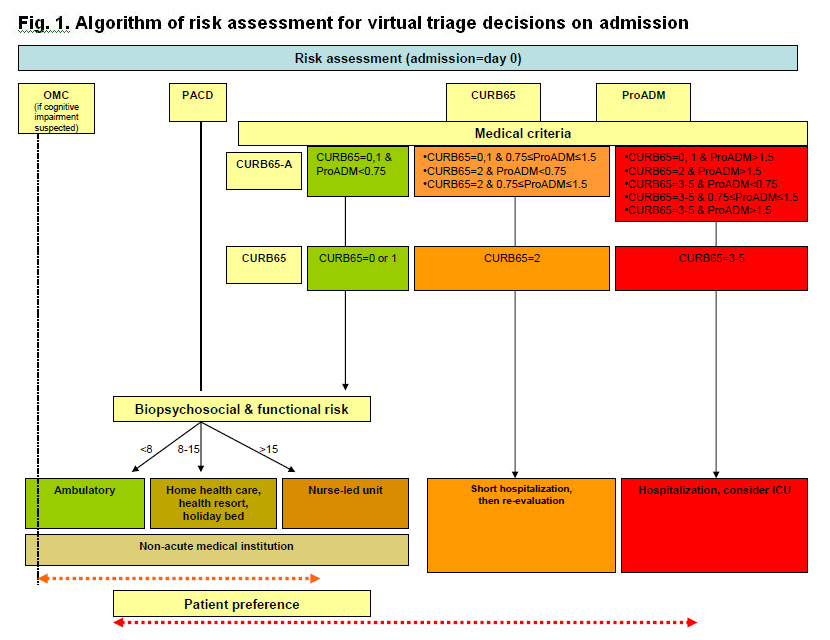
Figure 1
Algorithm of risk assessment for virtual triage decisions on admission.
Triage algorithm based on medical and functional risk assessment for patients with lower respiratory tract infection on admission. OMC – orientation memory concentration test for assessment of cognitive impairment; PACD – post acute care discharge score; ProADM – proadrenomedullin; ICU – intensive care unit
Treating physicians were encouraged to assess medical stability daily based on current IDSA/ATS criteria [26]. If a patient remained hospitalised despite fulfilling stability criteria, the physician was asked to provide the main reason for ongoing hospitalisation.
Patients were monitored from admission to hospital discharge. Psychosocial and functional assessments were performed upon hospital admission (PACD) and during hospitalisation (PACD day 3, SPI on days 2 ± 1, 5 ± 1, 10 ± 1, 17 ± 1 and within 2 days of discharge). Medical assessment was performed daily during hospitalisation to determine clinical stability and overruling criteria, if appropriate. As patients who were ill enough to require hospitalisation due to medical problems still require hospitalisation regardless of other non-medical issues, data on functional and biopsychosocial factors and nursing level requirement was obtained for all patients, but only facilitated the triage decision for those with low medical risk. Patients were interviewed after they had reached medical stability to assess their preference for site of care and feasibility of outpatient care at this time. All patients underwent a follow up phone interview 30 days after enrolment.
This survey was part of a large quality improvement project to inform hospital and regional administration in the light of chronic bed shortages and the impending introduction of DRGs in Switzerland. Thus, representative numbers of patients actually presenting to the hospital ER and occupying hospital beds in real life were necessary and any bias due to the need for informed consent might have significantly impaired our ability to predict actual and representative numbers. Obtaining informed consent is notoriously difficult in patients with reduced level of consciousness, frailty, dementia or difficulty in communication due to language barriers. However, these patients in particular should not be excluded from our analyses since they are the most vulnerable to changes in triage practice. Accordingly, the local Institutional Review Board (Kantonale Ethikkommission Aargau) classified this study as observational quality surveillance and waived the need for patient informed consent (EK AG 2009/074). Patients were informed that their routinely obtained data would be used for quality improvement purposes and that they would be contacted for a brief phone interview at a later date.
Risk scores
CURB65-A score
A novel combined risk score CURB65-A [20], consisting of the CURB65 score and admission values for ProADM was batch-measured at the end of the study for all patients who had stored specimens available. Patients were classified into three medical risk categories: CURB65-A class I (low-risk, appropriate for treatment as an outpatient or in a non-acute medical facility): CURB65 score of 0–1 and ProADM ≤0.75 nmol/l; CURB65-A class II (intermediate-risk, appropriate for short-term hospitalisation for 48 hours): CURB65=2 and ProADM ≤1.5 nmol/l; CURB65-A class III (high-risk, requiring regular hospitalisation): CURB65 ≥3 and / or ProADM ≥1.5 nmol/l.
Biopsychosocial and functional risk scores
The PACD score [22] was determined as a surrogate for biopsychosocial and functional status and nursing level requirement. Patients with a low medical risk (CURB65-A class I) were appropriate for care in non-acute medical institutions (e.g. rehabilitation facility) and were further subgrouped into three risk categories according to PACD scores. We defined low biopsychosocial and functional risk (PACD <8), appropriate for outpatient treatment; intermediate biopsychosocial and functional risk (PACD 8-15), appropriate for outpatient treatment with home health aid, a holiday bed or stay in a health resort; or high biopsychosocial and functional risk (PACD >15), appropriate for treatment in a proposed nurse-led unit (NLU). If the SPI-Index [21] was <32 in patients with a low PACD score (<8), the biopsychosocial and functional risk was considered intermediate. Cognitive function was assessed in case of suspicion for impairment, using the Short Orientation Memory Concentration test [27].
Methods of ProADM measurement
ProADM was batch-analysed from EDTA serum left over from a routinely collected phlebotomy specimen on admission (day 0) using a sandwich immunoassay with an analytical detection limit of 0.08 nmol/L [28]. Results were not available at the time of hospitalisation of the patients and, thus, physicians and patients were blinded to its results. If specimens were missing, this occurred at random; therefore no systematic bias was expected.
Definitions
LRTI comprised acute bronchitis, acute exacerbation of obstructive pulmonary disease (AECOPD) and CAP. LRTI was defined as the presence of at least one respiratory symptom (cough, sputum production, dyspnoea, pleuritic pain) plus at least one auscultatory finding or sign of infection (core body temperature >38 °C or <36 °C, shivers, leukocyte count >10 g/L or <4 g/L cells) [29]. CAP was defined as a new or increased infiltrate on chest radiograph; GOLD criteria were used to define COPD as an FEV1/FVC ratio <70% [30]; acute bronchitis was defined as LRTI in the absence of an underlying lung disease, focal chest signs or radiological infiltrates [31].
Medical stability was defined according to IDSA/ATS criteria for CAP as fulfilment for more than 24 hours of the following: maintaining oral intake, stable vital signs (temperature ≤37.8 °C, pulse ≤100/min, respiratory rate ≤24/min, O2-saturation ≥90% or pO2≥60 mm Hg on room air or return to baseline, systolic blood pressure ≥90 mm Hg), and return to baseline mental status [26]. Patients were considered to qualify for discharge from the acute medical care if they were medically stable and did not require any further intensive medical treatment.
Complications were evaluated 30 days after enrolment and included any of the following: admission to intensive care unit (ICU), need of vasopressors, mechanical ventilation, acute respiratory distress syndrome (ARDS), empyema, sepsis, adverse reaction to antibiotics, relapse or persistence of LRTI, and mortality from any cause.
We prespecified optional overruling criteria which could be used by the treating physician to justify ongoing hospitalisation despite formally fulfilling medical stability criteria. This overruling was virtual since some of these transfer options were not yet available, e.g. the planned but not yet existing NLU, or at times unavailable due to bed shortage.
Medical overruling criteria included: (1) admission to ICU for (a) respiratory (respiratory rate ≥30/min and/or O2-saturation <90% with 6L O2/min) or (b) haemodynamic instability (systolic blood pressure <90 mm Hg for ≥1 hour despite adequate volume resuscitation or vasopressor requirement); (2) imminent death; (3) complications (abscess, empyema); (4) COPD GOLD class III or IV; O2-saturation <90% despite 30 minutes of intensive treatment; (5) acute illness requiring hospitalisation independent from LRTI; (6) comorbidity, i.e. immunodeficiency (neutrophils <500/μL; if HIV+: CD4<350/μL, leukaemia, lymphoma, myeloma, cytotoxic medications, haemodialysis), pneumonia within last six weeks, antibiotics or hospitalisation (independent of indication) within seven days, other significant lung disease (cancer, fibrosis, bronchiectasis, tuberculosis, pulmonary embolism, cavitary lung disease); (7) confusion, delirium or intravenous drug use.
Nursing and organisational overruling criteria were applicable when a patient was medically stable and allowed an increase in level of care up to the level of NLU: (1) SPI-Index <32; (2) criteria requiring intensive nursing care, i.e. dementia, recurrent falls, decubitus ulcer and inability to reliably take medications; (3) waiting for non-acute medical care, i.e. holiday bed, rehabilitation, nursing home, home health care; (4) deficit of mobility or self-care requiring treatment; (5) other reasons, such as inconvenient timing (weekend, night). We documented if hospitalisation was prolonged by waiting for examinations or treatments which might also be performed as outpatient.
Patients’ and relatives’ preferences were documented: (1) concern about safety at home; (2) lack of supporting social network; (3) other reasons.
Endpoints
Our primary endpoint was to compare the percentage of patients allocated to the treatment locations based on the algorithm (fig. 1) with the percentage of patients actually treated in these sites. Secondary endpoints were the correlation of biomarkers, clinical and functional scores with site of care decisions; determination of length of hospitalisation before and after medical stability; identification of main reasons for discrepancy between actual and virtual treatment sites; and correlation of patient’s outcomes (adverse events) with CURB65 and CURB65-A.
Statistical analyses
Discrete variables were expressed as counts (percentage) and continuous variables as medians or means and standard deviations or interquartile range, unless stated otherwise. Frequency comparison was done by chi-square test. Chi-square test for trend was used to compare proportions over time. Two-group comparison of normally distributed data was performed by Student´s t-test. For data not normally distributed, the Mann-Whitney-U test was used. Statistical analyses were performed using SAS (SAS® Institute, Cary, NC, USA) and EpiInfo (version 3.5.1, CDC, USA). All testing was two-tailed and P-values < 0.05 were considered to indicate statistical significance.
Results
Baseline characteristics
Two hundred and fifty three patients were included in this survey (mean age of 64.5 years; 56.1% male). ProADM was measured on presentation to the ED in the subgroup of 146 patients (mean age: 63.6 years; 58.2% men) (table 1). The two groups (with and without measurement of ProADM) were comparable (data not shown). In this report, we restrict our analyses to those 146 patients, who had a ProADM value available.
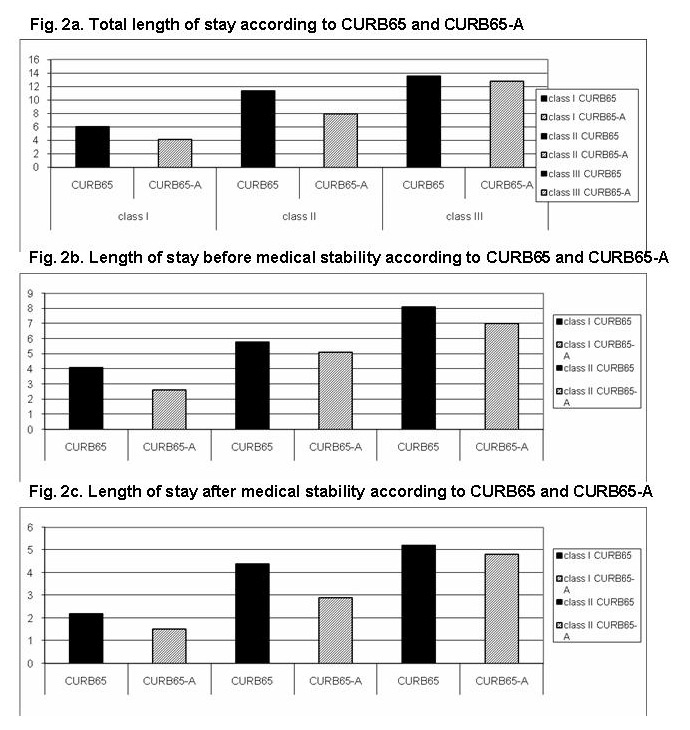
Figure 2
Length of stay according to CURB65 and CURB65-A.
Figure 2a
Total length of stay according to CURB65 and CURB65-A.
Figure 2b
Length of stay before medical stability according to CURB65 and CURB65-A.
Figure 2c
Length of stay after medical stability according to CURB65 and CURB65-A.
Length of stay (2a. total length of stay, 2b. before medical stabilization was reached, 2c. after medical stabilization was reached) stratified for risk score according to CURB65 and CURB65-A.
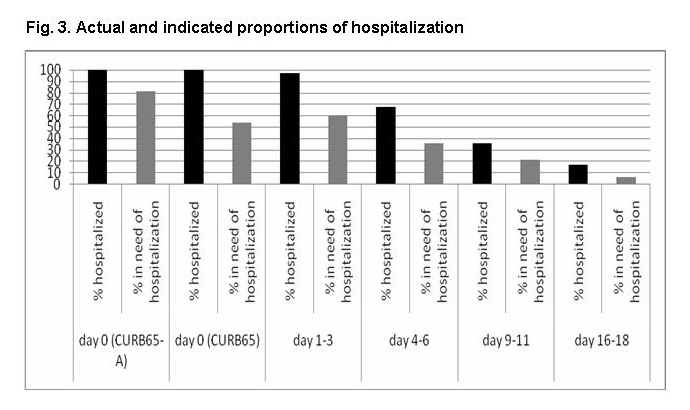
Figure 3
Actual and indicated proportions of hospitalisation.
Proportions of patients hospitalised in reality and proportions of patients in need of hospitalisation based on CURB65-A and CURB65 (on admission = day 0) and based on medical stability criteria during hospitalisation. Proportions are depicted as percentage of patients who were initially hospitalised (p for trend <0.001 for CURB65-A; p = 0.83 for CURB65).
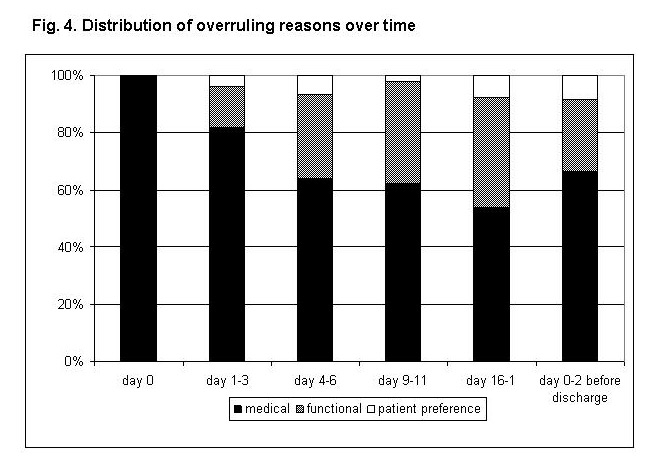
Figure 4
Distribution of overruling reasons over time.
Proportions of reasons indicated by the treating physician as responsible for virtual algorithm overruling over time.
Allocation to treatment sites according to virtual triage algorithm
According to CURB65, which was calculated in 138 patients, 63 patients (45.7%) had a low-risk (i.e. CURB65 score 0 or 1 = CURB65 class I, qualifying for outpatient treatment), 34 patients (24.6%) an intermediate-risk (CURB65 score 2 = CURB65 class II, qualifying for short-hospitalisation) and 41 patients (29.7%) a high-risk (CURB65 score 3–5 = CURB65 class III, qualifying for regular hospitalisation). In eight patients, no CURB65 could be assessed due to missing data.
According to CURB65-A, 24 of 138 patients (17.4%) had a low medical risk (CURB65-A class I), possibly qualifying for outpatient treatment with or without home health aid, management in an NLU or other non-acute medical care; 47 patients (34.1%) belonged to the “intermediate-risk group” (CURB65-A class II) and therefore would have qualified for short-hospitalisation; 67 patients (48.6%) were allocated to the “high-risk group” (CURB65-A class III) requiring regular hospitalisation; in eight patients, CURB65-A could not be calculated due to missing data. Significantly fewer patients were in a low CURB65-A class I compared with a low CURB65 class (0,1) (17.4% vs. 46.3%, p<0.001). In reality, only ten patients with available ProADM values were treated as outpatients, 5/24 (20.8%) patients of the low-risk group CURB65-A class I, 5/47 (10.6%) of the intermediate risk group (CURB65-A class II) and no patients in CURB65-A risk class III. All others were hospitalised.
Of the 54 patients in the medical low-risk group with sufficient information to calculate the PACD and CURB65 score, 48 (88.9%) would have qualified for treatment at home; 5 patients (9.3%) for outpatient treatment with home-health, and one patient (1.9%) for treatment in an NLU (table 2).
Combining the CURB65-A with the PACD score in the 138 inpatients with available information showed the following proportions: 24 (17.4%) patients would qualify for outpatient treatment, 47 (34.1%) for short-hospitalisation and 67 (48.6%) for hospitalisation (table 2).
Adverse events
There were complications present in 40 (27.4%) of the 146 patients who had a ProADM available (tab. 3). There was a trend for a lower complication rate in the 24 patients with a low CURB65-A (class I) than in the 63 patients with a low CURB65 score (0, 1) (any complication in 4.2% vs. 14.3%, p = 0.10). Patients with intermediate risks according to CURB65-A and CURB65 had similar complication rates (21.3% vs. 23.5%; p = 0.81). Patients with high risk according to CURB65-A and CURB65 had similarly high complication rates (26/67 [38.8%] vs. 20/41 [48.8%], respectively, p = 0.32) and mortality rates (9.0% vs. 14.7%, respectively, p = 0.37) (table 3). There was no loss-to-follow-up for any patient with an available ProADM measurement at the 30-day interview.
Length of acute hospital stay
Patients with CURB65 class I (n = 63) were hospitalised on average for 6.2 days, patients with a CURB65 class II (n = 34) for 11.4 days and patients in CURB65 class III (n = 41) for 13.5 days (overall length of stay (LOS): 9.8 days).
According to CURB65-A, patients had shorter length of stay in the respective categories: 4.1 days in CURB65-A class I (p = 0.01; n = 24), 7.9 in class II (p = 0.04; n = 47) and similar length of stay in class III (12.8 days, p = 0.73; n = 67) (fig. 2a).
Time to medical stability
Time to medical stability increased with increasing severity: 4.1 days in CURB65 class I, 5.8 days in class II and 8.1 days in class III (5.6 days in the entire cohort); and 2.6 days (p = 0.03), 5.1 days (p = 0.71) and 7.0 days (p = 0.29) in the CURB65-A classes, respectively (fig. 2b).
We also evaluated LOS after having reached medical stability, indicating the number of hospital days that could possibly be avoided. Overall patients remained in hospital for 3.6 days (2.1 days for CURB65 class I, 4.8 days for CURB65 class II and 4.7 days for CURB65 class III) after reaching medical stability. In comparison, according to CURB65-A classes the possible reduction of hospital stay was 1.5 days (p = 0.13), 2.7 days (p = 0.03) and 4.7 days (p = 0.98) (fig. 2c).
Of patients who were initially hospitalised, 81% and 54%, respectively, would have required hospitalisation based on the CURB65-A and CURB65. The proportion of patients who should be hospitalised according to medical stability criteria, as a percentage of the patients who were actually hospitalised, decreased over time since admission (p for trend <0.001; fig. 3).
Overruling criteria
In 88 (60.3%) of all 146 patients hospital discharge was prolonged after stability criteria were overruled at least once (overall 138 times) for the following reasons: medical overruling criteria in 75.4%, nursing and organisational overruling criteria in 18.8% and patients’ preferences in 5.8%. With increasing duration of hospitalisation, nursing factors became relatively more important (p for trend: <0.001; fig. 4).
Acute other medical problems were the most frequent medical overruling criterion. The most frequent nursing or organisational overruling criterion was waiting for placement in a non-acute medical care facility. Patients’ preferences would have been the primary reasons in eight cases, mostly (n = 6) due to concerns about safety at home (tab.4).
Site of discharge
After discharge from acute hospital, most patients were discharged to home (71.9%), 8.9% to pulmonary rehabilitation and 13.7% to another institution, including other hospitals or nursing homes.
|
Table 1: Baseline characteristics (diagnosis at discharge). |
| |
All patients
(n = 146)
|
CAP
(n = 83)
|
Bronchitis
(n = 15)
|
COPD
(n = 23)
|
Other diagnosis
(n = 25)
|
|
Demographic characteristics
|
|
|
|
|
|
| Age (years) |
64 |
64 |
61 |
71 |
57 |
| Sex (male), no. (%) |
85 (58.2) |
47 (56.6) |
8 (53.3) |
14 (60.9) |
16 (64.0) |
|
Coexisting illnesses, no. (%)
|
|
|
|
|
|
| Coronary heart disease |
25 (28.8) |
18 (21.7) |
|
3 (13.0) |
4 (16.0) |
| Cerebrovascular disease |
9 (6.2) |
7 (8.4) |
|
1 (4.3) |
1 (4.0) |
| Renal dysfunction |
43 (29.5) |
23 (27.7) |
5 (33.3) |
8 (34.8) |
7 (28.0) |
| Pneumopathy |
61 (41.8) |
23 (27.7) |
5 (33.3) |
20 (87.0) |
13 (52.0) |
| Lung cancer |
7 (4.8) |
5 (6.0) |
1 (6.7) |
|
1 (4.0) |
| Other malignancy |
11 (7.5) |
6 (7.2) |
2 (13.3) |
1 (4.3) |
2 (8.0) |
| Diabetes |
27 (18.5) |
15 (18.1) |
2 (13.3) |
4 (17.4) |
6 (24.0) |
| any, no. (%) |
104 (71.2) |
51 (61.4) |
11 (73.3) |
21 (91.3) |
20 (80.0) |
|
Clinical findings
|
|
|
|
|
|
| Confusion (%) |
15.1 |
16.9 |
13.3 |
13 |
12 |
| Respiratory rate (breaths/min.) |
20 (16–30) |
20 (16–30) |
18 (10-20) |
25 (18–34) |
20 (16–25) |
| Systolic blood pressure (mm Hg) |
125 (112–140) |
125 (112–135) |
121 (110–143) |
130 (118–146) |
133 (111–140) |
| Heart rate (beats/min.) |
100 (81–115) |
100 (80–117) |
86 (74–100) |
108 (80–117) |
103 (88–111) |
| Body temperature (°C) |
38 (36.9–38.7) |
38.1 (37.3–38.8) |
37 (36.5–38.5) |
37.4 (36.7–38) |
38.2 (37–39) |
|
Laboratory findings
|
|
|
|
|
|
| PCT (µg/L) |
0.315 (0.1–0.935) |
0.62 (0.23–4.14) |
0.16 (0.07–0.33) |
0.1 (0.07–0.27) |
0.12 (0.08–.035) |
| ProADM (nmol/l) |
1.118 (0.808–2.105) |
1.284 (0.873–2.511) |
1.122 (0.595–1.556) |
1.007 (0.851–1.558) |
0.997 (0.733–1.825) |
| Other diagnoses included asthma, cryptic organizing pneumonia, gastroenteritis, influenza, interstitial pneumonia, leukaemia, lung cancer, perimyocarditis, pleuritis, pulmonary embolism, radiation pneumonitis, restrictive lung disease, Staphylococcus aureus bacteraemia, Sweet syndrome, urinary tract infections. |
|
Table 2: Distribution of actual and virtual treatment sites according to clinical scores. |
| |
Home
|
Home health aid, post-per acute care management, NLU
|
Short hospitalisation
|
Hospitalisation
|
| |
Triage according to CURB65 and PACD (n = 129)* |
| Suggested treatment site n (%) |
48/129
(37.2%) |
6/129
(4.7%) |
34/129
(26.4%) |
41/129
(31.8%) |
| Actual treatment site n |
39 inpatients
vs.
9 outpatients |
6 inpatients
vs.
0 outpatients |
33 inpatients
vs.
1 outpatients |
41 inpatients
vs.
0 outpatients |
| |
Triage according to CURB65-A and PACD (n = 138)* |
| Suggested treatment site n (%) |
24/138
(17.4%) |
|
47/138
(34.1%) |
67/138
(48.6%) |
| Actual treatment site n |
19 inpatients
vs.
5 outpatients |
|
42 inpatients
vs.
5 outpatients |
67 inpatients
vs.
0 outpatients |
| *The PACD score could not be calculated due to missing information in 9 patients; in all of these 9 patients, the CURB65 score was 0 or 1 and ProADM >0.75 nmol/l. Therefore a treatment site could be suggested in 129 patients according to CURB65 and PACD. The elevated PACD determined the suggested treatment site regardless of the missing PACD in 138 patients according to CURB65-A and PACD. |
|
Table 3:Adverse events stratified for CURB65 or CURB65-A scores. |
|
According to CURB65
(n = 146)
|
CURB65 0-1 (n = 63)
|
CURB65 2 (n = 34)
|
CURB65 3-5 (n = 41)
|
no CURB65 (n = 8)
|
Overall
|
| Re-hospitalisation |
|
|
1 (2.5%) |
|
1 (0.7%) |
| ICU admission |
3 (4.8%) |
7 (20.6%) |
6 (14.6%) |
2 (25.0%) |
18 (12.3%) |
| Vasopressors |
1 (1.6%) |
3 (8.8%) |
4 (9.8%) |
1 (12.5%) |
9 (6.2%) |
| Mechanical ventilation |
3 (4.8%) |
5 (14.7%) |
5 (12.2%) |
2 (25.0%) |
15 (10.3%) |
| ARDS |
1 (1.6%) |
|
|
|
1 (0.7%) |
| Empyema |
|
|
1 (2.5%) |
1 (12.5%) |
2 (1.4%) |
| Sepsis |
|
1 (2.9%) |
5 (12.2%) |
1 (12.5%) |
7 (4.8%) |
| Adverse reaction to antibiotics |
1 (1.6%) |
|
|
|
1 (0.7%) |
| Death from LRTI |
1 (1.6%) |
|
4 (9.8%) |
|
5 (3.4%) |
| Death from other cause |
|
|
2 (4.9%) |
1 (12.5%) |
3 (2.1%) |
| Relapse |
2 (3.2%) |
|
5 (12.2%) |
|
7 (4.8%) |
| Persistence |
2 (3.2%) |
2 (5.9%) |
|
1 (12.5%) |
5 (3.4%) |
| Any complication |
9 (14.3%) |
8 (23.5%) |
20 (48.8%) |
3 (37.5%) |
40 (27.4%) |
|
According to CURB65-A (n = 146)
|
CURB65-A I (n = 24) |
CURB65-A II (n = 47) |
CURB65-A III (n = 67) |
no CURB65-A (n = 8) |
Overall |
| Re-hospitalisation |
|
|
1 (1.5%) |
|
1 (0.7%) |
| ICU admission |
|
4 (8.5%) |
12 (17.7%) |
2 (25%) |
18 (12.3%) |
| Vasopressors |
|
1 (2.1%) |
7 (10.4%) |
1 (12.5%) |
9 (6.2%) |
| Mechanical ventilation |
|
4 (8.5%) |
9 (13.4%) |
2 (25%) |
15 (10.3%) |
| ARDS |
|
1 (2.1%) |
|
|
1 (0.7%) |
| Empyema |
|
|
1 (1.5%) |
1 (12.5%) |
2 (1.4%) |
| Sepsis |
|
|
6 (9.0%) |
1 (12.5%) |
7 (4.8%) |
| Adverse reaction to antibiotics |
|
1 (2.1%) |
|
|
1 (0.7%) |
| Death from LRTI |
|
1 (2.1%) |
4 (6.0%) |
|
5 (3.4%) |
| Death from other cause |
|
|
2 (3.0%) |
1 (12.5%) |
3 (2.1%) |
| Relapse |
1 (4.2%) |
1 (2.1%) |
5 (7.5%) |
|
7 (4.8%) |
| Persistence |
|
3 (6.4%) |
1 (1.5%) |
1 (12.5%) |
5 (3.4%) |
| Any complication |
1 (4.2%) |
10 (21.3%) |
26 (38.8%) |
3 (37.5%) |
40 (27.4%) |
|
Table 4: Reasons to overrule triage algorithm after medical stabilization. |
| |
n = 146 (%)
|
|
Medical overruling criteria, no. (%)
|
|
| Admission to ICU |
|
| Life-threatening co-morbidity |
1 (1.0) |
| Complications |
2 (1.9) |
| COPD GOLD III & IV, SaO2 <90% despite 30 minutes intensive treatment |
5 (4.8) |
| Acute illness requiring hospitalisation independent from LRTI |
96 (92.3) |
| Comorbidity |
|
| Confusion, delirium or intravenous drug use |
|
| Total |
104 |
|
Nursing and organisational overruling criteria, no. (%)
|
|
| SPI-Index <32 |
|
| Criteria requiring intensive nursing care |
2 (7.7) |
| Waiting for placement in a non-acute medical care facility |
20 (76.9) |
| Deficit of mobility or self-care requiring treatment |
4 (15.4) |
| Other reasons |
|
| Total |
26 |
|
Patient's preferences, no. (%)
|
|
| Concern about safety at home |
6 (75.0) |
| Lack of supporting social network |
|
| Other reasons |
2 (25.0) |
| Total |
8 |
Discussion
In this observational assessment of the current practice at a Swiss medical university department, we detected a higher rate of hospitalisation and a longer LOS than recommended by prognostic clinical systems. More than half of all patients remained hospitalised after they had become medically stable. Both higher hospitalisation rates and longer duration of stay were observed with increased severity of illness. This was true regardless of whether assessment had been performed using the guideline recommended CURB65 score or the CURB65-A. Fewer patients were in a low CURB65-A class I compared to a low CURB65 class (0, 1) but a low CURB65-A class was better at predicting low risk of poor outcome.
Most low risk patients with CAP preferred treatment at home rather than in hospital [32]. Carratala and colleagues randomized patients with low risk CAP (PSI risk classes II and III) into outpatient and inpatient management and showed that both groups had similar positive outcomes [9]. Patient satisfaction was even higher in outpatients than inpatients [9, 33]. In observational cohorts, up to 13–14% of patients with severe CAP could be safely treated as outpatients [34, 35]. Innovative pathway bundles were shown to reduce hospitalisation for CAP particularly in low risk patients, decrease antibiotic use and overall costs while achieving similar quality of life and patient outcomes [36, 37]. Unfortunately, such interventions are highly resource-intensive and implementation in clinical routine is doubtful in most settings. Thus novel solutions and practicable, more generalisable triage algorithms are needed.
The primary aim of this observation was to identify the percentage of patients qualifying for different treatment sites as defined in a novel patient pathway using interdisciplinary, innovative triage criteria. We achieved an interdisciplinary recommendation for ideal treatment site by combining medical (CURB65-A) and biopsychosocial (PACD, SPI, OMC) criteria and considered patient preferences. This recommendation was, at this stage, still virtual due to the lack of an NLU. We previously showed that the CURB65-A score safely and effectively predicted complications and mortality in patients with LRTI, who were part of the large randomized double blind Swiss multicentre ProHOSP study [11] thus allowing 20% and 40% of hospitalised patients to undergo ambulatory treatment or only short term hospitalisation [20]. Our current data confirmed that non-acute medical treatment or short hospitalisation would have been possible in 17.4% and 34.1%, respectively. Given 9% actual outpatient treatment and 91% hospitalisation, this would allow a large proportion of patients to be treated outside the acute care hospital with subsequent advantages including reduction of costs [38], lower risks of nosocomial infections including those with resistant pathogens [39], falls or deconditioning [40], thromboembolic events, delirium and associated complications. As we found a particularly long LOS after reaching medical stability (3.6 days), there is a great potential for reducing LOS, especially in patients with high CURB65 or CURB65-A classes.
Patients with low CURB65-A score had a trend towards lower complication rates compared to patients with a low CURB65 score. This indicates that patients with low CURB65-A might indeed be safely treated as outpatients, whilst low risk patients according to the CURB65 alone would have a potential risk of complications. Increasing CURB65-A scores furthermore correlated well with increasing rates of severe adverse events, in-hospital and 30 day mortality. Physicians often follow their own judgment about hospitalisation rather than guideline recommendations based on clinical scores, as documented in the ProHOSP study, where only 20% of low risk CURB65 patients were treated as outpatients [20]. Others have confirmed poor confidence in clinical scores alone given hospitalisation rates of 31% [41] to 43% [36] in so-called low-risk patients with CAP. Conversely, 27% of patients with CAP requiring ICU admission were in low-risk PSI classes [42]. New objective scores with a high accuracy for high-risk and low-risk LRTI patients would be important advances in order to achieve greater confidence for physicians to recommend, and for patients and relatives to accept, non-acute hospital management and higher efficiency in allocating health-care resources [10]. A formal cost-effectiveness analysis was beyond the scope of the current paper. Indirect conclusions might be drawn from another Swiss prospective randomized controlled trial, which showed that B-type natriuretic peptide (BNP) for assessment of acute dyspnoea was associated with both a reduction in hospital days and a 25% reduction in treatment costs [43]. These results were confirmed by others [44, 45].
Medical criteria, most importantly acute non-LRTI illnesses, were the main reasons justifying hospitalisation in patients with low CURB65-A or “stable” LRTI. Waiting for placement in a non-acute medical care facility represented approximately 77% all instances, when functional criteria predominated. Our results suggest that with ongoing hospitalisation, non-medical factors gain increasing importance in the discharge planning. This is intuitive and most likely related to successful treatment of acute medical conditions. However, this finding indicates at least two non-exclusive possibilities. Firstly, the high nursing and functional demands of patients presenting with LRTIs, which remain or become increasingly prominent after resolution of the acute medical condition, and secondly, an insufficient or delayed emphasis on social services and organisational problems highlighting societal unpreparedness to receive patients after effective medical care. This underlines the potential of an NLU in this setting.
One of the strengths of this survey is its novel and innovative concept including the individualised interdisciplinary and biomarker-enhanced risk assessment. We included all consecutive patients without exclusion criteria independent of severity of illness, cognitive status and comorbidities, thus strengthening the generalisability to different settings and populations including the frail and cognitively impaired elderly, who otherwise are frequently excluded from randomized controlled trials despite representing a large fraction of patients hospitalised with LRTI [35]. Another strength is that we were able to follow-up all patients at the day 30 interview.
There are limitations of our observational survey. Some outpatients might not have been enrolled in the survey, as indicated by a relatively low proportion of outpatients. This might partially be explained by the high severity of illness, the large proportion of underlying comorbidities and the presence of a general medicine office, which is associated with our emergency room which primarily took care of low risk patients, who were not included in our survey. Secondly, overruling criteria were only provided by the treating physician, and other overruling criteria were mainly asked for once medically stable. Thus, medical overruling criteria might be overestimated in comparison to nursing and organisational criteria and patients’ preferences as we did not interview nurses and patients directly. Overruling criteria were only assessed after medical stabilization and not in the emergency department. This explains the low virtual assignment to non-acute medical care on admission. Only a relatively small sample size of patients had ProADM measurements. However, there was no clinical difference between patients with and patients without available ProADM levels (data not shown). Some of the provided options (e.g. NLU) were not available at the time. This underlines the importance of future interventional studies with availability of all triage options and real-time measurement of ProADM. Finally, this data was used to test prospectively the feasibility of previously developed algorithms [20] in an independent patient cohort in preparation for a (currently ongoing randomized controlled) intervention study. For this purpose, actual data and absolute numbers are required. More detailed analyses will be possible in an intervention study. Even though we feel that the data should be fairly generalisable, external validity has to be proven in other settings.
In conclusion, our results demonstrate the large potential of an interdisciplinary and biomarker-enhanced triage to avoid not-indicated hospital admissions, shorten length of stay and to improve patient care by individualizing triage decisions and better allocation of resources. Addition of ProADM to CURB65 might increase confidence in objective triage decisions compared to CURB65 alone. This pragmatic observational survey lays the scientific and logistic foundation for an interventional study to confirm the CURB65-A as a superior risk assessment tool and evaluate novel triage pathways.
The authors gratefully thank the following people without whom this survey would not have been possible:
– Ruth Schweingruber and Gabriela Wallimann for support with data collection and entry and logistic and secretarial support,
– Dr. Ulrich Buergi, Petra Tobias and the staff in the Emergency Department of the Kantonsspital Aarau,
– Renate Hunziker and the staff in the central laboratories of the Kantonsspital Aarau,
– Susanne Schirlo, Dr. Petra Schäfer and the medical, nursing and physiotherapy staff in the Department of Medicine of the Kantonsspital Aarau,
– Renata Kleeb and Omar Gloor from the Department of Finance and Controlling of the Kantonsspital Aarau,
– PD Dr. Sarosh Irani and Dr. Marc Maurer, Pulmonary Division, Kantonsspital Aarau and Dr. Martin Frey, Pulmonary Division, Klinik Barmelweid for expert clinical advice,
– Dr. Max Neuhaus and Therese Matter of the Department für Gesundheit und Soziales and Regierungsrätin Susanne Hochuli for logistic and financial support,
– Florian Dürr for IT support,
– The patients, family members, and caregivers who participated in the study.
Contributors:
WA, PS, BM had the idea, wrote the protocol and initiated the study,
WA, RB, KR, KrR, FD, US, AC designed the questionnaire, managed the trial and collected data, WA, FD and KrR performed the statistical analyses, WA, KrR drafted the manuscript, all other authors amended and commented on the manuscript. All authors approved the final version.
References
1 Niederman MS, McCombs JS, Unger AN, Kumar A, Popovian R. The cost of treating community-acquired pneumonia. Clin Ther. 1998;20(4):820–37.
2 Welte T, Suttorp N, Marre R. CAPNETZ-community-acquired pneumonia competence network. Infection. 2004;32(4):234–8.
3 Almirall J, Bolibar I, Vidal J, Sauca G, Coll P, Niklasson B, Bartolome M, Balanzo X. Epidemiology of community-acquired pneumonia in adults: a population-based study. Eur Respir J. 2000;15(4):757–63.
4 Aliyu ZY, Aliyu MH, McCormick K. Determinants for hospitalization in “low-risk” community acquired pneumonia. BMC Infect Dis. 2003;3:11.
5 Marrie TJ. Risks and outcomes in community acquired pneumonia. Can Respir J. 1999;6(Suppl A):6A–9A.
6 Fine MJ, Auble TE, Yealy DM, Hanusa BH, Weissfeld LA, Singer DE, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336(4):243–50.
7 Lim WS, van der Eerden MM, Laing R, Boersma WG, Karalus N, Town GI, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58(5):377–82.
8 Aujesky D, McCausland JB, Whittle J, Obrosky DS, Yealy DM, Fine MJ. Reasons why emergency department providers do not rely on the pneumonia severity index to determine the initial site of treatment for patients with pneumonia. Clin Infect Dis. 2009;49(10):e100–108.
9 Carratala J, Fernandez-Sabe N, Ortega L, Castellsague X, Roson B, Dorca J, et al. Outpatient care compared with hospitalization for community-acquired pneumonia: a randomized trial in low-risk patients. Ann Intern Med. 2005;142(3):165–72.
10 Baehni C, Meier S, Spreiter P, Schild U, Regez K, Bossart R, et al. Which patients with lower respiratory tract infections need inpatient treatment? Perceptions of physicians, nurses, patients and relatives. BMC Pulm Med. 10:12.
11 Schuetz P, Christ-Crain M, Thomann R, Falconnier C, Wolbers M, Widmer I, et al. Effect of procalcitonin-based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA. 2009;302(10):1059–66.
12 Christ-Crain M, Morgenthaler NG, Stolz D, Muller C, Bingisser R, Harbarth S, et al. Pro-adrenomedullin to predict severity and outcome in community-acquired pneumonia [ISRCTN04176397]. Crit Care. 2006;10(3):R96.
13 Christ-Crain M, Stolz D, Jutla S, Couppis O, Muller C, Bingisser R, et al. Free and total cortisol levels as predictors of severity and outcome in community-acquired pneumonia. Am J Respir Crit Care Med. 2007;176(9):913–20.
14 Gruber M, Christ-Crain M, Stolz D, Keller U, Muller C, Bingisser R, et al. Prognostic impact of plasma lipids in patients with lower respiratory tract infections – an observational study. Swiss Med Wkly. 2009;139(11–12):166–72.
15 Schuetz P, Stolz D, Mueller B, Morgenthaler NG, Struck J, Mueller C, et al. Endothelin-1 precursor peptides correlate with severity of disease and outcome in patients with community acquired pneumonia. BMC Infect Dis. 2008;8:22.
16 Huang DT, Angus DC, Kellum JA, Pugh NA, Weissfeld LA, Struck J, et al. Midregional proadrenomedullin as a prognostic tool in community-acquired pneumonia. Chest. 2009;136(3):823–31.
17 Schuetz P, Wolbers M, Christ-Crain M, Thomann R, Falconnier C, Widmer I, et al. Prohormones for prediction of adverse medical outcome in community-acquired pneumonia and lower respiratory tract infections. Crit Care. 14(3):R106.
18 Becker KL, Nylen ES, White JC, Muller B, Snider RH, Jr. Clinical review 167: Procalcitonin and the calcitonin gene family of peptides in inflammation, infection, and sepsis: a journey from calcitonin back to its precursors. J Clin Endocrinol Metab. 2004;89(4):1512–25.
19 Bunton DC, Petrie MC, Hillier C, Johnston F, McMurray JJ. The clinical relevance of adrenomedullin: a promising profile? Pharmacol Ther. 2004;103(3):179–201.
20 Albrich WC, Dusemund F, Rüegger K, Christ-Crain M, Zimmerli W, Bregenzer T, et al. Enhancement of CURB65 score with proadrenomedullin (CURB65-A) for outcome prediction in lower respiratory tract infections: Derivation of a clinical algorithm. BMC Infect Dis. 2011;11:112.
21 große Schlarmann J. Der CMS© im ePA©. Verschiedene Qualitätsdimensionen eines Instruments. Eine empirische Analyse. Gelsenkirchen: Private Universität Witten/Herdecke gGmbH; 2007.
22 Louis Simonet M, Kossovsky MP, Chopard P, Sigaud P, Perneger TV, Gaspoz JM. A predictive score to identify hospitalized patients’ risk of discharge to a post-acute care facility. BMC Health Serv Res. 2008;8:154.
23 Briel M, Schuetz P, Mueller B, Young J, Schild U, Nusbaumer C, et al. Procalcitonin-guided antibiotic use vs a standard approach for acute respiratory tract infections in primary care. Arch Intern Med. 2008;168(18):2000–2007; discussion 2007–2008.
24 Stolz D, Christ-Crain M, Gencay MM, Bingisser R, Huber PR, Muller B, et al. Diagnostic value of signs, symptoms and laboratory values in lower respiratory tract infection. Swiss Med Wkly. 2006;136(27-28):434–40.
25 Schuetz P, Widmer I, Chaudri A, Christ-Crain M, Zimmerli W, Mueller B. Prognostic value of procalcitonin in community-acquired pneumonia. Eur Respir J. 2010.
26 Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27–72.
27 Goring H, Baldwin R, Marriott A, Pratt H, Roberts C. Validation of short screening tests for depression and cognitive impairment in older medically ill inpatients. Int J Geriatr Psychiatry. 2004;19(5):465–71.
28 Morgenthaler NG, Struck J, Alonso C, Bergmann A. Measurement of midregional proadrenomedullin in plasma with an immunoluminometric assay. Clin Chem. 2005;51(10):1823–9.
29 Niederman MS, Mandell LA, Anzueto A, Bass JB, Broughton WA, Campbell GD, et al. Guidelines for the management of adults with community-acquired pneumonia. Diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am J Respir Crit Care Med. 2001;163(7):1730–54.
30 Marrie TJ. Community-acquired pneumonia in the elderly. Clin Infect Dis. 2000;31(4):1066–78.
31 Gonzales R, Sande MA. Uncomplicated acute bronchitis. Ann Intern Med. 2000;133(12):981–91.
32 Coley CM, Li YH, Medsger AR, Marrie TJ, Fine MJ, Kapoor WN, et al. Preferences for home vs hospital care among low-risk patients with community-acquired pneumonia. Arch Intern Med. 1996;156(14):1565–71.
33 Fried TR, van Doorn C, O’Leary JR, Tinetti ME, Drickamer MA. Older person’s preferences for home vs hospital care in the treatment of acute illness. Arch Intern Med. 2000;160(10):1501–6.
34 Marrie TJ, Wu L. Factors influencing in-hospital mortality in community-acquired pneumonia: a prospective study of patients not initially admitted to the ICU. Chest. 2005;127(4):1260–70.
35 Marrie TJ, Huang JQ. Admission is not always necessary for patients with community-acquired pneumonia in risk classes IV and V diagnosed in the emergency room. Can Respir J. 2007;14(4):212–6.
36 Mandell LA, Marrie TJ, Grossman RF, Chow AW, Hyland RH. Summary of Canadian Guidelines for the Initial Management of Community-acquired Pneumonia: An evidence-based update by the Canadian Infectious Disease Society and the Canadian Thoracic Society. Can J Infect Dis. 2000;11(5):237–48.
37 Loeb M, Carusone SC, Goeree R, Walter SD, Brazil K, Krueger P, et al. Effect of a clinical pathway to reduce hospitalizations in nursing home residents with pneumonia: a randomized controlled trial. JAMA. 2006;295(21):2503–10.
38 Labarere J, Stone RA, Scott Obrosky D, Yealy DM, Meehan TP, Auble TE, et al. Factors associated with the hospitalization of low-risk patients with community-acquired pneumonia in a cluster-randomized trial. J Gen Intern Med. 2006;21(7):745–52.
39 Mandell L. Decisions about treating community-acquired pneumonia. Ann Intern Med. 2005;142(3):215–6.
40 Kortebein P, Ferrando A, Lombeida J, Wolfe R, Evans WJ. Effect of 10 days of bed rest on skeletal muscle in healthy older adults. JAMA. 2007;297(16):1772–4.
41 Atlas SJ, Benzer TI, Borowsky LH, Chang Y, Burnham DC, Metlay JP, et al. Safely increasing the proportion of patients with community-acquired pneumonia treated as outpatients: an interventional trial. Arch Intern Med. 1998;158(12):1350–6.
42 Angus DC, Marrie TJ, Obrosky DS, Clermont G, Dremsizov TT, Coley C, et al. Severe community-acquired pneumonia: use of intensive care services and evaluation of American and British Thoracic Society Diagnostic criteria. Am J Respir Crit Care Med. 2002;166(5):717–23.
43 Mueller C, Laule-Kilian K, Schindler C, Klima T, Frana B, Rodriguez D, et al. Cost-effectiveness of B-type natriuretic peptide testing in patients with acute dyspnoea. Arch Intern Med. 2006;166(10):1081–7.
44 Adlbrecht C, Huelsmann M, Berger R, Moertl D, Strunk G, Oesterle A, et al. Cost analysis and cost-effectiveness of NT-proBNP-guided heart failure specialist care in addition to home-based nurse care. Eur J Clin Invest. 2010;41(3):315–22.
45 Reinhold T, Berghofer A, Willich SN. Is the determination of biomarkers worth its price? Review of the literature taking brain natriuretic peptides (BNP) as an example. Herz. 2010;35(1):1–10.



