Population-based age stratified morbidities of premature infants in Switzerland
DOI: https://doi.org/10.4414/smw.2011.13212
NM
Bajwa, M
Berner, S
Worley, RE
Pfister
Summary
OBJECTIVE: To provide population-based, gestational age (GA) stratified incidence of mortality and morbidities.
METHODS:Population-based prospective observational study of infants born between 23 0/7 and 31 6/7 weeks GA in the years 2000–2004 in all Swiss neonatal intensive care units. Outcomes measured were: mortality, severe intraventricular haemorrhage (IVH), periventricular leukomalacia (PVL), necrotizing enterocolitis (NEC), moderate/severe bronchopulmonary dysplasia (BPD) and free of major complications.
RESULTS: Mortality was 19% of 3083 infants. Mortality (95% CI) decreased from 95% (88%, 99%) at 23 weeks to 3% (2%, 5%) at 31 weeks. Short-term survival free of major complications was 66% (65%, 68%) overall and increased from 2%(0%, 9%) to 89% (87%, 92%). Rate of IVH was 8% (7%, 9%), PVL 2% (2%, 3%), NEC 3% (3%, 4%) and BPD 11% (10%, 12%). Males had more IVH than females (9% vs. 6%). Antenatal steroids were associated with lower mortality (11% vs. 18%) and IVH (5% vs. 12%). Odds of free of major complications (OR, 95%CI) were positive for female gender 1.2 (1.0, 1.5), steroids 1.3 (1.1, 1.5), multiple gestation 1.3 (1.0, 1.6), not small for gestational age 2.7 (2.0, 3.5), and each additional week of GA 1.6 (1.5, 1.7).
CONCLUSION: Mortality and incidence of morbidities known to influence outcome show a weekly decline with increasing gestational age, except for PVL. Gestational age stratified data are a key component for prenatal counselling.
Abbreviations:
GA Gestational Age
BPD Bronchopulmonary Dysplasia
IVH Intraventricular Haemorrhage
PVL Periventricular Leukomalacia
Introduction
The care of the extremely premature infant requires a multidisciplinary and collaborative approach by an experienced perinatal team. The dialogue between parents, obstetricians and neonatologists is complex because the decisions that are taken are of long term consequence [1]. Legally, parents are entitled to make decisions in the best interest of their future newborn but this requires that they are objectively informed on the potential factors influencing outcomes [2, 3]. Up to date mortality and morbidity data specific to the local population must be provided. As the incidence of most neonatal pathologies varies considerably with gestational age (GA), differences of one or two weeks significantly affect mortality and morbidity inside the large strata usually reported as very low birth weight or extremely low birth weight infants. Estimating the risk for each infant with the information available before birth (GA, estimated birth weight, singleton or multiple sex, and prenatal steroid treatment) is more accurate than with the use of GA or birth weight alone [4, 5].
The objective of this study is to provide population-based (Switzerland), age stratified incidences for mortality and morbidities for neonates born below 32 weeks GA.
Methods
Subjects
The Swiss Neonatal Network maintains a database of all Swiss infants born before 32 weeks GA. Our analysis included neonates born between January 1, 2000 and December 31, 2004. To assure that all infants (≥23 weeks GA and alive at the onset of labour), were included in the analysis, the birthing log books or electronic data bases at all participating hospitals were verified by the Swiss Society of Neonatology assuring that still born infants were also included. Mortality rates were based on all infants alive at the onset of labour, whereas morbidity rates were based on all infants admitted to the neonatal intensive care unit (NICU).
Centralised data set
The data were collected through a computerised questionnaire distributed to collaborators from each centre. Completed data forms were centralised and checked for incongruities. Across all participating centres, GA was based on completed weeks and days of gestation based on the first day of the last menstrual period and confirmed by a first trimester ultrasound (performed routinely since 1980) [6].
Among the data collected, we focused on the occurrence of the following diagnoses: 1) mortality defined by death after birth or stillborn; 2) grade 3 and 4 intraventricular haemorrhage (IVH) using the classifications defined by Papile et al. [7], based on the most severe ultrasound result during the hospital stay (per protocol performed by a paediatric radiologist or neonatologist at 24 hours, day 3 and 7 and then every one to two weeks until final discharge); 3) cystic periventricular leukomalacia (PVL) as defined by de Vries et al. [8]; 4) necrotizing enterocolitis (NEC) defined as clinical signs (abdominal distension, bilious aspirates and/or bloody stools) and confirmed by radiographic intramural gas, or at laparotomy; 5) moderate/severe bronchopulmonary dysplasia (BPD) defined as an oxygen requirement at 36 weeks gestational age according to the NICHD consensus conference paper [9] and 6) Free of major complications defined by survival during NICU stay, no BPD, no IVH 3/4, no PVL, and no NEC. Treatment information was available for antenatal steroid administration (defined as at least two doses given 48 hours before birth). Multiples were defined as any non-singleton birth. Small for gestational age (SGA) was defined according to weight <10th percentile for GA(10).
Ethics
Written permission was obtained from the national expert committee for professional confidentiality in medical research to collect data anonymously on stillborn and very low birth weight live born infants for the years 2000 to 2004.
Statistical analysis
Groups were compared on categorical variables using the Pearson’s χ2 test or Fisher’s exact test, as appropriate, and on continuous variables using the two-sample t-test. The Agresti-Coull score method was used to compute 95% confidence intervals (95% CI) for mortality and morbidity rates for the entire cohort and for GA subgroups. To assess the association between GA and mortality rate, we used a logistic regression model with a piecewise linear spline function for GA, using the Hosmer-Lemeshow goodness-of-fit test. A multivariable logistic regression model for complication-free survival was created by entering all variables of interest and then using backward selection to eliminate all variables not significant at the 0.05 level. All analyses were performed on a complete-case basis and all tests were two-tailed and performed at a significance level of 0.05. Analyses were performed using SPSS 15.0 (SPSS Inc, Chicago, IL) and SAS 9.2 software (SAS Institute, Cary, NC).
Results
Among the 368,055 infants born in Switzerland between the years 2000 and 2004, 3083 (0.8% of all births) were born before 32 weeks of GA. 2896 NICU admissions were included in the analysis of secondary outcomes (BPD, IVH, PVL, NEC and free of major complications), after excluding 187 (6%) labour ward deaths (stillborns and deaths occurring shortly after delivery). Missing data for outcomes was less than 0.2% of patients and missing data for antenatal steroid treatment was less than 1%. NICU admissions were more likely to be Caesarean deliveries, to have received antenatal corticosteroids and to be older and higher in birth weight. Singleton birth or gender differences between labour ward deaths and NICU admissions were insignificant (table 1).
The median gestational age in weeks (1st quartile, 3rd quartile) was 29 (27,30) weeks. The median birth weight (1st quartile, 3rd quartile; range) was 1180 g (900 g, 1490 g; 370–3180 g). Of NICU admissions, overall survival at discharge from the hospital was 86% (2503/2896). Overall mortality rates for males were similar to females, 14% (219/1556) vs. 13% (174/1339) respectively (p = 0.40).
|
Table 1: Characteristics at birth. |
|
Variable
|
All Infants
(N = 3083)
|
Infants who received
intensive care
(N = 2896)
|
Infants who did not receive
intensive care
(N = 187)
|
P-Value
|
| Caesarean delivery (%) |
74% |
75% |
43% |
<0.01 |
| Use of antenatal corticosteroids (%) |
61% |
63% |
7% |
<0.01 |
| Singleton birth (%) |
71% |
71% |
77% |
0.27 |
| Female sex (%) |
46% |
46% |
41% |
0.30 |
| Gestational age (wk) |
29 |
29 |
25 |
<0.01 |
| Average birth weight (g) |
1194 g |
1207 g* |
824 g |
<0.01 |
|
*11% of infants receiving intensive care were small for gestational age.
|
Mortality (fig. 1)
The overall mortality rate (95% CI) for infants born at <32 weeks GA in Switzerland was 19% (17%, 20%). Mortality rates were 95% (88%, 99%) for infants born at 23 weeks and decreased to 3% (2%, 5%) for infants born at 31 weeks GA. Between 23 to 26 weeks GA the overall decrease in mortality was 23% for each additional week of GA, which was a 3% daily decrease in mortality. The odds ratio for death (95% CI) was 0.34 (0.28, 0.40, P <0.001) for each additional week meaning that the odds of dying decreased by 66% for each additional week of GA. Between 27 and 31 weeks, the mortality decreased 5% for each week. Odds ratio for death for each additional week of GA was 0.60 (0.55, 0.65 P <0.001) meaning that the odds of dying decreased by 40% for each additional week of GA during this range.
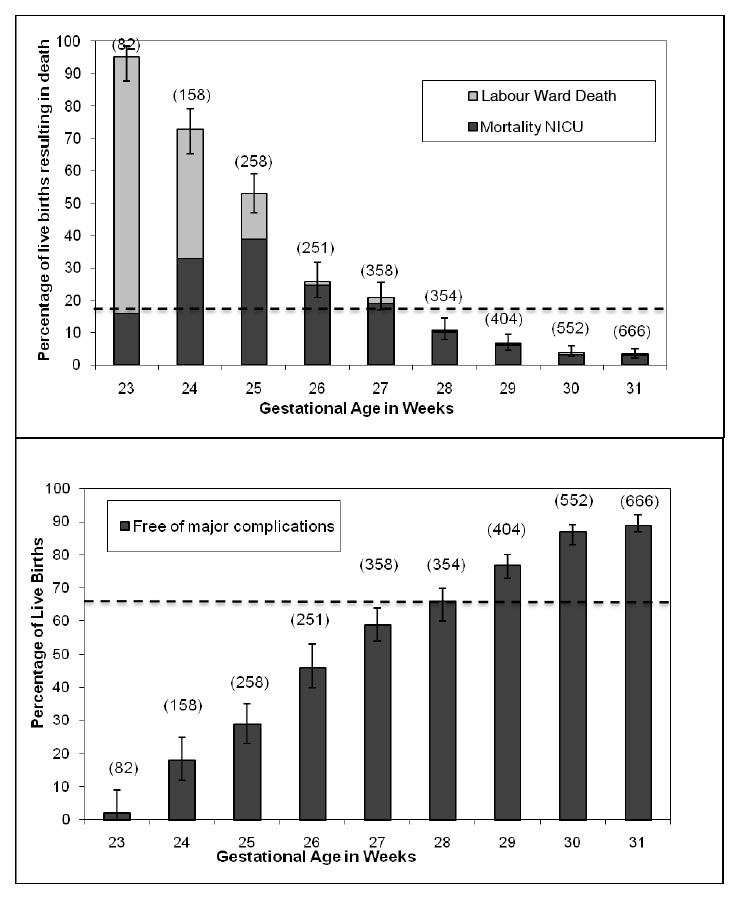
Figure 1
Gestational age stratified mortality rates and rates of free of major complications. CI for mortality is indicated by the error bar. Absolute number of total births by stratum is indicated by the N in (). Overall rate is indicated with the dashed line, 19% mortality rate and 66% free of major complications.
Free of major complications (fig. 1)
The overall rate (95% CI) was 66% (65%, 68%). The incidence of neonates free of major complications increased from 2% (0%, 9%) at 23 weeks to 59% (54%, 64%) at 27 weeks and 89% (87%, 92%) at 31 weeks. The proportion of free of major complications increased with increasing GA. This percentage increased almost linearly by an overall rate of 11% each week.
Morbidity (fig. 2)
2896 infants were admitted to NICU, 8% (221) had grade 3 or 4 IVH, 2.4% (69) had PVL, 3% (100) had NEC, and 11% (318) had moderate/severe BPD. The rates of BPD, IVH, and NEC decreased with increasing GA. Rates of BPD decreased approximately 5% with each additional week of GA after 25 weeks GA. Rates of IVH decreased 3.5% with each additional week of GA. Rates of NEC decreased 1% per week after 24 weeks GA. The rate of PVL varied minimally throughout. The rates for each morbidity stratified by gestational age can be found in figure 2.
Effect of gender, antenatal steroids, multiples, malformations, SGA and GA on individual morbidities
63% (1805/2860) of our study population received a complete course of antenatal steroids. Patients receiving antenatal steroids had lower rates of mortality (11% vs. 18%, P <0.001), IVH (5% vs. 12%, P <0.001), and higher rates of free of major complications (75% vs. 67%, P <0.001) but there was no difference in terms of NEC, BPD or PVL. Males had more IVH than females (9% vs. 6%, P = 0.003) and were less likely to be free of major complications (71% vs. 74%, P = 0.047). Males exposed to antenatal steroids had lower rates of mortality (11% vs. 20%, P <0.001), lower rates of IVH (6% vs. 14%, P <0.001) and higher rates of BPD (13% vs. 9%, P = 0.018) and free of major complications (74% vs. 66%, P = 0.001) than those without steroids. Females exposed to antenatal steroids had lower rates of mortality (11% vs. 16%, P = 0.003) and IVH (4% vs. 9%, P <0.001) and higher rates of free of major complications (78% vs. 68%, P <0.001).
Table 2 summarizes the multivariable model for the effect of the above variables on free of major complications. There was a significant interaction effect between SGA and GA. The association between advancing GA and better outcome was stronger in SGA subjects compared to not SGA subjects. The association between SGA and worse outcome was strongest for subjects with shorter GA.
|
Table 2: Rates of Mortality and Free of Major Complications by Gestational Age. |
|
Gestational age in weeks
|
Number of total births
|
Mortality rate
(95% CI)
|
Labour ward deaths
(95% CI)
|
Free of major complications
(95% CI)
|
| 23–31 combined |
3083 |
19%
(18%, 20%) |
6%
(5%, 7%) |
68%
(66%, 70%) |
| 23 |
82 |
95%
(88%, 99%) |
79%
(69%, 87%) |
2%
(0%, 9%) |
| 24 |
158 |
72%
(65%, 79%) |
40%
(33%,48%) |
19%
(13%, 26%) |
| 25 |
258 |
54%
(47%, 60%) |
15%
(11%, 20%) |
29%
(23%, 35%) |
| 26 |
251 |
26%
(21%, 32%) |
1%
(0%, 4%) |
47%
(41%, 54%) |
| 27 |
358 |
21%
(17%, 26%) |
2%
(1%, 4%) |
61%
(55%, 66%) |
| 28 |
354 |
11%
(8%, 14%) |
1%
(0%, 2%) |
67%
(62%, 72%) |
| 29 |
404 |
7%
(5%, 10%) |
1%
(0%, 2%) |
80%
(75%, 84%) |
| 30 |
552 |
4%
(3%, 6%) |
1%
(0%, 2%) |
88%
(85%, 91%) |
| 31 |
666 |
3%
(2%, 5%) |
0.3%
(0%, 1%) |
91%
(88%, 93%) |
Discussion
Switzerland has a population of approximately 7.5 million people, with an overall infant mortality rate of 4.5 (4.17F, 5.34M) per 1000 live births [11]. The medical system is regionalised with maternal transfer to a perinatal centre beginning at 24 weeks of completed gestation to allow counselling for parents. The Swiss Society of Neonatology recommends that parents of infants born at the limit of viability, 24 0/7 to 25 6/7 weeks GA, are offered initiation of intensive care measures considering the prenatal risk factors [12]: sex, prenatal steroids and singleton or multiple gestations.
Comparison of mortality and morbidity in time and between regions
A survey of premature infants born in 1996 in Switzerland found that the mortality rate at 24 weeks was 86% and 52% at 25 weeks [13]. When comparing our 2000–2004 cohort with that in 1996, the mortality rate at 24 weeks has decreased by around 13%, but the mortality rate between the two time periods for those infants born at 25 weeks has remained stable.
Overall mortality rates were similar to those found by other European groups [14, 15] . However, mortality rates between 23 to 25 weeks gestational age were slightly higher [4, 16–19] . This is most likely a reflection of Swiss guidelines which recommend palliative comfort care before 24 weeks, and offer the option of comfort care to parents until 26 weeks [12]. We had no documentation of positive non-intervention for our cohort. After 26 weeks GA our mortality rates are comparable or better than those found in the literature [14, 16, 18–20].
In terms of morbidity, the reported rates of BPD, IVH, NEC and PVL are comparable in the literature reported global rates as GA stratified data remains limited [15–18, 21].
Importance of prenatal counselling
Having reliable population-based mortality and morbidity data stratified by GA is an essential tool when informing parents of the risks implicated with extreme preterm birth. Guidelines for resuscitation at lower GAs have become an ethical issue where the opinion of the parents and the medical team is of utmost importance [22]. Population-based information aids in making these guidelines evidence-based. Switzerland’s current guidelines for palliative care before the age of 24 weeks is consistent with that of other countries [22]. The information transmitted to future parents is complex and care should be taken to use mortality and morbidity information in a clear manner so that this will aid and not hinder the decision making process. Future parents should also be counselled that decision plans for treatment made before delivery are subject to changes based on the condition of the infant at birth and the success of resuscitation.
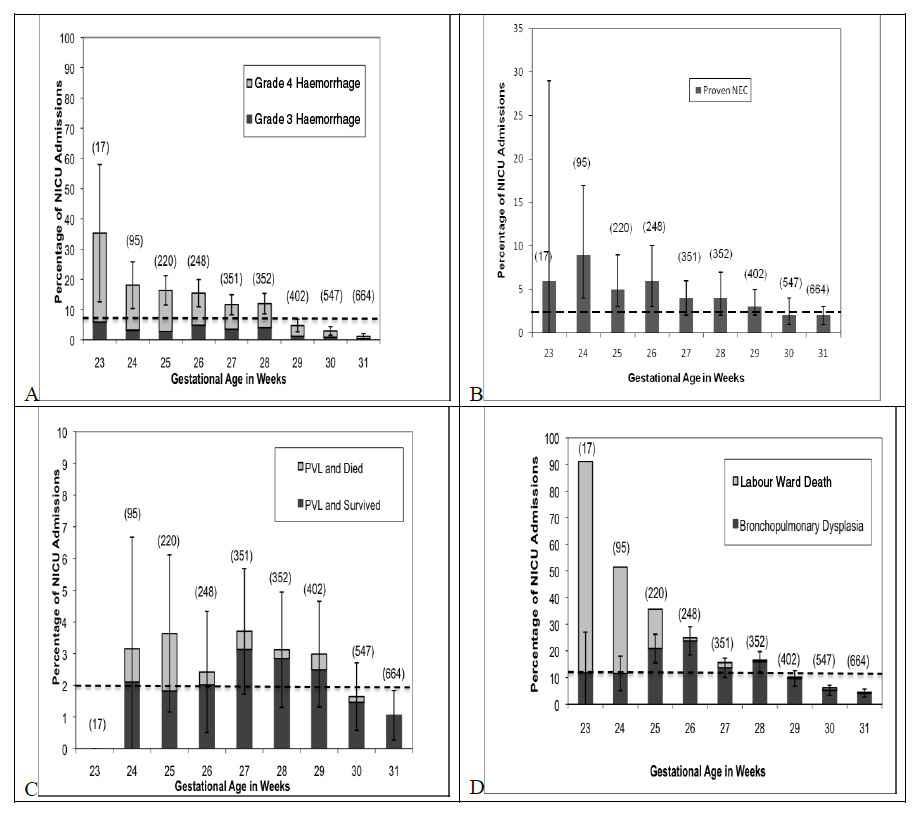
Figure 2
A: Age stratified rates of intraventricular haemorrhage. Overall rate of severe IVH is 8%. B: Age stratified rates of periventricular leukomalacia. Overall rate of PVL is 2%. C: Age stratified rates of necrotizing enterocolitis. Overall NEC rate is 3%. D: Age stratified rates of moderate/severe bronchopulmonary dysplasia. Overall BPD rate is 11.0%.
Confidence intervals are shown by the error bar. Absolute number of NICU admissions is indicated by the N in (). Overall rates are shown by the dashed line.
In counselling parents, the most relevant information communicated before delivery is the mortality rate and the percentage of infants free of major complications based on GA (fig. 1). Precise GA stratified outcomes are relevant and give more precise information compared to weight grouped classification. At the limit of viability, it is crucial that this information be age stratified as the overall rate for a population has a significant bias due to larger numbers of infants being born at a later GA. For example, the overall percentage of infants free of major complications based on traditional birth weight group classification risks giving an overly optimistic picture of outcomes compared to that given when analysing outcomes by GA.
Multivariable models that take into account all information available before delivery such as gestational age, gender, antenatal corticosteroid treatment, singleton or multiple birth and weight should be favoured over those using only one variable [4]. The odds ratios related in table 2 show that being of female sex, having received prenatal steroids, multiple births and not being small for gestational age at all GA strata have a positive influence on the rate of short term free of major complications.
|
Table 3: Rates of IVH, PVL, NEC, and BPD by gestational age. |
|
Gestational age in weeks
|
Number of NICU admissions
|
IVH
(95% CI)
|
PVL
(95% CI)
|
NEC
(95% CI)
|
BPD
(95% CI)
|
| 23–31 combined |
2896 |
8%
(7%, 9%) |
2%
(2%, 3%) |
3%
(3%, 4%) |
11%
(10%, 12%) |
| 23 |
17 |
35%
(13%, 58%) |
0%
(0%, 0%) |
6%
(0%, 29%) |
12%
(-4%, 27%) |
| 24 |
95 |
18%
(10%, 26%) |
3%
(0%, 7%) |
9%
(4%, 17%) |
12%
(5%, 18%) |
| 25 |
220 |
16%
(11%, 21%) |
4%
(1%, 6%) |
5%
(3%, 9%) |
21%
(16%, 26%) |
| 26 |
248 |
15%
(11%, 20%) |
2%
(0%, 4%) |
6%
(3%, 10%) |
24%
(19%, 29%) |
| 27 |
351 |
12%
(8%, 15%) |
4%
(2%, 6%) |
4%
(2%, 6%) |
14%
(10%, 17%9 |
| 28 |
352 |
12%
(9%, 15%) |
3%
(1%, 5%) |
4%
(2%, 7%) |
16%
(12%, 19%) |
| 29 |
402 |
5%
(3%, 7%) |
3%
(1%, 5%) |
3%
(2%, 5%) |
10%
(7%, 13%) |
| 30 |
547 |
3%
(2%, 4%) |
2%
(1%, 3%) |
2%
(1%, 4%) |
5%
(3%, 7%) |
| 31 |
664 |
1%
(0%, 2%) |
1%
(0%, 2%) |
2%
(1%, 3%) |
4%
(3%, 6%) |
Strengths and limitations
Our study is strengthened by several factors. The data is population-based and thus gives the most accurate view of outcomes for our nation and is not biased by individual centre results. Mortality and morbidity statistics are stratified by GA and not by birth weight thus limiting an important bias in prenatal counselling due to the influence of intra-uterine growth retardation on mortality and morbidity [23–25] . Our study included all infants alive at the onset of labour and mortality statistics do not exclude those infants who died in the delivery room in order to eliminate selection bias for survival data at the limits of viability [26].
Despite a five year pooling of population based data, gestational age stratified subgroups for gender, antenatal steroids and multiple pregnancies did not yield sufficient numbers to be analysed meaningfully. In addition, this study could have been improved if we had had precise information on maternal antenatal characteristics and ethnicity data [27], as these factors also have a significant effect on outcomes in studies from other developed countries. However, Switzerland has a relatively homogeneous Caucasian population [28]. Long term outcome data is currently being collected but not yet available. As this information becomes available, it will be necessary to integrate this data into the decision making process before delivery.
Conclusion
Our population-based statistics for the premature neonatal population serve several purposes. GA stratified data will help to counsel parents before a premature delivery and will facilitate the decision making process in the delivery room for the medical staff. This will also allow the surveillance of changes in morbidity and mortality over time. Population based information will permit individual centres to benchmark their own results with that of the Swiss population.
The following investigators and hospitals participated in the Swiss Neonatal Network:
Aarau:Kantonsspital Aarau, Kinderklinik (Georg Zeilinger); Basel:Universitäts-Kinderspital beider Basel, Abteilung fürNeonatologie (Christoph Bührer); Bern:Frauenklinik und Medizinische Kinderklinik, Abteilung für Neonatologie(Mathias Nelle), Inselspital, Abt. für Intensivpflege, Universitätskinderklinik (Bendicht Wagner); Chur:RätischesKantons- und Regionalspital, Kinderklinik (Walter Bär); Lausanne:CHUV, Département de pédiatrie (Adrien Moessinger), CHUV, SI pédiatriques, médico-chirurgicaux (Jacques Cotting); Luzern:Kantonsspital Luzern, Kinderspital Pädiatrie (Thomas M. Berger); St. Gallen:Kantonsspital St. Gallen, Klinik für Geburtshilfe und Gynäkologie (Andreas Malzacher), Ostschweizer Kinderspital, Intensivpflege- und Frühgeburtenstation (John P. Micallef); Zürich:UniversitätsSpital, Klinik für Neonatologie, (Romaine Arlettaz, Hans-Ulrich Bucher), Central data manager (Mark Adams).
References
1 Lorenz JM, Paneth N, Jetton JR, den Ouden L, Tyson JE. Comparison of Management Strategies for Extreme Prematurity in New Jersey and the Netherlands: Outcomes and Resource Expenditure. Pediatrics. 2001;108(6):1269–74.
2 Batton DG, Committee on F, Newborn. Antenatal Counselling Regarding Resuscitation at an Extremely Low Gestational Age. Pediatrics. 2009;124(1):422–7.
3 MacDonald H, Committee on F, Newborn. Perinatal Care at the Threshold of Viability. Pediatrics. 2002;110(5):1024–7.
4 Tyson JE, Parikh NA, Langer J, Green C, Higgins RD, the National Institute of Child H, Human Development Neonatal Research N. Intensive Care for Extreme Prematurity – Moving beyond Gestational Age. N Engl J Med. 2008;358(16):1672–81.
5 Ambalavanan N, Baibergenova A, Carlo WA, Saigal S, Schmidt B, Thorpe KE. Early prediction of poor outcome in extremely low birth weight infants by classification tree analysis. The Journal of Pediatrics. 2006;148(4):438–???????????.
6 Encyclopedia ADAMM. Gestational Age. Atlanta: A.D.A.M., Inc.; 2005 [updated 12.20.2009; cited 2010 12.12.2010]; Available from: http://www.nlm.nih.gov/medlineplus/ency/article/002367.htm.
7 Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular haemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92(4):529–34.
8 de Vries LS, Eken P, Dubowitz LM. The spectrum of leukomalacia using cranial ultrasound. Behav Brain Res. 1992;49(1):1–6.
9 Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163(7):1723–9.
10 Voigt M, Fusch C, Olbertz D, Hartmann K, Rochow N, Renken C, et al. Analysis of the Neonatal Collective in the Federal Republic of Germany 12th Report: Presentation of Detailed Percentiles for the Body Measurement of Newborns. Geburtsh Frauenheilk 2006;66:956–70.
11 Kohli R. Tables de mortalité pour la Suisse 1998/2003. Neuchâtel-Switzerland: Office fédéral de la statistique (OFS); 2005. p. 10.
12 Berger TM BV, Fauchère JC, Holzgreve W, Kind C, Largo R, Moessinger A ZR. Care of infants born at the limit of viability (22 to 26 weeks of gestation). Swiss Society of Neonatology [Guidelines]. 2002.
13 Bucher HU, Ochsner Y, Fauchere JC. Two years outcome of very pre-term and very low birthweight infants in Switzerland. Swiss Med Wkly. 2003;133(5-6):93–9.
14 Draper ES, Zeitlin J, Fenton AC, Weber T, Gerrits J, Martens G, et al., on behalf of the Mrg. Investigating the variations in survival rates for very preterm infants in 10 European regions: the MOSAIC birth cohort. Arch Dis Child Fetal Neonatal Ed. 2009;94(3):F158–63.
15 Zeitlin J, Ancel P-Y, Delmas D, Breart G, Papiernik E. Changes in care and outcome of very preterm babies in the Parisian region between 1998 and 2003. Arch Dis Child Fetal Neonatal Ed. 2009:adc.2008.156745.
16 Vanhaesebrouck P, Allegaert K, Bottu J, Debauche C, Devlieger H, Docx M, et al., for the ESG. The EPIBEL Study: Outcomes to Discharge From Hospital for Extremely Preterm Infants in Belgium. Pediatrics. 2004;114(3):663–75.
17 Herber-Jonat S, Schulze A, Kribs A, Roth B, Lindner W, Pohlandt F. Survival and major neonatal complications in infants born between 22 0/7 and 24 6/7 weeks of gestation (1999–2003). Am J Obstet Gynecol. 2006;195(1):16–22.
18 The EG. One-Year Survival of Extremely Preterm Infants After Active Perinatal Care in Sweden. JAMA. 2009;301(21):2225–33.
19 Markestad T, Kaaresen PI, Ronnestad A, Reigstad H, Lossius K, Medbo S, et al., on behalf of the Norwegian Extreme Prematurity Study G. Early Death, Morbidity, and Need of Treatment Among Extremely Premature Infants. Pediatrics. 2005;115(5):1289–98.
20 Larroque B, Breart G, Kaminski M, Dehan M, Andre M, Burguet A, et al. Survival of very preterm infants: Epipage, a population based cohort study. Arch Dis Child Fetal Neonatal Ed. 2004;89(2):F139–44.
21 Zeitlin J, Draper ES, Kollee L, Milligan D, Boerch K, Agostino R, et al., and the Mrg. Differences in Rates and Short-term Outcome of Live Births Before 32 Weeks of Gestation in Europe in 2003: Results From the MOSAIC Cohort. Pediatrics. 2008;121(4):e936–44.
22 Pignotti MS, Donzelli G. Perinatal Care at the Threshold of Viability: An International Comparison of Practical Guidelines for the Treatment of Extremely Preterm Births. Pediatrics. 2008;121(1):e193–8.
23 Kamoji VM, Dorling JS, Manktelow BN, Draper ES, Field DJ. Extremely Growth-Retarded Infants: Is There a Viability Centile? Pediatrics. 2006;118(2):758–63.
24 Men-Jean LEE, Ellen LC, Lama C, James R. Woods JR, Giuseppe P. A Critical Birth Weight and Other Determinants of Survival for Infants with Severe Intrauterine Growth Restriction. Annals of the New York Academy of Sciences. 2001;943(HUMAN FERTILITY AND REPRODUCTION: THE OOCYTE, THE EMBRYO, AND THE UTERUS):326–39.
25 Wold SHW, Sommerfelt K, Reigstad H, Ronnestad A, Medbo S, Farstad T, et al. Neonatal mortality and morbidity in extremely preterm SGA infants: A population based study. Arch Dis Child Fetal Neonatal Ed. 2009:adc.2009.157800.
26 Evans DJ, Levene MI. Evidence of selection bias in preterm survival studies: a systematic review. Arch Dis Child Fetal Neonatal Ed. 2001;84(2):F79–84.
27 Levene M. Is intensive care for very immature babies justified? Acta Paediatr. 2004;93(2):149–52.
28 Heiniger M ME, Rausa F. La population étrangère en Suisse. Edition 2004. Office fédéral de la statistique (OFS); 2004.

