Low-dose recombinant factor VIIa for massive bleeding
DOI: https://doi.org/10.4414/smw.2011.13213
A
Mordasini, M
Luginbühl, B
Regli, HP
Kohler, D
Inderbitzin, B
Lämmle, L
Alberio
Summary
QUESTIONS UNDER STUDY: recombinant activated factor VII (rFVIIa) is used off-label for massive bleeding. There is no convincing evidence of the benefits of this practice and the minimal effective dose is unknown. The aim of the study was to evaluate our in-house guideline recommending a low dose of 60 μg/kg for off-label use of rFVIIa.
METHODS:observational cohort study at the Inselspital Bern, a tertiary care University Hospital in Switzerland. All patients with massive bleeding treated off-label with rFVIIa between January 2005 and December 2007 were included.Survival, change of bleeding and transfusion rates, coagulation parameters and complications were analysed.
RESULTS: seventy-three patients received rFVIIa. Severe haemorrhage was documented by a bleeding rate of 1000 mL/h (median; interquartile range 350–3000) and total volume replacement of 11.9 L (6.6–15.2) before administration of rFVIIa. The median rFVIIa-dose was 64 μg/kg (56–71). rFVIIa was administered once in 79% patients, twice in 18%. The bleeding rate was reduced in 82% of the patients. Transfused packed red blood cells decreased from 14 units (8–22) over 4.9 h (2.5–8.8) before rFVIIa to 2 (0–6) in 24 h thereafter, platelet concentrates from 2 units (1–3) to 1 (0–2) and FFP from 11 units (6–16) to 2 (0–9). In-hospital mortality was 14% within 24 h and 32% at day 30. There were two arterial thromboembolic complications possibly related to rFVIIa.
CONCLUSION: a single injection of 60 μg/kg rFVIIa, a lower dose than usually recommended, appears to be efficacious in controlling massive bleeding with a very low complication rate.
A single centre observational cohort study with 73 patients
Poster presentation
Parts of these results were presented as a poster at the 52nd conference of the German-Swiss-Austrian Society for Thrombosis and Haemostasis Research (“Gesellschaft für Thrombose- und Hämostaseforschung” GTH) in Wiesbaden, Germany, February 2008.
Abbreviations
aPTT activated partial thromboplastin time
ECC extracorporeal circulation
FFP fresh frozen plasma
IQR interquartile range
PLT platelet concentrate
PT prothrombin time (“Quick”)
RBC packed red blood cells
rFVIIa recombinant activated factor VII
Introduction
Recombinant activated factor VII (rFVIIa) has been licensed in Europe for use in patients with haemophilia A or B with inhibitory alloantibodies, with acquired haemophilia, with congenital deficiency of coagulation factor VII, or with Glanzmann’s thrombasthenia [1]. Additionally, rFVIIa has been employed for prevention or treatment of massive bleeding in non-haemophilic patients as well [2, 3]. In contrast to the large number of published case reports and retrospective cohort studies on successful use of rFVIIa in various off-label situations, randomised placebo controlled trials report limited effects at best [4–6]. According to the missing evidence for rFVIIa off-label use, a recently published European guideline on management of bleeding following major trauma states that rFVIIa should be considered only if first-line treatment with a combination of surgical approaches, best-practice use of blood products, the use of antifibrinolytics and correction of severe acidosis, severe hypothermia and hypocalcaemia fail to control bleeding [7].
Measurable effects of rFVIIa administration have been observed in several populations, such as reduced growth of haematoma in patients with intracerebral haemorrhage [8, 9] and reduced need for blood products in trauma patients [10–12], in cardiac surgery [13], in obstetrics [14] or in urology [15]. Furthermore, there are reports suggesting that early use of rFVIIa may prevent massive transfusion coagulopathy [16]. Nevertheless, many studies failed to show a significant impact of rFVIIa on relevant clinical outcomes, in particular mortality [5, 17]. A meta-analysis of 22 randomised controlled trials in patients without haemophilia found a non-significant decrease in mortality and a significant reduction in the need for blood transfusion both in prophylactic and therapeutic trials [4]. A recently published meta-analysis of 26 randomised controlled studies in non-haemophilic patients found in 12 therapeutic trials a non-significant decrease in mortality and no difference in control of bleeding or red blood cell administration [6].
Side effects of rFVIIa, predominantly thromboembolic events, have been reported in off-label use, with various incidences ranging from <1% to 44% [18–21]. While published meta-analyses did show no significant difference between patients who received rFVIIa and patients who received placebo with respect to the incidence of venous thromboembolic events [4, 22], treatment with rFVIIa significantly increased the risk of arterial thromboembolic events [4, 22], in particular among elderly patients [22]. There was a significantly higher frequency of coronary events and a statistically non-significant trend toward an increased rate of cerebrovascular events among patients treated with rFVIIa compared to placebo [4, 22]. As for rFVIIa dose, among patients with central nervous system bleeding who received rFVIIa, there was a significant correlation between dose and rates of arterial thromboembolic events [22].
Although many publications on off-label use of rFVIIa reported an effective median dose similar to that of standard indications, 90 μg/kg body weight [4, 23], or even much higher, up to 200 μg/kg body weight [10], there are some studies indicating a therapeutic effect already at lower doses, in particular in certain indications, such as acute intracerebral haemorrhage [8], traumatic bleeding [11, 12], retropubic prostatectomy [15] or cardiac surgery [24, 25]. In 2004, we had chosen to employ for off-label use at our institution a low rFVIIa dose of 60 μg/kg because it leads to a plasmatic rFVIIa concentration which still is 5-6 times higher than the minimal effective level [26], and because high rFVIIa doses could theoretically lead to excessive thrombin generation with consecutive activation of the anticoagulant protein C pathway by means of thrombin binding thrombomodulin [27].
In order to regulate off-label use of rFVIIa at the University Hospital of Bern, an interdisciplinary working group was instituted in 2004. Representatives of the departments of anaesthesiology, emergency medicine, intensive care medicine, visceral surgery, paediatrics and haematology reached a consensus guideline for the administration of rFVIIa in patients with massive bleeding (table 1). This guideline is congruent with the recently published European guideline on management of bleeding after major trauma [7]. The aim of the present study was to evaluate effect and safety of this consensus in-house guideline at the University Hospital of Bern.
Patients and methods
Study design
This is a single-centre observational cohort study performed at the Inselspital, a tertiary care university hospital in Bern, Switzerland. Since it had been impossible to design and conduct a prospective randomised controlled study, the interdisciplinary working group agreed to prospectively document the experience collected since implementation of the institutional guideline for off-label use of rFVIIa (table 1). All patients treated with rFVIIa from January 1, 2005 to December 31, 2007 were included into this study. The treating physicians decided on the indication to administer rFVIIa. The haematologist on call verified the indication and provided the correct dose. This was possible since rFVIIa is stored at the Central Haematology Laboratory. Implementing a gatekeeper strategy, a haematology medical consultation is necessary in order to administer plasma derived or recombinant coagulation factor concentrates, including rFVIIa.
|
Table 1: Guideline for off-label use of rFVIIa for treatment of massive bleeding at the Inselspital, University Hospital, Bern, Switzerland. |
|
Topic
|
|
Comment
|
| Information |
Contact |
Early contact with haematologist on call |
| |
Diagnostics |
Surgical bleeding?
Coagulation disorder that can be targeted?
Anticoagulant drug that can be antagonised?
Acquired coagulopathy?
Acidosis? |
| |
Laboratory |
Haemoglobin?
Platelet count?
Coagulation tests?
Draw blood for coagulation tests before rFVIIa |
| |
Treatment already given |
Number of blood products (RBC, FFP, PLT)?
Antifibrinolytics (tranexamic acid, aprotinin)?
Haemostatic drugs (DDAVP, vWF/FVIII, Fibrinogen, Fibrin “glue”)?
Surgery? |
| Preconditions |
Inclusion criteria |
Persistent severe bleeding (i.e. ≥4 mL/kg/h) after 8 RBC and 4 FFP units
Exclusion of an otherwise treatable bleeding
Optimal treatment with blood products, haemostatic drugs, and surgery
Platelet count >20 x109/L |
| |
Exclusion criteria |
Patient with a non-treatable condition likely to lead to death soon, such as non-treatable metastatic cancer, severe liver cirrhosis without indication for liver transplantation, very severe traumatic brain injury, pre-terminal chronic disease |
| |
Absolute contraindications |
Non-compensated DIC or severe sepsis
Acute myocardial infarction
Acute ischaemic cerebral stroke |
| |
Relative contraindications |
Severe coronary heart disease
Previous ischaemic cerebral insult |
| |
Antifibrinolytic drug |
The use of an antifibrinolytic drug like tranexamic acid is recommended before the use of rFVIIa |
| |
Haematologist consultation |
No rFVIIa without consultation |
| Dosing |
First dose |
60 μg/kg body weight i.v. [35, 36] |
| |
Second dose |
Repeat same dose after 60 to 120 minutes if the bleeding is persistent but has decreased; in case of unchanged bleeding a repeat dose of 90 μg/kg body weight is allowed |
| |
Third dose |
No third dose is advised [37] |
| Follow-up |
Laboratory |
Obtain blood sample for coagulation tests 1 h and 12 h after use |
| |
Documentation |
Return completed data sheet with clinical data |
Study population
Patients from all clinical departments were included into this observational cohort study (table 2). This is a very heterogeneous population, exactly representing everyday practice at a tertiary care hospital. rFVIIa was also used off-label in 6 patients due to refusal of blood products by the patient; these patients were excluded from this analysis to avoid confounding of the data. The study was approved by the responsible Ethics committee (Kantonale Ethikkomission Bern) and conducted according to the institutional guidelines on observational studies.
|
Table 2: Baseline characteristics of the patients receiving recombinant factor VIIa for massive hemorrhage. |
|
Characteristic
|
Study population*
|
| Basic epidemiological data |
| No. of patients |
73 |
| Sex (female / male), no. |
35 / 38 |
| Age, years, median (IQR, range) |
44 (31–59, 4–89) |
| Weight, kg, median (IQR, range) |
72.5 (59.0–84.0, 16–170) |
| Medical category |
| Surgery |
35 / 73 (48%) |
| of these with ECC |
15 / 35 (43%) |
| Obstetrics |
20 / 73 (27%) |
| Trauma |
14 / 73 (19%) |
| Medicine |
4 / 73 (6%) |
| Severity of bleeding before use of rFVIIa |
| Total volume replacement, L, median (IQR) |
11.9 (6.6–15.2) |
| Estimated bleeding rate,† L/h, median (IQR) |
1.0 (0.3–3.0) |
| Treatment with anticoagulant and antiplatelet drugs before bleeding episode |
| Any‡ |
18 / 73 (25%) |
| Vitamin K antagonist |
3 / 73 (4%) |
| Unfractionated heparin |
11 / 73 (15%) |
| Low-molecular-weight heparin |
7 / 73 (10%) |
| Antiplatelet drugs |
6 / 73 (8%) |
| * All values represent no. / total no. (%) unless otherwise specified.
† Estimated bleeding rate was documented in 23 patients
‡ Some patients had combinations of various anticoagulant / antiplatelet drugs.
ECC = extracorporeal circulation; IQR = interquartile range; rFVIIa = recombinant factor VIIa |
Data collection
All clinical data were documented by the treating physician(s). A standardised data collection form was used. Missing clinical data were searched for in the original patient documentation, by the study group. Qualitative change of bleeding 30 minutes after rFVIIa administration (decrease, without change, increase) was assessed and documented by the treating physician. For some patients detailed quantitative data were available as bleeding rates (ml per hour) before and after rFVIIa administration. According to the current practice at our institution bleeding volume was estimated by means of the suction volume over time, the number of blood soaked gauze pads and serial haemoglobin measurements. Results of laboratory analyses were retrieved from the electronic data management system. Time and number of transfused packed red blood cells (RBC), fresh frozen plasma (FFP), and platelet concentrates (PLT) for all patients were available from transfusion service records. The end-points for follow up of the patients were death, hospital-discharge or survival at day 30, whichever came first.
Statistical methods
Clinical and laboratory data were collected in a database worksheet with Microsoft Excel (Redmond, USA). Endpoints were the number of transfused RBC, FFP and PLT before and after the application of rFVIIa, qualitative change of bleeding after rFVIIa administration (decrease, without change, increase), side effects of rFVIIa and in-hospital mortality on days 1 and 30. Statistical analysis was performed with MedCalc (Mariakerke, Belgium). Due to the number of patients and the non-normal distribution of some parameters (D'Agostino-Pearson test), non-parametric tests were used for the statistical analysis of quantitative variables. The Kruskal-Wallis test was used for comparisons between various groups. A Wilcoxon signed rank test was used for paired samples, whilst a Mann-Whitney U test was used for independent samples. A p value of <0.05 was considered significant.
Results
Severity of bleeding before the use of rFVIIa
During the 3 years, 79 patients were treated with rFVIIa off-label. In 6/79 patients (7.6%) rFVIIa was given because of refusal of blood products; these patients were excluded from analysis. Baseline characteristics of the 73 patients who received rFVIIa according to the guideline (table 1) are shown in table 2. Severe bleeding before use of rFVIIa had been documented by an estimated bleeding rate of 1000 mL/h (median; IQR 350–3000; n = 23) and a total volume replacement per patient of 11.9 L (median; IQR 6.6–15.2; n = 73) with crystalloids, colloids (predominantly hydroxyethyl starch, HES; only 4 cases with gelatine), RBC, and FFP over 4.9 hours (median; IQR 2.5–8.8). Specifically, 14 RBC units (median; IQR 8–22), 11 FFP units (median; IQR 6–16), and 2 PLT units (median; IQR 1–3) had been administered before infusion of rFVIIa (Table 3). Eight patients with a median platelet count of 125 x109/L (IQR: 81–208, range: 69–385) before rFVIIa did not receive any PLT units. Key laboratory parameters are summarised in table 4.
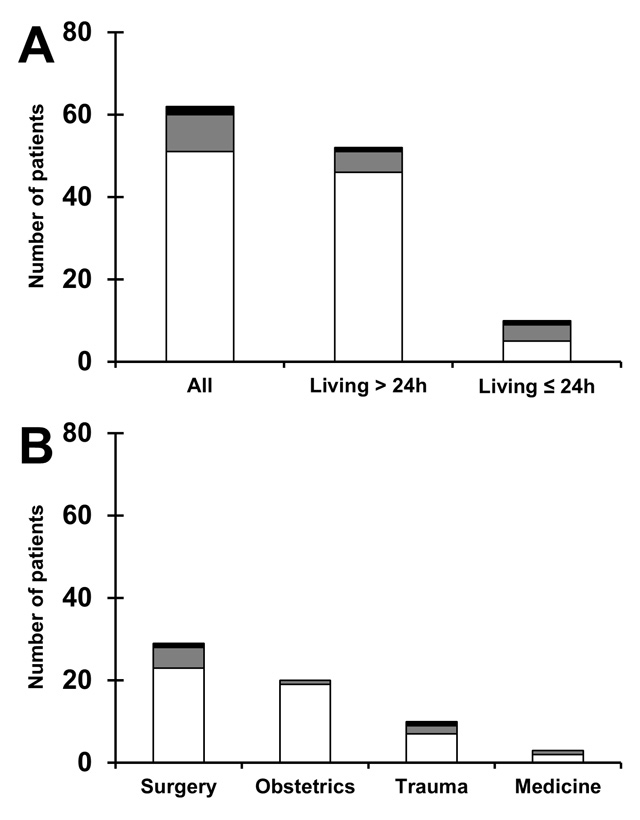
Figure 1
Qualitative bleeding change after application of rFVIIa.
(A) Qualitative change of bleeding 30 minutes after rFVIIa administration is shown for all patients with known information (n = 62), patients living longer than one day (n = 52), and patients deceased within 24 hours after application of rFVIIa (n = 10). (B) Same information according to patient category. Patients with decreased bleeding are shown in white, patients without change of bleeding are shown in grey, and patients with increased bleeding after rFVIIa are shown in black.
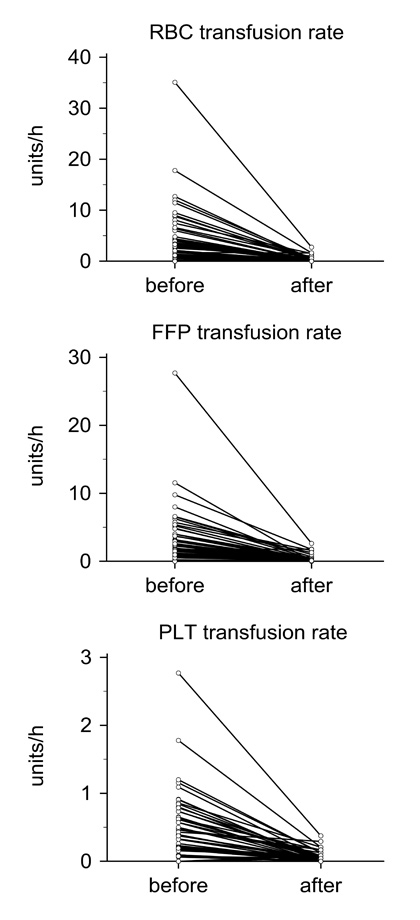
Figure 2
Transfusion requirement before and after rFVIIa.
Change in transfusion rates of packed red blood cells (RBC), fresh frozen plasma (FFP), and platelet concentrates (PLT) from a time window of 4.9 hours (median; range: 2.5–8.8) before administration of rFVIIa compared to 24 hours thereafter for patients living more than one day after treatment (n = 63).
Treatment before the use of rFVIIa
Haemostatic drugs employed to control bleeding are shown in table 3. Tranexamic acid was used in 27% of patients. Protamine sulphate and aprotinin (before retraction) were predominantly used in patients after extracorporeal circulation (ECC). Desmopressin was mainly used in hepatic surgery. The number of surgical interventions to control bleeding included currently on-going surgery when rFVIIa was used during the operation (table 3). Although therapeutic interventions before administration of rFVIIa could prevent a further deterioration of haemoglobin and coagulation parameters (table 4, compare “Closest to start of bleeding” with “Before rFVIIa”), they could not satisfactorily control bleeding.
Application of rFVIIa
The first dose of rFVIIa was 64 μg/kg (median; IQR 56–71) and was applied 4.9 hours (median; IQR 2.5–8.8) after onset of bleeding. In 58/73 patients (79%) only a single dose was used; 13/73 patients (18%) received a second dose of 64 μg/kg (median; IQR 56–75). Only two patients (2.7%) got rFVIIa more than twice (table 3).
Effect of the use of rFVIIa
The severity of bleeding was reduced in 82% of the patients after application of rFVIIa (fig. 1). The estimated bleeding rate decreased from 2750 mL/h (median; IQR 1000–4000) to 300 mL/h (IQR 50–2000; n = 14; p= 0.0005). The number of transfused RBC, FFP and PLT units (table 3) was significantly lower during the 24 hours after rFVIIa compared to the 4.9 hours (median; IQR 2.5–8.8) before rFVIIa (n = 73; p<0.0001, p<0.0001, p= 0.0094, respectively). The transfusion rates before and 24 hours after rFVIIa are shown in figure 2 for the patients still alive more than one day after treatment (n = 63). Despite greatly decreased transfusion requirements, haemoglobin and haematocrit levels remained stable during the 24 hours after rFVIIa administration (table 4). Coagulation studies show the typical rapid normalisation of PT due to high circulating rFVIIa levels. Of note, there is a spontaneous increase in “clottable” fibrinogen levels from 1.48 g/l (median; IQR 1.22–1.74) just before rFVIIa to 2.76 g/l (median; IQR 1.97-4.76; n = 12; p = 0.0024) 24 hours thereafter also in those patients not receiving further FFP after rFVIIa.
In-hospital mortality on day 1 (10/73, 14%) and up to day 30 (23/73, 32%) is shown in figure 3. Median follow-up time was 2 days (IQR 1–11) for the 23 patients who died during follow-up and 16 days (IQR 11–30; 30 days equals the study limit) for the surviving 50 patients; cumulative follow-up time on all patients was 1048 days. It is noteworthy that no obstetric patient died. Data of all 10 patients who died within 24 hours after administration of rFVIIa were closely reviewed (table 5). rFVIIa could not possibly have been effective in uncontrolled bleeding from larger vessels during surgery or after polytrauma in 4 patients. In five instances, bleeding decreased after rFVIIa but patients died because of other reasons. In one patient with mediastinitis and valvular prosthesis infection (table 5, patient 10) no significant reduction of bleeding after 65 μg/kg rFVIIa was documented and he died following acute myocardial infarction (see below). It is noteworthy that the ten patients who died within 24 hours did not receive rFVIIa later than the other patients; they received the first dose 3.6 hours (median; IQR 2.0–7.2) after onset of bleeding compared to 5.1 hours (median; IQR 2.7–8.9) for the 63 survivors (p= 0.25).
Side effects of the use of rFVIIa
A potential complication of rFVIIa was considered in 6/73 patients (8.2%). Two reports were related to patients who died within 24 hours after rFVIIa administration. One patient died from acute myocardial infarction (table 5, patient 10); although he had pre-existing massive left heart hypertrophy with a postulated reduced myocardial perfusion reserve, the acute myocardial infarction might be considered a rFVIIa side-effect. One patient died from massive pulmonary embolism after polytrauma (table 5, patient 4); postmortem examination revealed central and peripheral fat embolism, thus ruling out a side effect of rFVIIa. Four other patients with suspected rFVIIa related side-effects survived beyond the first 24 hours. In three instances, reported potential rFVIIa complications were either not confirmed or more likely to have occurred for other reasons. In one patient the clinical suspicion of deep vein thrombosis was not confirmed by imaging studies. Another patient did not awake from anaesthesia after surgery of an extended adenocarcinoma of the distal oesophagus; prolonged hypotension to 80/45 mmHg was documented during the massive bleeding episode, suggesting hypoxic brain damage. Another patient developed critical ischaemia of a lower limb, probably related to infrarenal aortic dissection. Finally, one patient developed hemiparesis after ECC for aortic and mitral valve replacement; although stroke is not infrequent after open heart surgery [28], the aetiology of this hemiparesis could not conclusively be determined. In summary, we registered two arterial thromboembolic complications possibly related to the use of rFVIIa: a lethal myocardial infarction (table 5, patient 10) and a post ECC-surgery stroke. None of the 20 patients treated with tranexamic acid (table 3) developed thromboembolic events.
|
Table 3: Treatments applied in 73 patients with massive bleeding. |
|
|
No. of patients (%)
|
Time / Volume / Dose*
|
| Volume replacement and blood products before rFVIIa |
| Time from start of bleeding to
first dose of rFVIIa, h |
– |
4.9 (2.5–8.8) |
| Crystalloids, L |
– |
1.8 (1.0–2.5) |
| Colloids, L |
– |
1.5 (0.5–2.5) |
| RBC, units |
– |
14 (8–22) |
| FFP, units |
– |
11 (6–16) |
| PLT, units |
– |
2 (1–3) |
| Drugs |
| Tranexamic acid, mg |
20 / 73 (27%) |
500 (500–750) |
| Protamine sulphate, x103 U |
13 / 73 (18%) |
35 (25–50) |
| Aprotinine, x106 U |
12 / 73 (16%) |
2.0 (1.3–3.3) |
| Desmopressin, μg |
7 / 73 (10%) |
24 (21–32) |
| Fibrinogen concentrate, g, median (range) |
3 / 73 (4%) |
1.0 (1.0–1.3) |
| Prothrombin complex concentrate, U, value |
1 / 73 (1.5%) |
1200 |
| Use of rFVIIa |
| 1 application |
58 / 73 (79%) |
– |
| 2 applications |
13 / 73 (18%) |
– |
| 3 applications |
1 / 73 (1.5%) |
– |
| 4 applications |
1 / 73 (1.5 %) |
– |
| First rFVIIa application |
| Dose, mg |
73 / 73 |
4.8 (3.6–4.8) |
| Weight adjusted dose, μg/kg |
– |
64 (56–71) |
| Second rFVIIa application |
| Dose, mg |
15 / 73 (21%) |
4.8 (3.6–4.8) |
| Weight adjusted dose, μg/kg |
– |
64 (56–75) |
| Time from 1st to 2nd application, h |
– |
3.7 (1.0–8.0) |
| Replacement of blood components during 24 hours after rFVIIa |
| RBC, units |
– |
2 (0–6) |
| FFP, units |
– |
2 (0–9) |
| PLT, units |
– |
1 (0–2) |
| Surgery |
| No. of surgical interventions, median (IQR, range) |
– |
1 (1–1, 0–3) |
| * All values represent median (IQR) unless indicated otherwise.
FFP = fresh frozen plasma; h = hours; IQR = interquartile range; PLT = platelet concentrates; RBC = packed red blood cells; rFVIIa = recombinant factor VIIa |
|
Table 4: Key laboratory values before and after recombinant factor VIIa administration*. |
| |
Normal values
|
Closest to start of bleeding
|
Before rFVIIa
|
After rFVIIa
|
Day 1 after rFVIIa
|
| Time point of blood sampling relative to application of rFVIIa |
|
– 4.9 h (2.5–8.8) |
– 1.0 h (0.5–1.7) |
+ 1.4 h (0.8–2.3) |
+ 23.9 h (18.9–27.1) |
| Cellular components |
|
|
|
|
|
| Haemoglobin level, g/L |
female: 121–154
male: 135–168 |
74 (59–90)
91 (65–127) |
82 (61–92)
83 (70–95) |
88 (82–103)
94 (86–107) |
88 (84–100)
97 (88–104) |
| Haematocrit, L/L |
female: 0.36–0.44
male: 0.40–0.50 |
0.22 (0.18–0.27)
0.27 (0.22–0.37) |
0.24 (0.18–0.28)
0.25 (0.20–0.28) |
0.25 (0.23–0.29)
0.27 (0.25–0.31) |
0.26 (0.24–0.29)
0.27 (0.25–0.30) |
| Platelet count, x109/L |
140–380 |
118 (80–172) |
82 (66–110) |
76 (54–105) |
74 (54–98) |
| Coagulation parameters |
|
|
|
|
|
| PT, % |
70–130 |
50 (33–85) |
58 (46–73) |
100 (95–100) |
93 (56–100) |
| aPTT, s |
25.0–36.0 |
70.0 (45.3–90.0) |
56.9 (41.6–75.5) |
45.7 (37.3–57.4) |
43.4 (36.3–55.2) |
| Fibrinogen, g/L |
1.75–3.75 |
1.24 (0.89–1.70) |
1.48 (1.21–1.69) |
1.76 (1.39–2.23) |
2.43 (1.92–3.93) |
| Acid-base status |
|
|
|
|
|
| pH |
7.35–7.45 |
7.34 (7.22–7.41) |
7.26 (7.21–7.35) |
7.35 (7.27–7.40) |
n/a |
| * All values represent median (IQR); no. of values is 23-71 dependent on parameter and time point
aPTT = activated partial thromboplastin time; h = hours; IQR = interquartile range; n/a = not available; PT = prothrombin time; rFVIIa = recombinant factor VIIa |
|
Table 5: Detailed analysis of the 10 patients who died during the first 24h after use of recombinant factor VIIa. |
| # |
Diagnosis; cause of bleeding |
Use of rFVIIa |
Cause of death |
Evaluation* |
| Time to dose (h) |
Dose(μg/kg) |
Bleeding after rFVIIa |
| 1 |
Hepatic surgery, metastatic adrenocortical carcinoma; bleeding from hepatic vein |
1.5
2.5 |
55
55 |
no changeno change |
Surgically not controllable abdominal bleeding |
A |
| 2 |
Polytrauma; bleeding from several larger arteries |
2.0 |
55 |
increased |
Hypoxic brain oedema |
A |
| 3 |
Cerebral shooting trauma; disseminated intravascular coagulation |
0.3 |
87 |
no change |
Brain lesion and oedema |
A |
| 4 |
Polytrauma; chest artery and pulmonary bleeding |
3.4 |
64 |
no change |
Central and peripheral pulmonary fat embolism |
A |
| 5 |
Polytrauma, head injury; disseminated intravascular coagulation |
2.1 |
73 |
reduced |
Brain death |
B |
| 6 |
Klatskin tumor, hemi-hepatectomy and Whipple surgery; bleeding from splenic artery |
4.2
18.8 |
67
89 |
reduced
reduced |
Prolonged tissue hypoperfusion with acute multi-organ failure |
B |
| 7 |
Heart transplantation; generalised capillary leak syndrome |
14.7 |
69 |
reduced |
Fulminant graft failure |
B |
| 8 |
Advanced gallbladder carcinoma; disseminated intravascular coagulation |
3.7
7.5 |
60
60 |
reduced |
Sepsis, liver failure |
B |
| 9 |
Septic shock, liver cirrhosis; diffuse bleeding in the left hemithorax |
11.8 |
28 |
reduced |
Cardiac electro-mechanic dissociation, hypoxic brain damage |
B |
| 10 |
Mediastinitis, heart valve prosthesis infection; increasingly coagulation disorder |
7.2 |
65 |
no change |
Massive left heart hypertrophy, Myocardial infarction |
C |
| * Evaluation codes: A = no effect of rFVIIa was possible, due to uncontrolled surgical or traumatic bleeding (n = 4); B = rFVIIa was effective, other cause of death than bleeding (n = 5), C = potential rFVIla side-effect (n = 1). rFVIIa, recombinant factor VIIa. |
Discussion
The present observational cohort study in a mixed population of a tertiary care hospital shows that 60 μg/kg rFVIIa was effective and safe in massive bleeding. Clinically relevant bleeding before the use of rFVIIa was documented by an estimated bleeding rate of 1 L/h, a total volume replacement of median 11.9 L (table 2), and the number of administered blood products: median 14 RBC, 11 FFP and 2 PLT units (table 3). The majority of the patients (79%) received just one application of rFVIIa and only two patients received more than two doses (table 3). After rFVIIa the estimated bleeding was reduced in 82% (fig. 1), significantly fewer blood products were applied (fig. 2 and table 3), haematocrit remained stable and fibrinogen levels increased (table 4), indicating that bleeding was satisfactorily controlled. Our results are consistent with former reports of rFVIIa in off-label indications, which however, used much higher doses [10, 29, 30]. In addition, we observed an in-hospital mortality of 14% during the first 24 hours and 32% at day 30, which compares very well with the hospital mortality rates reported by several recent studies in massive transfusion [31].
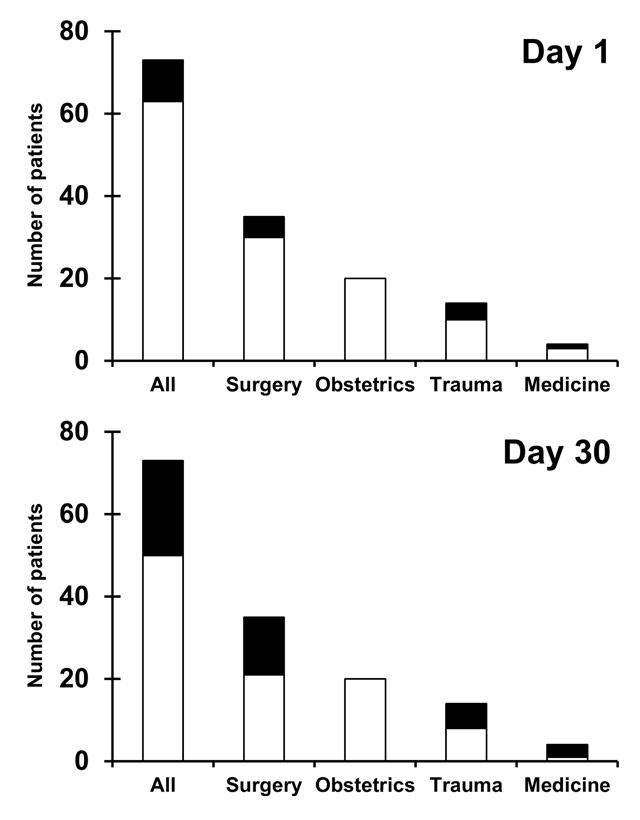
Figure 3
Survival at day 1 and day 30.
Survival and in-hospital mortality data on day 1 and up to day 30 for all patients and for categories of patients after off-label use of recombinant factor VIIa (rFVIIa). Deceased patients are shown in black, living patients are shown in white.
The incidence of possible rFVIIa related thromboembolic complications among the 73 patients in our study – one lethal acute myocardial infarction in a patient with massive left ventricular hypertrophy and a hemiparesis after open heart surgery – was low (2.7%). This is at the lower end of the reported incidence of thromboembolic complications after rFVIIa, ranging from <1% up to 44% [18–21], and compares very well with the rate of 3.2% arterial thromboembolic complications reported in placebo groups [22]. Even if all six recorded complications were considered to be due to rFVIIa, their proportion (6/73, 8.2%) is well in line with the rate of 8.7% thromboembolic events, both venous and arterial, observed among patients who received placebo [22].
Administration of 60 μg/kg rFVIIa theoretically leads to a plasmatic rFVIIa concentration which still is 5–6 times higher than the minimal effective level [26]. A few studies have shown an effect of rFVIIa in even lower doses in specific populations: e.g. Friederich et al. with 20 or 40 μg/kg in patients after prostatectomy [15], Mayer et al. with a minimum of 40 μg/kg in intracerebral haemorrhage [8] or Stein et al. with a fixed dose of 1.2 mg in trauma patients with coagulopathy [12]. The results of the present study indicate that the frequently used dose of 90 μg/kg can be safely reduced to 60 μg/kg in off-label indications. In our experience this dose was able to control bleeding, as subjectively judged by the treating physicians and objectively documented by the number of transfused blood products. In addition, the risk of thromboembolic complications is likely to be lower at a lower rFVIIa dose, as indicated by the significant dose-effect correlation observed among patients treated with rFVIIa for central nervous system bleeding [22]. Finally, rFVIIa efficacy may even be better with lower doses, as shown by a meta-analysis – reporting a significant reduction of transfusion requirements if the rFVIIa dose was ≤ 90 μg/kg but not if it exceeded 90 μg/kg [4] – and suggested by a post-hoc analysis of patients receiving rFVIIa for bleeding after haematopoietic stem-cell transplantation [32].
Limitations of this study
The present study is an observational study and not a randomised placebo controlled trial. Therefore, the effectiveness of rFVIIa compared to placebo cannot be assessed. The number of investigated patients is small and represents a heterogeneous group. Complete laboratory values were available only for a subset of the patients.
Strengths of this study
The present study reflects “real-life” in a tertiary care hospital, at which it had been possible to develop an interdisciplinary consensus guideline. All patients receiving off-label rFVIIa during a three year period were included in our registry. Complete data for demographics, clinical charts, procoagulant-drug treatment, transfusion requirement, qualitative bleeding, observed complications, and mortality were available. Of particular note: 1. We extended the observational period till death, hospital-discharge or day 30, whichever came first; 2. we present details on all patients who died during the first 24 hours after administration of rFVIIa; and 3. we openly discuss all patients with perceived complications due to rFVIIa.
Hypotheses generated by this study
Firstly, we observed that transfusion requirements significantly decreased after administration of rFVIIa. This suggests that early use of an effective procoagulant drug such as rFVIIa in massive bleeding may actually be cost-efficient – particularly if lower doses can be used [12, 33] – by preventing administration of additional blood products and procoagulant drugs, further surgical interventions, and/or medical complications. Secondly, we have been able to show that rFVIIa could control clinically severe bleeding in patients with median fibrinogen levels of 1.48 g/L (IQR: 1.21–1.69). This suggests that rFVIIa can be effective without the need of aggressive fibrinogen substitution, as indicated by a recent study in a porcine model [34] . None of the 20/73 (27%) patients, who had received tranexamic acid developed thromboembolic events, suggesting that combining low dose rFVIIa with this antifibrinolytic drug is safe. All these hypotheses should be tested in appropriate prospective randomised trials.
Conclusions
This observational single-centre cohort study documents that 60 μg/kg rFVIIa, a significantly lower dose than commonly used, is effective in reducing massive bleeding and transfusion requirements in off-label use, with a low complication rate. The heterogeneous population, which represents everyday practice at a tertiary care hospital, may allow a wide applicability of our study results. However, there is a clear need for large controlled randomised trials powered to evaluate clinical relevant endpoints. rFVIIa should still be used restrictively in off-label indications before having more evidence on efficacy and safety.
PS created the database, collected data, performed the statistical analysis, analysed and interpreted the data, wrote and revised the paper; AM collected data, analysed and interpreted the data, wrote and revised the paper; ML, BR, HPK, HZ, DI, AH, and BL determined the guidelines (table 1) and revised the paper; LA conceived the study, determined the guidelines (table 1), analysed and interpreted the data, wrote and revised the paper; all authors gave final approval.
The authors would like to thank Gabriela Barizzi, Dr. Raffaella Hesse, Therese Jost and Valérie Kurta for the excellent support in data acquisition. The authors acknowledge the work of the staff physicians in returning clinical information with the registry forms.
References
1 Hoots WK. Challenges in the therapeutic use of a “so-called” universal hemostatic agent: recombinant factor VIIa. Hematology Am Soc Hematol Educ Program. 2006:426–31.
2 MacLaren R, Weber LA, Brake H, Gardner MA, Tanzi M. A multicenter assessment of recombinant factor VIIa off-label usage: clinical experiences and associated outcomes. Transfusion. 2005;45:1434–42.
3 Fishman PE, Drumheller BC, Dubon ME, Slesinger TL. Recombinant activated factor VII use in the emergency department. Emerg Med J. 2008;25:625–30.
4 Hsia CC, Chin-Yee IH, McAlister VC. Use of recombinant activated factor VII in patients without hemophilia: a meta-analysis of randomized control trials. Ann Surg. 2008;248:61–8.
5 Johansson PI. Off-label use of recombinant factor VIIa for treatment of haemorrhage: results from randomized clinical trials. Vox Sang. 2008;95:1–7.
6 Lin Y, Stanworth S, Birchall J, Doree C, Hyde C. Use of recombinant factor VIIa for the prevention and treatment of bleeding in patients without hemophilia: a systematic review and meta-analysis. CMAJ. 2011;183:E9–19.
7 Rossaint R, Bouillon B, Cerny V, Coats TJ, Duranteau J, Fernandez-Mondejar E, et al. Management of bleeding following major trauma: an updated European guideline. Crit Care. 2010;14:R52.
8 Mayer SA, Brun NC, Begtrup K, Broderick J, Davis S, Diringer MN, et al. Recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2005;352:777–85.
9 Mayer SA, Brun NC, Begtrup K, Broderick J, Davis S, Diringer MN, et al. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2008;358:2127–37.
10 Boffard KD, Riou B, Warren B, Choong PI, Rizoli S, Rossaint R, et al. Recombinant factor VIIa as adjunctive therapy for bleeding control in severely injured trauma patients: two parallel randomized, placebo-controlled, double-blind clinical trials. J Trauma. 2005;59:8–15.
11 Harrison TD, Laskosky J, Jazaeri O, Pasquale MD, Cipolle M. “Low-dose” recombinant activated factor VII results in less blood and blood product use in traumatic hemorrhage. J Trauma. 2005;59:150–4.
12 Stein DM, Dutton RP, Hess JR, Scalea TM. Low-dose recombinant factor VIIa for trauma patients with coagulopathy. Injury. 2008;39:1054–61.
13 Hyllner M, Houltz E, Jeppsson A. Recombinant activated factor VII in the management of life-threatening bleeding in cardiac surgery. Eur J Cardiothorac Surg. 2005;28:254–8.
14 Franchini M, Lippi G, Franchi M. The use of recombinant activated factor VII in obstetric and gynaecological haemorrhage. BJOG. 2007;114:8–15.
15 Friederich PW, Henny CP, Messelink EJ, Geerdink MG, Keller T, Kurth KH, et al. Effect of recombinant activated factor VII on perioperative blood loss in patients undergoing retropubic prostatectomy: a double-blind placebo-controlled randomised trial. Lancet. 2003;361:201–5.
16 Levy JH. Massive transfusion coagulopathy. Semin Hematol. 2006;43:S59–63.
17 Dutton R, Hauser C, Boffard K, Dimsitts J, Bernard G, Holcomb J, et al. Scientific and logistical challenges in designing the CONTROL trial: recombinant factor VIIa in severe trauma patients with refractory bleeding. Clin Trials. 2009;6:467–79.
18 O’Connell KA, Wood JJ, Wise RP, Lozier JN, Braun MM. Thromboembolic adverse events after use of recombinant human coagulation factor VIIa. JAMA. 2006;295:293–8.
19 Raivio P, Suojaranta-Ylinen R, Kuitunen AH. Recombinant factor VIIa in the treatment of postoperative hemorrhage after cardiac surgery. Ann Thorac Surg. 2005;80:66–71.
20 Mazer CD, Leong-Poi H, Mahoney J, Latter D, Strauss BH, Teitel JM. Vascular injury and thrombotic potential: a note of caution about recombinant factor VIIa. Semin Cardiothorac Vasc Anesth. 2007;11:261–4.
21 Howes JL, Smith RS, Helmer SD, Taylor SM. Complications of recombinant activated human coagulation factor VII. Am J Surg. 2009;198:895–9.
22 Levi M, Levy JH, Andersen HF, Truloff D. Safety of recombinant activated factor VII in randomized clinical trials. N Engl J Med. 2010;363:1791–800.
23 Berkhof FF, Eikenboom JC. Efficacy of recombinant activated Factor VII in patients with massive uncontrolled bleeding: a retrospective observational analysis. Transfusion. 2009;49:570–7.
24 Masud F, Bostan F, Chi E, Pass SE, Samir H, Stuebing K, Liebl MG. Recombinant factor VIIa treatment of severe bleeding in cardiac surgery patients: a retrospective analysis of dosing, efficacy, and safety outcomes. J Cardiothorac Vasc Anesth. 2009;23:28–33.
25 Gill R, Herbertson M, Vuylsteke A, Olsen PS, von Heymann C, Mythen M, et al. Safety and efficacy of recombinant activated factor VII: a randomized placebo-controlled trial in the setting of bleeding after cardiac surgery. Circulation. 2009;120:21–7.
26 Monroe DM, Hoffman M, Oliver JA, Roberts HR. Platelet activity of high-dose factor VIIa is independent of tissue factor. Br J Haematol. 1997;99:542–7.
27 Alberio L, Lammle B, Esmon CT. Protein C replacement in severe meningococcemia: rationale and clinical experience. Clin Infect Dis. 2001;32:1338–46.
28 Barber PA, Hach S, Tippett LJ, Ross L, Merry AF, Milsom P. Cerebral ischemic lesions on diffusion-weighted imaging are associated with neurocognitive decline after cardiac surgery. Stroke. 2008;39:1427–33.
29 Filsoufi F, Castillo JG, Rahmanian PB, Scurlock C, Fischer G, Adams DH. Effective management of refractory postcardiotomy bleeding with the use of recombinant activated factor VII. Ann Thorac Surg. 2006;82:1779–83.
30 Isbister J, Phillips L, Dunkley S, Jankelowitz G, McNeil J, Cameron P. Recombinant activated factor VII in critical bleeding: experience from the Australian and New Zealand Haemostasis Register. Intern Med J. 2008;38:156–65.
31 Sihler KC, Napolitano LM. Massive transfusion: new insights. Chest. 2009;136:1654–67.
32 Pihusch M, Bacigalupo A, Szer J, von Depka Prondzinski M, Gaspar-Blaudschun B, Hyveled L, et al. Recombinant activated factor VII in treatment of bleeding complications following hematopoietic stem cell transplantation. J Thromb Haemost. 2005;3:1935–44.
33 Kissela BM, Eckman MH. Cost effectiveness of recombinant factor VIIa for treatment of intracerebral hemorrhage. BMC Neurol. 2008;8:17.
34 Grottke O, Braunschweig T, Zimmermann L, Kopp R, Lauritzen B, Coburn M, et al. Recombinant factor VIIa reduces bleeding after blunt liver injury in coagulopathic, hypofibrinogenaemic pigs. Br J Anaesth. 2010;105:789–97.
35 Kenet G, Walden R, Eldad A, Martinowitz U. Treatment of traumatic bleeding with recombinant factor VIIa. Lancet. 1999;354:1879.
36 Martinowitz U, Kenet G, Segal E, Luboshitz J, Lubetsky A, Ingerslev J, et al. Recombinant activated factor VII for adjunctive hemorrhage control in trauma. J Trauma. 2001;51:431–8.
37 O’Connell NM, Perry DJ, Hodgson AJ, O’Shaughnessy DF, Laffan MA, Smith OP. Recombinant FVIIa in the management of uncontrolled hemorrhage. Transfusion. 2003;43:1711–6.


