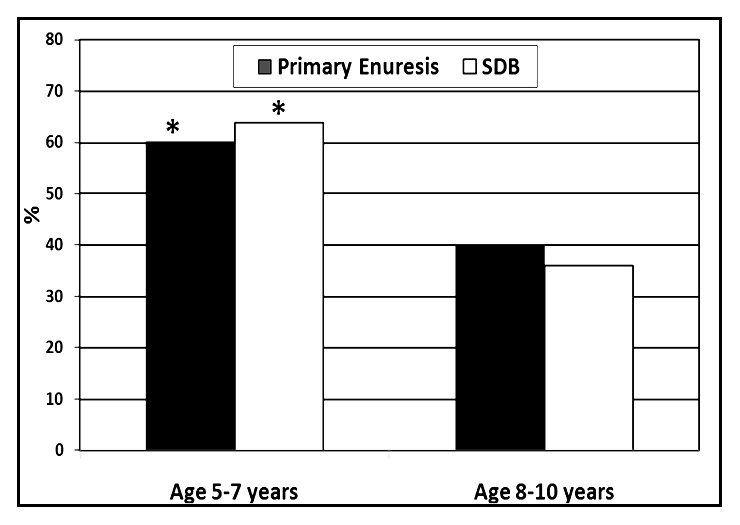
Figure 1
The incidence of primary nocturnal enuresis and SDB in relation to age. (*indicates p <0.05).
DOI: https://doi.org/10.4414/smw.2011.13216
Nocturnal enuresis is a common and important problem in school children presenting to clinical settings [1]. It affects not only the child but the entire family, and it may adversely affect the relationship between the child and his parents. It has been reported that nocturnal enuresis could be considered as a public health problem, and therefore it is important to be fully aware of the prevalence, clinical features and symptoms of childhood nocturnal enuresis as well as the associated factors involved [2].
Nocturnal enuresis refers to involuntary loss of urine after the age of 5 years, when children are expected to have achieved full bladder control at night. In most cases, enuresis is ultimately categorised as primary, when documented by the absence of an overt urogenital pathology. In addition, enuresis is also classified as primary when the child has never achieved night time dryness and secondary when bed wetting occurs after being dry for ≥6 months. The prevalence of nocturnal enuresis in childhood is reported to be between 8–14% [1, 3, 4]and it is apparent that the parameters associated with nocturnal enuresis vary somewhat between different societies [5].
Causes of primary and secondary enuresis are multiple; among them are idiopathic causes, disorders of sleep arousal, nocturnal polyuria, small nocturnal bladder capacity, overactive bladder and dysfunctional voiding, cystitis, constipation, neurogenic bladder, urethral obstruction, ectopic ureter, diabetes insipidus, diabetes mellitus, psychological and seizure disorders [6].
Sleep-disordered breathing (SDB) is a common problem during childhood and is a term that describes a spectrum of upper airway obstructions during sleep from primary snoring, which is reported in 10% of preschool children [7], to obstructive sleep apnea (OSA) with community prevalence rates between 0.9% and 4.3% [8].
There is a recognised link between SDB and polyuria / nocturia in adults [9], thus it would be fair to hypothesize that the nocturnal polyuria frequently seen in children with primary nocturnal enuresis could be associated with SDB [10]. Compelling evidence for an association between SDB and nocturnal enuresis was reported in a large Greek community sample of children aged 5–14 years [7], where children with habitual snoring were four times more likely to have primary enuresis than those who did not regularly snore.
Brain-type natriuretic peptide (BNP) is a non-glycosylated peptide that is produced predominantly by ventricular myocytes and belongs to the natriuretic peptide family [11]. Since BNP is released from cardiac myocytes after cardiac wall distension, SDB could lead to increased urinary output and natriuresis by promoting the release of BNP through increased venous blood return and atrial distension in the context of the increased intrathoracic pressure swings associated with upper airway obstruction.
Plasma BNP concentrations vary among different age groups; in newborn infants the levels are relatively high, vary greatly, and decrease rapidly during the first week of life. In infants older than 2 weeks and in young children, the mean plasma concentration of BNP is lower than that in adults. There is also a sex related difference in the second decade of life (aged 10 years or older), with higher BNP concentrations in girls than in boys, and this is related to the feminine pubertal stage [12].
Based on the aforementioned considerations, the aims of the present study were to; (1) determine the prevalence of nocturnal enuresis and SDB in a sample survey of young school-age Egyptian children (age between 5 and 10 years), (2) determine whether higher BNP levels are present in children with SDB, particularly when enuresis is present, and (3) evaluate the response to surgical intervention of SDB in indicated enuretic patients.
This prospective study included children aged 5 to 10 years recruited from the General Paediatrics Outpatient Clinic, Ain Shams University Hospitals, Cairo, Egypt, during the period from June 2008 to November 2009. We intended to design a study that would provide, with a 95% certainty, a rate of enuresis with a confidence interval (CI) in the total sample no higher than ±2%, using the PS programme (version 2.1.31, 2004) (provided by the Vanderbilt University, USA) for the calculation of the sample power and size. Hence, the number to be included with approximation to the higher round figure was 1000 children.
Approval from the ethical and moral committee of the University for the design and protocol of the study was obtained, and informed consent was obtained from the parents or caregivers of each child before enrollment.
Parents of the 1000 children were invited to complete a detailed questionnaire (a previously validated questionnaire was used)[13, 14]. With emphasis on their child’s gender, sleeping habits, daytime wetting and nocturnal enuresis, and its frequency or grading were collected. A positive family history of enuresis was also investigated.
According to the survey's answers, snoring and its severity was graded as “never” or “rarely” (once per week), “occasionally” (twice per week), “frequently” (3–4 times per week), and “almost always” (>4 times per week). Children were subdivided according to their snoring patterns (as a symptom) into non-snorer children (responses of never or rarely snore in questionnaire) or habitual snoring children (responses of almost always [>3 nights per week] or always on snoring frequency and medium loud to loud on loudness of snoring)[11].
The frequency of enuresis was graded as “never,” or “rarely” (once per month), “occasionally” (twice per month), “frequently” (3–6 times per month) and “almost always” (>3 times per week). Enuresis was considered to be present when responses were in the frequently or almost always range[15].
For the surveyed children,inclusion criteria of the patientsincluded; having primary nocturnal enuresis, not receiving medical treatment for enuresis, and not being put on fluid restriction during the study period.
Exclusion criteria were patients with; secondary enuresis, urological anomalies and/or bladder instability, urinary tract infection, known genetic or craniofacial anomalies, or patients with a neurological abnormality or mental retardation. The presence of any cause of nasal obstruction, other than adenotonsillar hypertrophy, was also an exclusion criterion.
An additional control group was enrolled, including 30 non-snorer, otherwise healthy children with [n = 15; (50%)] and without enuresis [n = 15; (50%)]. They were age- and sex-matched to the studied patients.
All patients and control children were subjected to the following;
This included weight, height and body mass index (BMI) assessment, neurological examination and otolaryngologic assessment for grading of tonsillar hypertrophy. The tonsil size was graded by direct visualisation into 4 grades. Moderate to severe hypertrophy was defined as grade 3 or above [16]. Other otolaryngologic causes of nasal obstruction were looked for, as were nasal allergy or nasal polyposis, or undiagnosed choanal atresia.
As polysomnography was not available for all children, transcutaneous nocturnal pulse oximetry was used, as it is simple and easily interpreted for confirming or excluding the diagnosis of SDB and has an acceptable predictive value in the diagnosis of OSA. A positive nocturnal oximetry trend graph has at least a 97% positive predictive value, but a low (41%) negative predictive value (high false negative rate), [17], but Stradling and others showed that pulse oximetry had 93% sensitivity and 86% specificity for detecting OSAS [18].
Therefore, we depended on oximetry as a diagnostic test for straightforward SDB attributable to adenotonsillar hypertrophy in children older than 12 months [17]. The Invacare®Overnight Sleep Printing Pulse Oximeter, Model: INVIRC735 was used. This test grouped the children into SDB positive (i.e Oxygen Desaturation Index >5%) or negative (i.e Oxygen Desaturation Index <5%), as overnight pulse oximetry was defined as a decrease in the oxyhaemoglobin saturation (SpO2) of 4% or greater from baseline, to a value 90% or lower [19].
A. AP view on lumbosacral spine:to exclude any congenital anomalies.
B. Lateral neck radiograph: This was done for the measurement and grading of post-nasal space. Adenoid enlargement with adenoidal-nasopharyngeal ratio (ANR) <0.5 was considered as normal or mild while adenoid enlargement with ANR ≥0.5 was considered moderately or severely enlarged [20].
This was done to exclude urinary tract infections.
Sample collection : About two mL of fasting venous blood were collected aseptically in the morning from each control subject and the 33 patients with SDB and enuresis who underwent surgical intervention, pre- and post-operative.
The assay used was a sandwich enzyme immunoassay (ELISA) intended for the in vitro quantitative determination of uncomplexed human BNP in biological fluids (The Quantikine kit was developed and supplied by R & D Systems, Inc. McKinley Place N.E., Minneapolis, Minnesota, MN 55413 U.S.A).BNP was quantified by an enzyme catalysed color change, detectable on a standard ELISA reader at 450 nm against 690 or 620 nm as a reference.
1Apostoperative questionnaire that dealt with evaluation of (i) the frequency of enuresis (episodes of daytime, and nocturnal enuresis and number of voids per day and night), (ii) Improvement of snoring and symptoms of SDB.
2Postoperative Plasma Brain Natriuretic Peptide (BNP) assay.
All statistical analysis was carried out using SPSS software for Windows system (version 12) [SPPS Inc, Chicago, IL]. The presence or absence of SDB was the main outcome variable, and enuresis was the independent variable of interest. Parametric data were expressed as mean ± standard deviation (SD). Comparison of groups for continuous variables was conducted using Student’s ttests and for categorical characteristics using the X2 test (Yates’ correction). The odds ratios were determined and corresponding 95% confidence intervals were calculated using univariate logistic regression analysis. Multiple logistic regression analysis was then performed to adjust the odds ratio for age, gender, and ethnicity. The Pearson’s correlation coefficient (r)was used to inter-relate the numeric variables. A p value <0.05 was considered statistically significant.
The current study revealed that out of the 1000 children recruited from general paediatric clinic, after applying the inclusion and exclusion criteria, only 153 had primary nocturnal enuresis (NE). There were 80 males and 73 females.

Figure 1
The incidence of primary nocturnal enuresis and SDB in relation to age. (*indicates p <0.05).
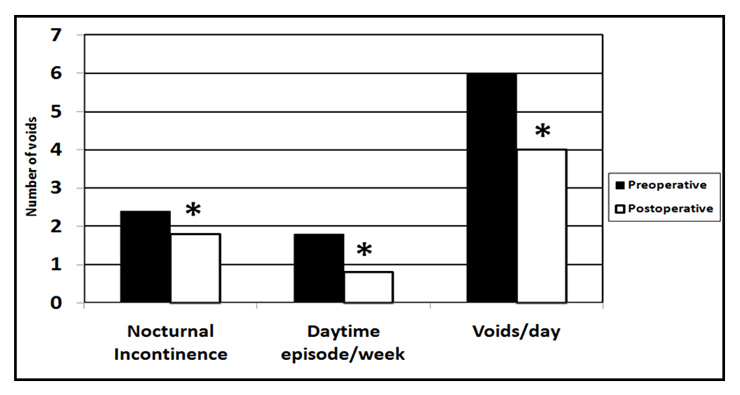
Figure 2
Incidence of nocturnal enuresis, daytime enuresis and voids per day in 33 enuretic children with SDB pre- and postoperatively (* p <0.05 = significant).
The frequency of enuresis and sleep disordered breathing were graded and summarised in table 1. The sociodemographic data and clinical characteristics ofenuretic children with and without SDB were summarised in table 2, where there were no significant differences between enuretic children with or without SDB with regard to male/female ratio, socioeconomic status (which was determined by the ratio of total family income/family size, in relation to the gross domestic product/capita for the year 2008) family history, BMI, frequency of enuresis. The only significant difference was the age factor.
A downward trend of enuresis and SDB were found as age increased, as shown in figure 1.
A total of 33 out of 47 enuretic children with SDB agreed and underwent surgical intervention after their parents’ consent was gained. A total of 20 children out of 33 (61%) with upper airway obstructive symptoms underwent adenotonsillectomy, 8 (24%) underwent adenoidectomy, and 5 (15%) underwent tonsillectomy only. No operative intervention was done for the other 14 children, because either their parents refused or they did not show up for the operation although surgically indicated.
Follow up of the 33 patients who underwent surgical intervention for the treatment of upper airway obstruction secondary to adenotonsillar hypertrophy was done for at least one year to assess the improvement of symptoms (as summarised in table 3).
Among the 15 otherwise healthy children with enuresis, morning plasma BNP levels were significantly higher when compared with the 15 control children who did not have enuresis (t = 3.5; p <0.001) (table 4).
Pre- and post-operative plasma BNP concentrations were significantly higher among the 33 enuretic children with SDB in comparison to all controls (independent of whether they had enuresis or not). The only exception was the post operative levels of patients and levels in the controls with enuresis, where both levels were statistically comparable (table 4).
In addition, children with SDB and enuresis graded “frequently” to “almost always” had higher levels of plasma BNP compared to children with enuresis graded “rarely” to “occasionally” both preoperatively (mean ± SD = 659 ± 153.2 versus 390 ± 98.34 pg/ml; p <0.01) and postoperatively (mean ± SD = 290 ± 76.9 versus 210 ± 45.86 pg/ml; p <0.05).
The postoperative questionnaire also dealt with episodes of daytime enuresis and number of voids per day. Interestingly, when calculating the average number of voids, the 29 out of 33 enuretic children with SDB who underwent surgery exhibited a significant reduction in daytime enuresis episodes, night time enuresis and voids per day postoperatively (fig. 2).
| Table 1: The frequency of enuresis and sleep disordered breathing. | ||
| Grading | No of patients with NE Total Number = 153 | No of patients with SDB &NE Total Number = 47 |
| Rarely/Occasionally | 31 (20.3%) | 10 (21%) |
| Frequently/Almost always | 121 (79%) | 37 (79%) |
| SDB: sleep disordered breathing, NE: nocturnal enuresis. | ||
| Table 2: Comparison between enuretic children with and without SDB with regard to socio-demographic data and clinical characteristics. | ||||
| Variable | Children with NE & SDB (n = 47) | Children with NE without SDB (n = 106) | Test of sig. | p |
| Age in years | 7.06 ± 1.59 | 7.76 ± 1.56 | 2.56 | 0.01* |
| M/F ratio | 20/27 | 57/49 | 1.64 | 0.20 |
| SES (Middle/Low) | 12/35 | 25/81 | 0.07 | 0.79 |
| +ve family history of enuresis | 30 (63.8%) | 67 (63.2%) | 0.005 | 0.94 |
| BMI | 18.77 ± 2.56 | 19.88 ± 3.92 | –1.77 | 0.08 |
| Frequency of NE (almost always/ rare) | 32/15 | 70/36 | 0.06 | 0.80 |
| M = male, F = Females, SES = socioeconomic status, BMI = body mass index, NE = nocturnal enuresis. * p <0.05 = significant. | ||||
| Table 3: The response to surgical intervention in enuretic children with SDB. | |||
| Number | Patients (%) | Duration of response | |
| Preoperative nocturnal enuresis | 33 | 100% | – |
| Postoperative status | |||
| Complete resolution | 6 | 18.5% | 7 days |
| 9 | 27% | 3 months | |
| Improved | 12 | 36.6% | 3 months |
| 2 | 6% | 1 year | |
| Unchanged | 4 | 12% | – |
| Total | 33 | 100% | – |
| Table 4: The expression of BNP in the studied subjects. Values are given as mean ± SD. | |||||
| Morning plasma BNP (pg/ml) | Enuresis and SDB (n = 33) | Controls (n = 30) | |||
| Preoperative | Postoperative | All controls (n = 30) | Enuresis (n = 15) | No enuresis (n = 15) | |
| Range | 180–873 | 120–620 | 60–321 | 150–321 | 60–130 |
| mean | 510.69a, b | 260.56a, c | 161.76 | 240.77 | 96.23 |
| ± (SD) | 131.94 | 60.97 | 31.8 | 35.67 | 11.6 |
| t = 2; p <0.001 | t = 3.5; p <0.001 | ||||
| a Statistical significant difference in comparison to all controls and to controls without enuresis (p <0.001 for all) b Statistical significant difference in comparison to controls with enuresis (t = 1.2; p <0.01) c Non-significant difference in comparison to controls with enuresis (t = 0.32; p >0.05) | |||||
Nocturnal enuresis refers to involuntary loss of urine after the age of 5 years, when children are expected to have achieved full bladder control at night. The minimal frequency of nocturnal urine loss required to diagnose this condition has varied in different studies from “once in the previous year” [2], to “at least twice a week”[3, 21]. In the present study, enuresis was considered to be present when the parental questionnaire responses were in the “frequently” or “almost always” range. The overall frequency in young Egyptian school-aged children (15.3%) was somewhat higher compared to the prevalence reported in other studies from all over the world (8–14%) [1, 3, 15, 22, 23].
In this study, the presence of mouth breathing, nightmares, intrusive naps, and occasional witnessed or observational apnea during sleep could not be related to, or did not seem to have a significant impact on the incidence of enuresis. This may be related to the small number of children with such variables as well as to subject inter-variability.
When assessing SDB, we depended on pulse oximetry, as overnight pulse oximetry is simple, cheap, and tends to have a high specificity but a low sensitivity when quantitative criteria are used [24, 25].
In the current study, 30.7% of children with nocturnal enuresis had SDB of whom 57% were boys. The over representation of the male gender among enuretic children was clearly present in most studies, as primary nocturnal enuresis was exceedingly more common in boys, with a 3:1 gender ratio [4, 26]. Moreover, there was a downward trend of enuresis and SDB as age increased, in keeping with that expected based on the literature. However, the rates of enuresis and SDB were higher than expected in each age group.
Other studies have examined the potential links between habitual snoring, as an indicator of the risk for SDB, and the presence of enuresis. Among those surveys that included habitual snoring as part of their questionnaires, an increased frequency of enuresis was generally reported among habitual snoring children [5, 22]. For example, Yeung et al. [22] reported that 46% of children with OSA diagnosed by polysomnography had nocturnal enuresis, whereas Alexopoulos et al. [26] found that 23.3% of children with nocturnal enuresis were habitual snorers. Taken together, it would seem that increased upper airway resistance during sleep, manifesting either as habitual snoring or as polysomnographically documented OSA, is indeed associated with an increased probability for enuresis, and the current findings further confirm this assumption.
In the current study, we found that 79% of children with SDB had enuresis graded as “frequently” to “almost always”, compared to 21% with enuresis graded as “rarely” to “occasionally”. Moreover, there was a significant direct correlation between the frequency of enuresis and each of the grades of snoring due to adenotonsillar hypertrophy. Therefore, it seems that the severity of SDB had a significant impact on the frequency of enuresis. However, other studies [11, 27, 28] have reported that the prevalence of nocturnal enuretic symptoms is increased among children with habitual snoring, but that the severity of SDB does not seem to impact on the frequency of enuresis. Furthermore, they reported that sleep architecture in habitually snoring children with enuresis is not markedly different from that of habitually snoring children without enuresis, although some subtle increases in sleep efficiency and delta-wave sleep are present.
Several different pathophysiological mechanisms have been proposed to explain such associations between increased upper airway resistance during sleep and nocturnal enuresis. Three major mechanisms have been implicated in uncomplicated nocturnal enuresis in children, namely, hyperactive detrusor activity, polyuria, and deep sleep with reduced arousability [29, 30]. The latter 2 mechanisms seem particularly propitious in the context of habitual snoring.
One of the potential mechanisms accounting for the increased prevalence of enuresis in the context of SDB may be related to the release of both atrial and brain natriuretic peptides. Therefore, we were stimulated to determine whether higher BNP levels were present in children with SDB, particularly when enuresis is present in comparison to a group of non-snorer, otherwise healthy children with and without enuresis.
Among the 15 otherwise healthy children with enuresis, morning plasma BNP levels were significantly higher when compared with the 15 control children who did not have enuresis (table 4). Pre-operative plasma BNP concentrations were significantly higher among the 33 enuretic children with SDB in comparison to all controls (independent of whether they had enuresis or not). Moreover, children with SDB and enuresis graded as “frequently” to “almost always” had higher levels of plasma BNP compared to children with enuresis graded as “rarely” to “occasionally”. This was in accordance with the literature that reported increased natriuresis in enuretic children [11, 31–33].
Since BNP is released from cardiac myocytes after cardiac wall distension, SDB could lead to increased urinary output and natriuresis by promoting the release of BNP. However, supportive evidence for such an assumption is somewhat conflictive. For example, Patwardhann et al. [33] did not find an association between natriuretic peptides and OSA in adult patients, and similarly, Pepperell et al. [34] reported the absence of any significant changes in BNP among adult patients with OSA after treatment with continuous positive airway pressure. Conversely, Tasci et al. [35] reported that, although no differences existed in BNP levels among OSA patients and control subjects, treatment with continuous positive airway pressure induced marked decreases in BNP levels in the OSA patients. Furthermore, Kaditis et al. [32] have recently shown that increased BNP levels are much more likely to occur among children with habitual snoring. Our current findings confirm this latter report and add additional insights into this particular issue because we not only confirm that BNP levels are increased in the presence of SDB but further show that enuretic children have markedly higher BNP levels compared with non-enuretic children at any degree of severity. It seems that BNP morning levels are markedly elevated in children with enuresis, and the presence of SDB leads to further increases in BNP plasma levels. Taken together, these findings suggest that increased release of BNP in the context of increased upper airway resistance during sleep may contribute to enuresis in children with SDB.
In the postoperative questionnaire that dealt with episodes of daytime enuresis and number of voids per day, interestingly, 33 enuretic children with SDB who underwent surgery exhibited a significant reduction in daytime enuresis episodes and voids per day postoperatively. In addition, decreases in the frequency or complete resolution of bed-wetting occurred after successful treatment of the breathing disorder during sleep. Indeed, other reports [22, 36] showed resolution or decreased frequency of primary nocturnal enuresis after relief of upper airway obstruction by adenotonsillectomy, and moreover, the severity of enuresis episodes, grading of adenotonsillar hypertrophy, apnea count, and grading of snoring did not predict the likelihood of resolution or improvement in either nocturnal or daytime enuresis. Similarly, Brooks and Topol [21] did not find any differences in the prevalence of enuresis among children with OSA when the latter were subdivided into respiratory disturbance severity categories. It is possible that the presence of upper airway obstruction alone is sufficient to saturate the effect of SDB on enuretic-prone children.
It seems that children with a pre-existing propensity for enuresis had higher levels of BNP that may be further enhanced by upper airway obstruction and, therefore, tip the balance in favour of more pronounced enuretic symptoms contributing to the continuance of enuresis. However, for enuretic children with SDB improved after surgical treatment of the airway obstruction, it was found that daytime voiding dysfunction also improved in these patients.
On the basis of the evidence in the literature and the results of this current study, it would seem that there could be an association between SDB and nocturnal enuresis in children. The importance of this finding is twofold. Firstly, nocturnal enuresis could be used as a predictor of SDB leading to earlier diagnosis and treatment, and hopefully reducing the significant morbidity associated with this condition. Secondly, the identification of SDB may open new therapeutic pathways, in terms of surgery for the upper obstructed airway, for those patients with nocturnal enuresis who do not respond to standard treatment.
In addition, we conclude that primary screening of all enuretic children should comprise of combined clinical, laboratory and radiological studies. This might be helpful to screen for children with clinically significant SDB who need earlier investigation and intervention. Further studies are warranted to determine the potential causative pathways and clinical relevance of these findings.
1 Stone J, Malone PS, Atwill D, McGrigor V, Hill CM. Symptoms of sleep-disordered breathing in children with nocturnal enuresis. J Pediatr Urol. 2008;4:197–202.
2 Butler RJ, Golding J, Heron J. ALSPAC Study Team: Nocturnal enuresis: a survey of parental coping strategies at 7½ years. Child Care Health Dev. 2005;31:659–67.
3 Kanaheswari Y. Epidemiology of childhood nocturnal enuresis in Malaysia. J Pediatr Child Health. 2003;39:118–23.
4 Ozkan KU, Garipardic M, Toktamis A, Karabiber H, Sahinkanat T. Enuresis prevalence and accompanying factors in school children: a questionnaire study from southeast Anatolia. Urol Int. 2004;73:149–55.
5 Chang P, Chen WJ, Tsai WY, Chiu YN. An epidemiological study of nocturnal enuresis in Taiwanese children. BJU Int. 2001;87:678–81.
6 Robson WM. Enuresis: overview, Differential Diagnoses & Workup, treatment, and follow up. Emedicine> pediatrics; surgery> urology Updated: Apr 7, 2010.
7 Kaditis AG, Finder J, Alexopoulos EI, Tanou K, Gampeta S, Agorogiannis E, et al. Sleep-disordered breathing in 3,680 Greek children. Pediatr Pulmonol. 2004;37:499–509.
8 Castronovo V, Zucconi M, Nosetti L, Marazzini C, Hensley M, Veglia F, et al. Prevalence of habitual snoring and sleep-disordered breathing in preschool aged children in an Italian community. J Pediatr. 2003;142:364–5.
9 Umlauf MG, Chasens ER. Sleep disordered breathing and nocturnal polyuria: nocturia and enuresis. Sleep Med Rev. 2003;7:403–11.
10 Djurhuus JC, Matthiesen TB, Rittig S. Similarities and dissimilarities between nocturnal enuresis in childhood and nocturia in adults. Br J Urol Int. 1999;84(Suppl 1):9–12.
11 Capdevila OS, Crabtree VM, Kheirandish-Gozal L, Gozal D. Increased morning brain natriuretic peptide levels in children with nocturnal enuresis and sleep- disordered breathing: A Community-based study. Pediatrics. 2008;121:1208–14.
12 Koch A, Singer H. Normal values of B type natriuretic peptide in infants, children, and adolescents. Heart. 2003;89(8):875–8.
13 Gozal D. Sleep-disordered breathing and school performance in children. Pediatrics. 1998;102(3 pt 1):616–20.
14 Montgomery-Downs HE, O’Brien LM, Holbrook CR, Gozal D. Snoring and sleep-disordered breathing in young children: subjective and objective correlates. Sleep. 2004;27(1):87–94.
15 Hjalmas K, Arnold T, Bower W. Nocturnal enuresis an international evidence based management strategy. J Urol. 2004;171:2545–61.
16 Li AM, Wong E, Kew J. Use of tonsil size in the evaluation of obstructive sleep apnea. Arch Dis Children. 2002;87:156–9.
17 Brouillette RT, Morielli A, Leimanis A, Waters KA, Luciano R, Ducharme FM. Nocturnal pulse oximetry as an abbreviated testing modality for pediatric obstructive sleep apnea. Pediatrics. 2000;105:405–12.
18 Stradling JR, Thomas G, Warley AR, et al. Effect of adenotonsillectomy on hypoxaemia, sleep disturbance and symptoms in snoring children. Lancet. 1990;335:249–53.
19 Collop N. Portable monitoring in obstructive sleep apnea in adults. http://www.uptodate.com, Last literature review in September 2010.
20 Goodwin JL, Kaemingk KL, Fregosi RF. Parasomnias and sleep disordered breathing in Caucasian and Hispanic children. The Tucson children’s assessment of sleep apnea study. BMC Med. 2004;2:14.
21 Brooks LJ, Topol HI. Enuresis in children with sleep apnea. J Pediatr. 2003;142:515–8.
22 Yeung CK, Sreedhar B, Sihoe JD, Sit FK, Lau J. Differences in characteristics of nocturnal enuresis between children and adolescents: a critical appraisal from a large epidemiological study. BJU Int.2006;97(5):1069–73.
23 Petit D, Touchette E, Tremblay RE, Boivin M, Montplaisir J. Dyssomnias and parasomnias in early childhood. Pediatrics. 2007;119:5.
24 Williams, AJ, Yu, G, Santiago, S, Stein, M. Screening for sleep apnea using pulse oximetry and a clinical score. Chest. 1991;100:631.
25 Gyulay, S, Olson, LG, Hensley, MJ, et al. A comparison of clinical assessment and home oximetry in the diagnosis of obstructive sleep apnea. Am Rev Respir Dis. 1993;147:50.
26 Alexopoulos EI, Kostadima E, Pagonari I, Zintzaras E, Gourgoulianis K, Kaditis AG. Association between primary nocturnal enuresis and habitual snoring in children. Urology.2006;68(2):406–9.
27 Firoozi F, Batniji R, Aslan AR, Longhurst PA, Kogan BA. Resolution of diurnal incontinence and nocturnal enuresis after adenotonsillectomy in children. J Urol. 2006;175:1885–8.
28 Harari MD, Moulden A. Nocturnal enuresis: What is happening? J Pediatr Child Health. 2000;36:78–81.
29 Neve’us T. The role of sleep and arousal in nocturnal enuresis. Acta Paediatr.2003;92(10):1118–23.
30 Wille S. Primary nocturnal enuresis in children: background and treatment. Scand J Urol Nephrol.1994;156(Suppl):1–48.
31 Kuznetsova AA, Natochin YV, Papayan AV. Osmoregulatory function of the kidney in enuretic children. Scand J Urol Nephrol. 1998;32(2):132–7.
32 Kaditis AG, Alexopoulos EI, Hatzi F. Overnight change in brain natriuretic peptide levels in children with sleep disordered breathing. Chest.2006;130(5):1377–84.
33 Patwardhann AA, Larson MG, Levy D. Obstructive sleep apnea and plasma natriuretic peptide levels in a community-based sample. Sleep. 2006;29(10):1301–6.
34 Pepperell J, Stradling J, Davies R. Brain natriuretic peptide is unchanged after 4 weeks of continuous positive airway pressure therapy. J Sleep Res. 2006;15(4):463–4.
35 Tasci S, Manka R, Scholtyssek S. NT-pro-BNP in obstructive sleep apnea syndrome is decreased by nasal continuous positive airway pressure. Clin Res Cardiol. 2006;95(1):23–30.
36 Alexopoulos EI, Kaditis AG, Kostadima E, Gourgoulianis K. Resolution of nocturnal enuresis in snoring children after treatment with nasal budesonide. Urology. 2005;66(1):194.
Funding / potential competing interests: No financial support and no other potential conflict of interest relevant to this article were reported.