Figure 1
DOI: https://doi.org/10.4414/smw.2011.13235
Practical consensus recommendations
Ulcerative colitis (UC) is a chronic relapsing and remitting inflammatory disorder of the gastrointestinal tract, and, besides Crohn’s disease (CD), it is one of the two major forms of inflammatory bowel disease (IBD) [1].
The aetiology of UC remains unclear. Medical therapies to completely cure the disease are not available. Genetic, immunologic and environmental factors are likely to play a role in UC pathogenesis. Currently, the most widely accepted hypothesis implicates a combination of the following factors: immune deregulation (caused by genetic or environmental factors), abnormal gastrointestinal (GI) tract luminal factors, (such as microorganisms constituting the GI tract flora, oxidative stress, and tumour necrosis factor (TNF) -alpha) and defects in the GI mucosal barrier that allow luminal factors to penetrate into the mucosa [2–7].
The clinical presentation of UC is characterised by abdominal pain, diarrhoea with or without hematochezia, and mucosal ulcerations [8]. The extent of disease varies from limited involvement of the rectum to involvement of the whole colon, and seldom of the terminal ileum (backwash ileitis).
Clinically, mild disease is associated with fewer than 4 bowel movements per day, with or without bloody stools but without systemic manifestations and blood tests are usually normal. Moderate disease is associated with more than 4 bowel movements a day with minimal systemic manifestations. The simplest clinical measure to distinguish moderate from mildly active colitis is the presence of mucosal friability (bleeding on light contact with the rectal mucosa at sigmoidoscopy) [9]. Severe disease describes disease associated with more than 6 bowel movements a day, with blood in the stool and with systemic involvement [10, 11] (table 1).
During relapses or flares, pharmacological or surgical intervention is needed to re-establish symptom free remission. Ideally, medical strategies are employed to maintain patients in long-term remission, while minimising steroid dependence and therapy-related toxicity.
While most acute flares of UC are mild and manageable on an ambulatory basis, more severe flares requiring hospitalisation appear in about 15% of all cases [12]. Thirty to forty years ago, mortality in these patients reached up to 25%. With the introduction of intravenous corticosteroids, modern intensive care treatment, earlier recognition of treatment failure and advanced surgical techniques, mortality has decreased to less than one percent in specialised centres [11, 13, 14].
In this review, we focus on the treatment of patients with moderate to severe UC. Four typical clinical scenarios are defined and discussed in detail. The recommendations are based on the current literature and published guidelines and reviews [15–20] (up to end of 2009). They were finally discussed at a consensus meeting (22nd June 2010) with the above mentioned IBD experts. Based on the discussion, a comprehensive treatment algorithm could be developed and was agreed upon by the board members. These treatment algorithms are aimed for daily clinical practice at colleagues who regularly treat patients with UC.
| Table 1: Disease Activity in UC, adapted from Truelove and Witts (11) | |||
| Patient characteristics | Mild | Moderate | Severe |
| Light clinical symptoms, but which can be worsened by the subjective perception of the patient. Especially urgencies contribute to a more severe perception of the disease. Localization of the disease is important as topical treatments will have to be adjusted accordingly. | Increased clinical symptoms but only mild systemic symptoms. Steroid use is a good criterion to classify a patient as moderate (or severe). Presence of mucosal friability (bleeding on light contact with the rectal mucosa on sigmoidoscopy). | Severe active ulcerative colitis is best defined by Truelove and Witts' Criteria. Patients should be admitted to hospital for intensive treatment | |
| Bloody stools per day | < 4 | ≥ 4 | ≥ 6 |
| Pulse | < 90 bpm | ≤ 90 bpm | > 90 bpm |
| Temperature | < 37.5 °C | ≤ 37.5 °C | > 37.8 °C |
| Hemoglobin | > 11.5 g/dl | ≥ 10.5 g/dl | < 10.5 g/dl |
| ESR | < 20 mm/h | ≤ 30 mm/h | > 30 mm/h |
| CRP | Normal | ≤ 30 mg/L | > 30 mg/L |
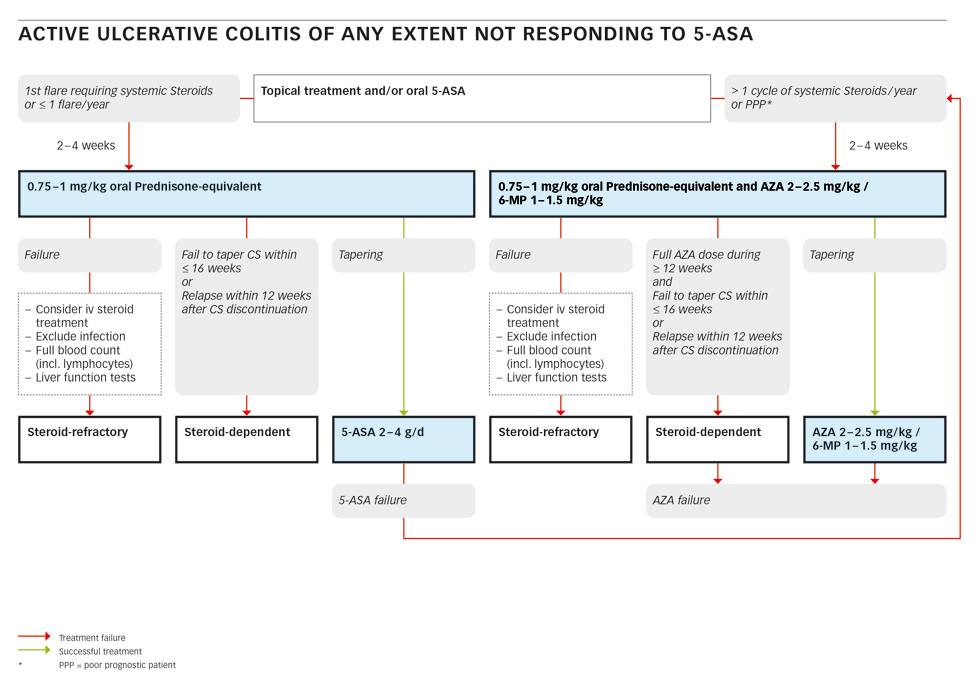
The first line treatment of mild to moderate left sided UC or pancolitis is combined topical and oral aminosalicylates [20]. The combination of oral and rectal mesalamine seems to be superior to either therapy alone in patients with extensive colitis [21]. 5-ASA is superior to placebo for induction of remission. In cases of failure to induce global or clinical improvement or remission, the pooled Peto odds ratio is 0.40 (95% confidence interval (CI), 0.30 to 0.53) [22].

Figure 1
Patients with a first flare or patients with a maximum of one flare per year requiring systemic steroids should be started on 0.75–1 mg/kg of oral prednisone-equivalent for two to four weeks [15, 20, 23–25]. After successful tapering of the steroids, maintenance therapy with topic and/or oral 5-ASA can be re-established [22, 26–28]. If remission cannot be maintained, the next course of oral steroids should be combined with azathioprine (AZA) or mercaptopurine (6-MP) at 2–2.5 mg/kg or 1–1.5 mg/kg, respectively [15, 20, 29, 30].
In a patient not responding to oral steroids, infection must be excluded. Stool cultures for bacteria and parasites and an assay for clostridium (Cl.) difficile must be obtained [31]. Sigmoidoscopy should be performed to confirm the diagnosis and to exclude cytomegalovirus (CMV) infection [32, 33]. If infection is excluded, treatment failure in this situation is called steroid-refractory. This topic will be discussed in the corresponding section below.
If a patient fails to taper corticosteroids within less than 16 weeks or a patient relapses within 12 weeks after corticosteroid discontinuation they are considered to be steroid-dependent (see the corresponding section below).
In a patient requiring more than one cycle of systemic steroids per year or in a patient with a poor projected prognosis (e.g., young age, severe first flare, severe systemic inflammation, slow response to steroids), oral prednisone-equivalent should be combined with azathioprine (AZA) or mercaptopurine (6-MP) at 2–2.5 mg/kg or 1–1.5 mg/kg, respectively [15, 20, 29, 30].
If tapering of the steroids is successful, maintenance-therapy with AZA/6-MP at the mentioned dose should be continued. In case of failure of this treatment including a trial with intravenous steroids (and excluding infection), the patient is considered as steroid-refractory. Further treatment will be discussed in the corresponding section. If a patient has had a full dose of AZA/6-MP for more than 12 weeks and fails to taper corticosteroids within 16 weeks, or relapses within 12 weeks after cessation of steroids, they are considered as steroid-dependent and an AZA/6-MP failure and should be treated according to the appropriate algorithm below.
Steroid-dependent ulcerative colitis defines a patient who fails to taper steroids below 10mg within 16 weeks from a starting dose of 0.75–1 mg/kg oral prednisone-equivalent, or who relapses within 12 weeks after steroid discontinuation [31]. In this situation, compliance to 5-ASA must be checked first. Infection as a cause for therapy-failure must be ruled out by appropriate stool tests. CMV colitis should be excluded by sigmoidoscopy with biopsies. Before introducing azathioprine or mercaptopurine, a full blood count including absolute lymphocyte numbers must be obtained. Measurement of the activity of Thiopurin-S-Methyltransferase (TPMT) can be considered to identify patients at high risk for severe myelodepression under therapy with thiopurines [34–36]. Alternatively, regular testing of the full white blood count (WBC) is mandatory. Azathioprine is significantly more effective than mesalazine at inducing clinical and endoscopic remission in the treatment of steroid-dependent UC [30].
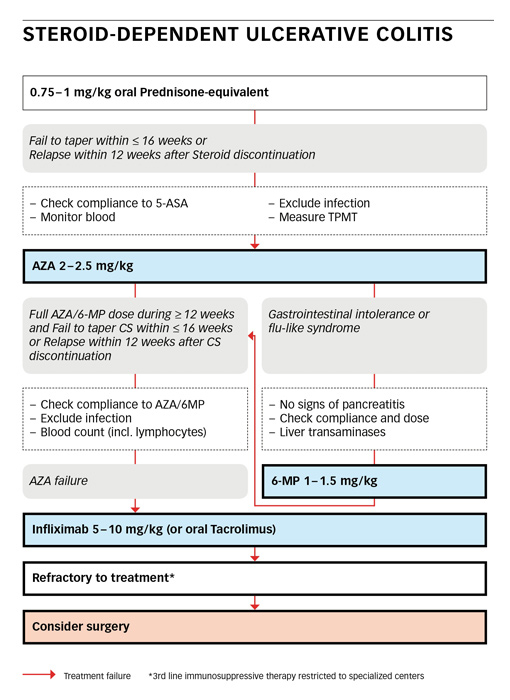
Figure 2
Any patient with steroid-dependent ulcerative colitis or with an early relapse is best started on azathioprine (AZA) at 2-2.5 mg/kg. In case of gastrointestinal intolerance or a flu-like syndrome, after exclusion of pancreatitis or hepatopathy, a switch to mercaptopurine (6-MP) at 1–1.5 mg/kg can be done. AZA/6-MP needs at least 12 weeks to be fully effective.
AZA/6-MP failure is present, if a patient fails to taper corticosteroids after 12–24 weeks of full dose therapy or if the patient relapses within 12 weeks after steroid tapering. Again, compliance must be secured and infection ruled out. In this clinical scenario, induction treatment with infliximab at 5–10 mg/kg can be considered with infusions given at week 0, 2 and 6 followed by an infusion every 8 weeks [37]. As an alternative, oral tacrolimus at 0.1–0.2 mg/kg may be tried [16, 38]. The targeted trough level for induction therapy is 10–15 ng/ml [39]. A patient who is still refractory to treatment should be referred to a specialised centre with profound experience in IBD and adequate infrastructure. Surgery must always be considered as an alternative in the refractory patient.
A patient not responding to 0.75–1 mg/kg of oral prednisone-equivalent within two to four weeks is defined as steroid-refractory ulcerative colitis [10, 31]. This diagnosis is made after exclusion of an infection and is best re-assessed by colonoscopy and biopsy to confirm the diagnosis and/or to rule out complications.
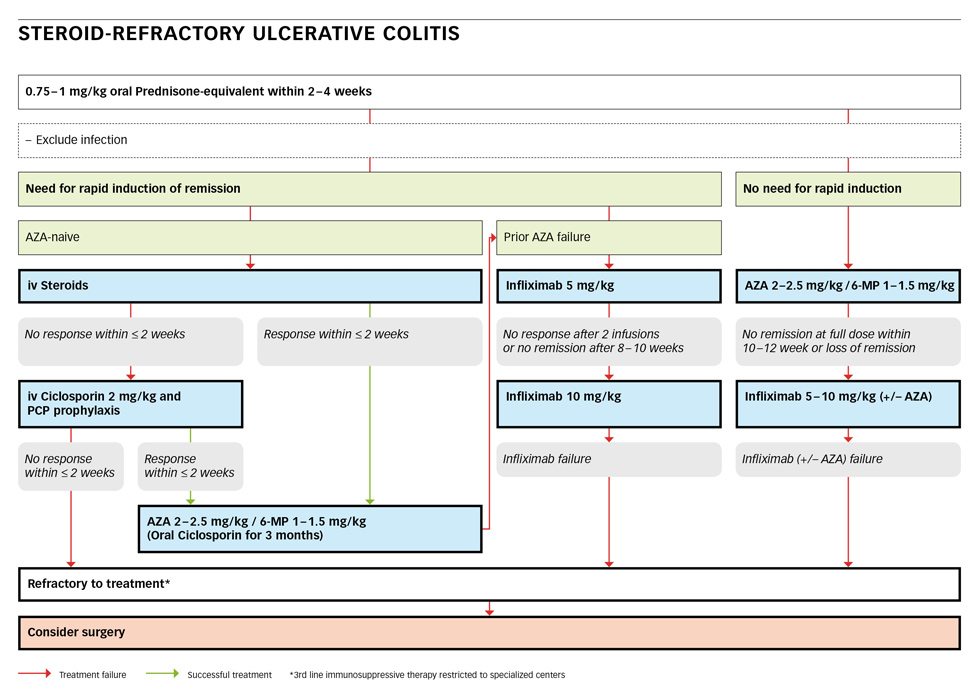
Figure 3
If the patient is in a stable clinical condition without the need for rapid induction, AZA at 2–2.5 mg/kg or 6-MP at 1–1.5 mg/kg should be added [20]. If there is still no remission after a full dose within 12–24 weeks, a switch to infliximab at 5–10 mg/kg at week 0, 2 and 6 followed by an infusion every 8 weeks [17, 37, 40] should be considered. If the patient is still refractory to this treatment, a third-line immunosuppressive therapy or surgery must be considered. This patient should or must be referred to a specialised centre.
If there is a need for rapid induction of remission in a severely ill patient and the patient is AZA-naïve, steroids must be given intravenously. Intravenous (iv) steroids (such as 60 mg of methylprednisolone or 400 mg of hydrocortisone daily) remain the mainstay of conventional therapy [20, 41]. Higher doses are not more effective, but lower doses are less effective [42, 43]. The expected overall response rate of steroids is 67% [43]. In case of response to treatment, AZA at 2–2.5 mg/kg or 6-MP at 1–1.5 mg/kg should be added and steroids can be slowly tapered. If there is no response to iv-steroids after 2 weeks, intravenous cyclosporin at a dose of 2 mg/kg should be started [20, 44, 45]. Cholesterol levels should be checked before the start of cyclosporine. A prophylaxis against pneumocystis jiroveci may be installed [46, 47] as a combined immunosuppression entails an increased risk. In case of response to i.v. cyclosporine, AZA at 2–2.5 mg/kg or 6-MP at 1–1.5 mg/kg should be added and oral cyclosporine should be continued for at least three month as a bridging therapy [16]. In case of non- response within two weeks, third-line immunosuppressive therapy or surgery must be considered at a specialised centre.
If a patient was a prior AZA/6-MP failure, infliximab at 5 mg/kg can be started [48–52]. In case of lack of response after two infusions or no remission after ten weeks, the dose should be doubled to 10 mg/kg. In a patient still not responding, third-line immunosuppressive therapy or surgery must be considered at a specialised centre.
Acute severe or fulminant ulcerative colitis is best defined according to the index of Truelove and Witts (table 1) [11]. It defines a patient with a bloody stool frequency of more than five per day AND tachycardia >90 bpm, OR a temperature >37.8°, OR anaemia with a haemoglobin <10.5 g/dl, OR an elevated CRP (>30 mg/l) or ESR (>30 mm/h), respectively. A patient fitting these criteria must be admitted to hospital and needs intensive treatment.
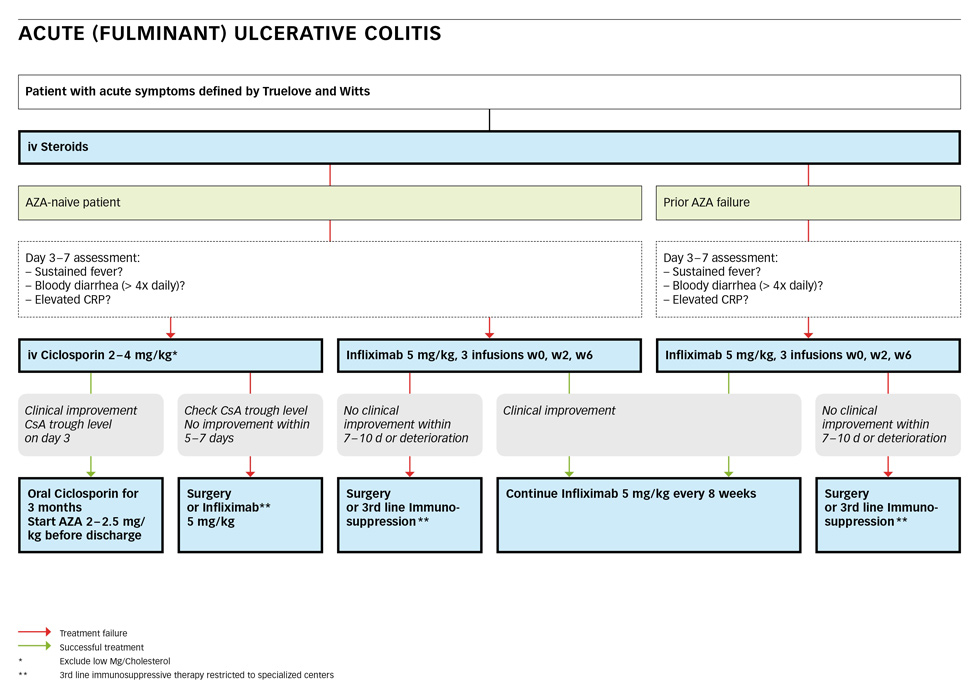
Figure 4
First line treatment is intravenous application of steroids such as 60 mg of methylprednisolone or 400 mg of hydrocortisone daily [20, 41]. If the AZA-naïve patient does not respond (sustained fever, bloody diarrhoea more than 4 times daily or continued elevated CRP) on day 3–7, iv ciclosporin or infliximab should be started [20]. Cyclosporin is given at a dose of 2 mg/kg [20, 44, 45] after exclusion of hypomagnesaemia. Cyclosporin trough levels must be checked on day 3, dependent on the assay and the local laboratory (it should be between 300 and 500 ng/ml). In case of clinical improvement, AZA at 2–2.5 mg/kg or 6-MP at 1–1.5 mg/kg should be started before discharge and oral cyclosporin should be continued for at least three months as a bridging therapy [16]. If no improvement is achieved within 5–7 days, infliximab may be tried or colectomy must be performed.
As an alternative to cyclosporine, infliximab may be started with 5 mg/kg at week 0, 2 and 6 [48–52, 54]. If no clinical improvement occurs within 7–10 days or a deterioration is developing, surgery must be considered. In case of clinical improvement, infliximab should be continued at 5 mg/kg every 8 weeks.
Patients who are already on a therapy with AZA/6-MP at the time of their severe flare seem to respond poorly to cyclosporin [55, 56]. If they fail iv-steroids, infliximab should be started at 5 mg/kg and continued every 8 weeks if an improvement occurs. In case of failure or deterioration, surgery must be considered. Tacrolimus may be an alternative in specialised centres [20, 39, 57, 58].
The care for a patient with moderate to severe ulcerative colitis remains challenging despite the fact that morbidity and mortality rates have been considerably reduced during the last 30 years. 5-ASA, thiopurines and infliximab are well established treatment regimens for the maintenance of remission. 5-ASA are considered the standard in treating mild to moderate UC. For inducing remission in refractory patients and in severe disease cases, corticosteroids remain the first line therapy. With the administration of cyclosporin and infliximab as rescue therapies in severe refractory cases, morbidity and mortality can be clearly reduced. The early detection and/or the prophylaxis of complications, better intensive care and the improvement of anaesthesiological and surgical techniques have contributed to better outcomes. One of the most important factors remains the close teamwork between the gastroenterologist and the surgeon at all times during the clinical course in order not to miss the best timing for colectomy, which will still be necessary in about 30% of the patients with severe ulcerative colitis.
1 Silverberg MS, Satsangi J, Ahmad T, Arnott ID, Bernstein CN, Brant SR, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: Report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Canadian journal of gastroenterology = Journal canadien de gastroenterologie. 2005;19(Suppl A):5–36.
2 Kucharzik T, Maaser C, Lugering A, Kagnoff M, Mayer L, Targan S, et al. Recent understanding of IBD pathogenesis: implications for future therapies. Inflamm Bowel Dis. 2006;12(11):1068–83.
3 Rahimi R, Nikfar S, Abdollahi M. Meta-analysis technique confirms the effectiveness of anti-TNF-alpha in the management of active ulcerative colitis when administered in combination with corticosteroids. Med Sci Monit. 2007;13(7):PI13–8.
4 Rahimi R, Nikfar S, Larijani B, Abdollahi M. A review on the role of antioxidants in the management of diabetes and its complications. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2005;59(7):365–73.
5 Rezaie A, Khalaj S, Shabihkhani M, Nikfar S, Zamani MJ, Mohammadirad A, et al. Study on the correlations among disease activity index and salivary transforming growth factor-beta 1 and nitric oxide in ulcerative colitis patients. Ann N Y Acad Sci. 2007;1095:305–14.
6 Jahanshahi G, Motavasel V, Rezaie A, Hashtroudi AA, Daryani NE, Abdollahi M. Alterations in antioxidant power and levels of epidermal growth factor and nitric oxide in saliva of patients with inflammatory bowel diseases. Dig Dis Sci. 2004;49(11-12):1752–7.
7 Rezaie A, Parker RD, Abdollahi M. Oxidative stress and pathogenesis of inflammatory bowel disease: an epiphenomenon or the cause? Dig Dis Sci. 2007;52(9):2015–21.
8 Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. 2007;369(9573):1641–57.
9 E.F. Stange SPLT, S. Vermeire, W. Reinisch, K. Geboes, A. Barakauskiene, R. Feakins, et al., for the European Crohn’s and Colitis Organisation (ECCO). European evidence-based Consensus on the diagnosis and management of ulcerative colitis: Definitions and diagnosis. Journal of Crohn’s and Colitis. 2008;2(1):1–23.
10 D’Haens G, Sandborn WJ, Feagan BG, Geboes K, Hanauer SB, Irvine EJ, et al. A review of activity indices and efficacy end points for clinical trials of medical therapy in adults with ulcerative colitis. Gastroenterology. 2007;132(2):763–86.
11 Truelove SC, Witts LJ. Cortisone in ulcerative colitis; final report on a therapeutic trial. British medical journal. 1955;2(4947):1041–8.
12 Farmer RG, Easley KA, Rankin GB. Clinical patterns, natural history, and progression of ulcerative colitis. A long-term follow-up of 1116 patients. Dig Dis Sci. 1993;38(6):1137–46.
13 Flatmark A, Fretheim B, Gjone E. Early colectomy in severe ulcerative colitis. Scand J Gastroenterol. 1975;10(4):427–31.
14 Sonnenberg A. Time trends of mortality from Crohn's disease and ulcerative colitis. Int J Epidemiol. 2007;36(4):890–9.
15 Travis SPL, Lémann M, Øresland T, Bemelman WA, Chowers Y, Colombel JF, et al., for the European Crohn’s and Colitis Organisation (ECCO) European evidence-based Consensus on the management of ulcerative colitis: Current management. Journal of Crohn’s and Colitis. 2008;2(1):24–62.
16 Ng SC, Kamm MA. Therapeutic strategies for the management of ulcerative colitis. Inflamm Bowel Dis. 2009;15(6):935–50.
17 Panaccione R, Rutgeerts P, Sandborn WJ, Feagan B, Schreiber S, Ghosh S. Review article: treatment algorithms to maximize remission and minimize corticosteroid dependence in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2008;28(6):674–88.
18 Carter MJ, Lobo AJ, Travis SP. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2004;53(Suppl 5):V1–16.
19 Hoffmann JC, Zeitz M, Bischoff SC, Brambs HJ, Bruch HP, Buhr HJ, et al. Diagnosis and therapy of ulcerative colitis: results of an evidence based consensus conference by the German society of Digestive and Metabolic Diseases and the competence network on inflammatory bowel disease. Z Gastroenterol. 2004;42(9):979–83.
20 Travis SPL, Stange EF, Lémann M M, Øresland T, Bemelman WA, Chowers Y, et al. European evidence-based Consensus on the management of ulcerative colitis: Current management. Journal of Crohn’s and Colitis. 2008;2(1):24–62.
21 Marteau P, Probert CS, Lindgren S, Gassul M, Tan TG, Dignass A, et al. Combined oral and enema treatment with Pentasa (mesalazine) is superior to oral therapy alone in patients with extensive mild/moderate active ulcerative colitis: a randomised, double blind, placebo controlled study. Gut. 2005;54(7):960–5.
22 Sutherland L, Macdonald JK. Oral 5-aminosalicylic acid for induction of remission in ulcerative colitis. Cochrane database of systematic reviews (Online). 2006(2):CD000543.
23 Lichtenstein GR, Sbreu MT, Cohen R, Tremaine W. American Gastroenterological Association Institute technical review on corticosteroids, immunomodulators, and infliximab in inflammatory bowel disease. Revista de gastroenterologia de Mexico. 2006;71(3):351–401.
24 Ho GT, Chiam P, Drummond H, Loane J, Arnott ID, Satsangi J. The efficacy of corticosteroid therapy in inflammatory bowel disease: analysis of a 5-year UK inception cohort. Aliment Pharmacol Ther. 2006;24(2):319–30.
25 Truelove SC, Witts LJ. Cortisone in ulcerative colitis; preliminary report on a therapeutic trial. British medical journal. 1954;2(4884):375–8.
26 Sutherland L, Macdonald JK. Oral 5-aminosalicylic acid for maintenance of remission in ulcerative colitis. Cochrane database of systematic reviews (Online). 2006(2):CD000544.
27 Bebb JR, Scott BB. How effective are the usual treatments for ulcerative colitis? Aliment Pharmacol Ther. 2004;20(2):143–9.
28 Kane SV, Bjorkman DJ. The efficacy of oral 5-ASAs in the treatment of active ulcerative colitis: a systematic review. Rev Gastroenterol Dis. 2003 Fall;3(4):210–8.
29 Kirk AP, Lennard-Jones JE. Controlled trial of azathioprine in chronic ulcerative colitis. British medical journal (Clinical research ed. 1982;284(6325):1291–2.
30 Ardizzone S, Maconi G, Russo A, Imbesi V, Colombo E, Bianchi Porro G. Randomised controlled trial of azathioprine and 5-aminosalicylic acid for treatment of steroid dependent ulcerative colitis. Gut. 2006;55(1):47–53.
31 Stange EF, Travis SPL, Vermeire S, Reinisch W, Geboes K, Barakauskiene A, et al. European evidence-based Consensus on the diagnosis and management of ulcerative colitis: Definitions and diagnosis. 2008 03/01;2(1):1–23.
32 Dimitroulia E, Spanakis N, Konstantinidou AE, Legakis NJ, Tsakris A. Frequent detection of cytomegalovirus in the intestine of patients with inflammatory bowel disease. Inflamm Bowel Dis. 2006;12(9):879–84.
33 Matsuoka K, Iwao Y, Mori T, Sakuraba A, Yajima T, Hisamatsu T, et al. Cytomegalovirus is frequently reactivated and disappears without antiviral agents in ulcerative colitis patients. Am J Gastroenterol. 2007;102(2):331–7.
34 Bruns T, Stallmach A. Drug monitoring in inflammatory bowel disease: helpful or dispensable? Dig Dis. 2009;27(3):394–403.
35 Teml A, Schaeffeler E, Herrlinger KR, Klotz U, Schwab M. Thiopurine treatment in inflammatory bowel disease: clinical pharmacology and implication of pharmacogenetically guided dosing. Clin Pharmacokinet. 2007;46(3):187–208.
36 Hindorf U, Lindqvist M, Peterson C, Soderkvist P, Strom M, Hjortswang H, et al. Pharmacogenetics during standardised initiation of thiopurine treatment in inflammatory bowel disease. Gut. 2006;55(10):1423–31.
37 Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353(23):2462–76.
38 Fellermann K, Tanko Z, Herrlinger KR, Witthoeft T, Homann N, Bruening A, et al. Response of refractory colitis to intravenous or oral tacrolimus (FK506). Inflamm Bowel Dis. 2002;8(5):317–24.
39 Ogata H, Matsui T, Nakamura M, Iida M, Takazoe M, Suzuki Y, et al. A randomised dose finding study of oral tacrolimus (FK506) therapy in refractory ulcerative colitis. Gut. 2006;55(9):1255–62.
40 Sands BE, Kaplan GG. The role of TNFalpha in ulcerative colitis. J Clin Pharmacol. 2007;47(8):930–41.
41 Truelove SC, Jewell DP. Intensive intravenous regimen for severe attacks of ulcerative colitis. Lancet. 1974;1(7866):1067–70.
42 Rosenberg W, Ireland A, Jewell DP. High-dose methylprednisolone in the treatment of active ulcerative colitis. J Clin Gastroenterol. 1990;12(1):40–1.
43 Turner D, Walsh CM, Steinhart AH, Griffiths AM. Response to corticosteroids in severe ulcerative colitis: a systematic review of the literature and a meta-regression. Clin Gastroenterol Hepatol. 2007;5(1):103–10.
44 Van Assche G, D’Haens G, Noman M, Vermeire S, Hiele M, Asnong K, et al. Randomized, double-blind comparison of 4 mg/kg versus 2 mg/kg intravenous cyclosporine in severe ulcerative colitis. Gastroenterology. 2003;125(4):1025–31.
45 Arts J, D’Haens G, Zeegers M, Van Assche G, Hiele M, D’Hoore A, et al. Long-term outcome of treatment with intravenous cyclosporin in patients with severe ulcerative colitis. Inflamm Bowel Dis. 2004;10(2):73–8.
46 Cohen RD, Stein R, Hanauer SB. Intravenous cyclosporin in ulcerative colitis: a five-year experience. Am J Gastroenterol. 1999;94(6):1587–92.
47 Epple HJ. Therapy- and non-therapy-dependent infectious complications in inflammatory bowel disease. Dig Dis. 2009;27(4):555–9.
48 Ferrante M, Vermeire S, Katsanos KH, Noman M, Van Assche G, Schnitzler F, et al. Predictors of early response to infliximab in patients with ulcerative colitis. Inflamm Bowel Dis. 2007;13(2):123–8.
49 Gisbert JP, Gonzalez-Lama Y, Mate J. Systematic review: Infliximab therapy in ulcerative colitis. Aliment Pharmacol Ther. 2007;25(1):19–37.
50 Jakobovits SL, Jewell DP, Travis SP. Infliximab for the treatment of ulcerative colitis: outcomes in Oxford from 2000 to 2006. Aliment Pharmacol Ther. 2007;25(9):1055–60.
51 Jarnerot G, Hertervig E, Friis-Liby I, Blomquist L, Karlen P, Granno C, et al. Infliximab as rescue therapy in severe to moderately severe ulcerative colitis: a randomized, placebo-controlled study. Gastroenterology. 2005;128(7):1805–11.
52 Kohn A, Daperno M, Armuzzi A, Cappello M, Biancone L, Orlando A, et al. Infliximab in severe ulcerative colitis: short-term results of different infusion regimens and long-term follow-up. Aliment Pharmacol Ther. 2007;26(5):747–56.
53 Manosa M, Lopez San Roman A, Garcia-Planella E, Bastida G, Hinojosa J, Gonzalez-Lama Y, et al. Infliximab rescue therapy after cyclosporin failure in steroid-refractory ulcerative colitis. Digestion. 2009;80(1):30–5.
54 Lees CW, Heys D, Ho GT, Noble CL, Shand AG, Mowat C, et al. A retrospective analysis of the efficacy and safety of infliximab as rescue therapy in acute severe ulcerative colitis. Aliment Pharmacol Ther. 2007;26(3):411–9.
55 Moskovitz DN, Van Assche G, Maenhout B, Arts J, Ferrante M, Vermeire S, et al. Incidence of colectomy during long-term follow-up after cyclosporine-induced remission of severe ulcerative colitis. Clin Gastroenterol Hepatol. 2006;4(6):760–5.
56 Moss AC, Peppercorn MA. Steroid-refractory severe ulcerative colitis: what are the available treatment options? Drugs. 2008;68(9):1157–67.
57 Baumgart DC, Macdonald JK, Feagan B. Tacrolimus (FK506) for induction of remission in refractory ulcerative colitis. Cochrane database of systematic reviews (Online). 2008(3):CD007216.
58 Baumgart DC, Pintoffl JP, Sturm A, Wiedenmann B, Dignass AU. Tacrolimus is safe and effective in patients with severe steroid-refractory or steroid-dependent inflammatory bowel disease – 2a long-term follow-up. Am J Gastroenterol. 2006;101(5):1048–56.
Funding / potential competing interests: The work was supported by an unrestricted grant from MSD. Data interpretation and writing of this report were done in collaboration with the co-authors independently of MSD. Christoph Beglinger is advisory board member at MSD and Abbott and has recieved grant support from MSD, Abbott and Roche. Michael Manz was supported by a grant from Essex. Pierre Michetti served as consultant for MSD, Abbott, UCB, Merck-Serono, Bertex, AstraZeneca. Gerhard Rogler has consulted to Abbott Switzerland, MSD Switzerland, Novartis, Roche, UCB Switzerland, Falk and Tillots; has recieved speaker honoraria from Abbott, Astra Zeneca, Essex/MSD, Falk, Tillots, UCB, Vifor; has recieved grant support from Abbott, Essex/MSD, Falk, Novartis, Roche, Tillots, UCB, Zeller.