Figure 1
All patients: effects of vitamin D3 supplementation.
DOI: https://doi.org/10.4414/smw.2011.13196
Vitamin D plays a major role in bone metabolism and neuromuscular function [1, 2]. Vitamin D deficiency leads primarily to osteomalacia, but has also been related to non specific symptoms such as pain and fatigue [3, 4], while supplementation with vitamin D (and calcium) has been shown to be effective for lowering the risk of fractures in patients with osteoporosis [5–9]. There is also increasing evidence for a much broader role of vitamin D, and an increasing numbers of studies investigating the effects of hypovitaminosis D and/or vitamin D supplementation on different health aspects have recently been published. Vitamin D deficiency has been correlated to a higher incidence of oncologic, cardiovascular and auto-immune diseases, as well as increased mortality [10–14]. Nevertheless, vitamin D deficiency and insufficiency often remain a neglected problem. Furthermore, there are now controversies about optimal vitamin D levels and mode of supplementation [15].
Vitamin D insufficiency has a high prevalence, with a gradient between regions depending on the latitude and amount of sunshine [16]. Hypovitaminosis D is highly prevalent in Swiss senior citizens, probably secondary to poor exposure to sunlight and decreased skin production (about 25% of young adults) [17–19]. The few studies among rheumatology outpatients have also shown a high prevalence of hypovitaminosis D [20–22], and systematic screening and correction of this problem could improve the management of these patients with chronic diseases at high risk of osteoporosis and fractures. An improvement in vitamin D levels could also improve the prognosis of patients with other diseases through other mechanisms as vitamin D levels have been shown to be inversely correlated with disease activity scores in inflammatory arthritis and systemic lupus erythematosus (SLE) [23–25]. We therefore systematically evaluated the vitamin D status in our outpatient rheumatology population to gain a better understanding of the severity of the problem in different categories of rheumatologic diseases and in relation to a current oral supplementation of vitamin D. We decided to use a widely accepted definition of hypovitaminosis D with vitamin D deficiency defined as a serum level of 25-OH vitamin D below 10 µg/l (ng/ml) (25 nmol/l) and vitamin D insufficiency characterised as serum level of 25-OH vitamin D of 10 to 30 µg/l (25 to 75 nmol/l) [26, 27].
During one month (November 2009), all physicians consulting at our Rheumatology Outpatient Clinic of Lausanne University Hospital (latitude 46.52°N) were suggested to offer a blood screening test to evaluate 25-OH vitamin D levels in all their patients. 25-OH vitamin D levels were determined using the radio-immunologic assay with extraction (25-hydroxyvitamin D 125I RIA Kit, DiaSorin®): the first step involves rapid extraction of 25-OH vitamin D and other hydroxylated metabolites from serum with acetonitrile. Following extraction, the treated sample is then assayed using an equilibrium RIA procedure based on an antibody with specificity to 25-OH vitamin D.
Results were classified as followed: deficiency of 25-OH vitamin D for levels <10 µg/l (25 nmol/l), insufficiency of 25-OH vitamin D for results between 10 µg/l to 30 µg/l (25 to 75 nmol/l) and a normal level of 25-OH vitamin D for results above 30 µg/l (>75 nmol/l).
Data on the use of daily oral vitamin D3 and calcium supplementation or a single high dose of oral or intramuscular vitamin D3 in the last 6 months were systematically collected, and patients were categorised into three non exclusive categories of rheumatologic diseases: inflammatory rheumatologic disease (IRD, including mainly rheumatoid arthritis, spondyloarthropathies and systemic lupus), degenerative disease (DD, including mainly chronic low back pain) and osteoporosis (OP, with or without fracture).
Student unpaired t-test were done for body mass index (BMI), age and 25-OH vitamin D level comparisons between groups.
Chi squared tests were performed for comparison of prevalence of hypovitaminosis D and vitamin D supplementation in different populations. Significance was reached with a p value <0.05. Statistical analyses were performed using Stata 8.1 software, College Station, TX, USA.
During the study period, 292 out of 784 attending patients (37%) had their level of 25-OH vitamin D determined. Many patients were not included because they did not get the blood test since many physicians forgot to offer the test to their patients. We excluded 20 patients who had received a single dose of 300’000 IU of vitamin D3 within the last 6 months, and only the 272 remaining patients were used for the analysis.

Figure 1
All patients: effects of vitamin D3 supplementation.
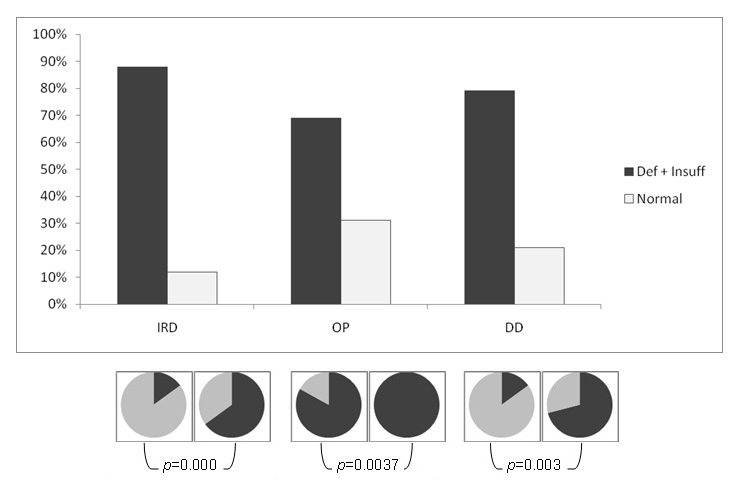
Figure 2
Underlying pathologies: effects of vitamin D3 supplementation. Circles : black : daily vitamin D3, grey : no supplementation Def + Insuff: Deficiency + Insufficiency, IRD: Inflammatory Rheumatic Disease, OP: Osteoporosis, DD: Degenerative Disease
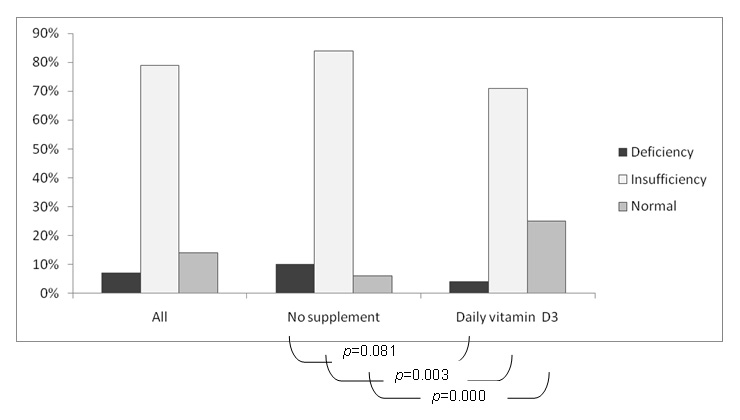
The mean 25-OH vitamin D level was 21 µg/l (range 1.5 to 45.9). True vitamin D deficiency was fairly rare found in only 20 patients (7%), whereas an insufficiency was the majority with 215 patients (79%) falling within this category. Only 37 patients (14%) had normal results.
25-OH vitamin D levels were not correlated to age or sex, with similar figures for the various categories of 25-OH vitamin D levels. The BMI was significantly higher in patients with an insufficiency compared with patients with normal results in our cohort.
Of the 272 patients, 104 (38%) were on daily oral supplementation of calcium and vitamin D3 (Calcium 500mg and vitamin D3 400 IU once or twice a day). A total of four (4%) of them still had a deficiency, 74 (71%) had an insufficiency and only 26 (25%) had normal results, compared to the 168 patients not receiving any supplementation where 16 (10%) had a deficiency, 141 (84%) had an insufficiency and 11 (6%) had a normal 25-OH vitamin D level.
Supplementation did somewhat improve the figures, with a significantly higher prevalence of normal results and a lower prevalence of insufficiency (p <0.05). The difference of prevalence of vitamin D deficiency did not reach statistical significance (p = 0.081), probably due to the small number of patients in this category.
Of these 272 patients, 219 had an inflammatory rheumatologic disease, 48 consulted for osteoporosis and 33 had a diagnosis of a degenerative rheumatologic pathology.
As expected, patients’ ages were significantly different between the three groups of diseases, with patients with OP being the oldest (mean age 67.7 (range 26.5 to 88.4)), and the patients with IRD (mean age 50.4 (range 17.7 to 88.4)) significantly younger, even compared to DD patients (mean age 56.4 (range 17.4 to 85.4)) (p <0.05).
In the small group of OP patients, the mean level of 25-OH vitamin D was the highest with a mean of 25 µg/l (range 1.5 to 45.9), significantly higher than in patients with IRD (p <0.05); an observation certainly explained by the high percentage of patients on oral daily supplementation of calcium and vitamin D3 (40 patients or 83%). Nevertheless, 3 patients still had deficient levels and 30 had insufficient levels of 25-OH vitamin D, an observation not completely explained by supplementation.
In the main group of younger IRD patients (44% rheumatoid arthritis, 34% spondylarthropathies, 4% systemic lupus, 28% others), the mean level of 25-OH vitamin D was surprisingly low to 20.5 µg/l (range 4.3 to 45.9), with only 26 patients with normal results (12%). A total of 19 had a deficiency and 174 an insufficiency. Again this observation is only partly explained by the low number of patients on oral daily supplementation (76 patients or 35%) with a mean level of 24.3 µg/l (range 8.3 to 45.9) for the supplemented group, compared to 18.5 µg/l (range 4.3 to 44) for the non-supplemented group.
In the last group of DD, the mean level of 25-OH vitamin D was 21.8 µg/l (12.1–40.8). None of the patients had a deficiency, 26 had an insufficiency and 7 had normal results, while 9 patients (27%) were on oral daily supplementation.
| Table 1: 25-OH vitamin D levels. | ||||||
| Number of patients | Mean 25-OH vitamin D level (µg/l) | Women | Age (years) | BMI (kg/m²) | Vitamin D3 Supplement | |
| All patients | 272 | 21 (1.5–45.9) | 71% | 52.6 | 25.9 | 38% |
| Deficiency (<10 µg/l) | 20 | 7.9 (1.5–9.9) | 70% | 49.6 | 24.9 | 20% |
| Insufficiency (10–30 µg/l) | 215 | 19.8 (10.2–29.6) | 70% | 52.5 | 26.4 | 34% |
| Normal (>30 µg/l) | 37 | 35.4 (30–45.9) | 76% | 55 | 23.4 | 70% |
| BMI: Body Mass Index | ||||||
| Table 2: Effects of underlying pathologies. | ||||||
| Number of patients | Mean 25-OH vitamin D level (µg/l) | Women | Age (years) | BMI (kg/m²) | Vitamin D3 Supplement | |
| All patients | 272 | 21 (1.5–45.9) | 71% | 52.6 | 25.9 | 38% |
| IRD | 219 | 20.5 (4.3–45.9) | 70% | 50.4 # | 26 | 35% |
| OP | 48 | 25 (1.5–45.9)∞ | 79% | 67.7 # | 25 | 83% |
| DD | 33 | 21.8 (12.1–40.8) | 70% | 56.4 # | 27.6 | 27% |
| # compared between disease groups: p <0.05 ∞ compared with IRD: p = 0.0003 BMI: Body Mass Index, IRD: Inflammatory Rheumatic Disease, OP: Osteoporosis, DD: Degenerative Disease | ||||||
The current study has many limitations. It was a simple cross-sectional study and less than 40% of the patients attending our clinic had their levels of vitamin D determined. Nevertheless, involvement in the study appeared to be directly related to the need of blood drawing for another purpose, and is unlikely to reflect a systematic bias. Data on compliance with supplementation, as well as on sun exposure and calcium and vitamin D3 intake in the diet were also of very limited quality and did not allow us to draw any conclusion from them. However, this is a true population-based study probably reflecting the reality of our current practice with all its incertitude, but also with very strong messages about the prevalence of hypovitaminosis D in a Swiss rheumatologic population and the value of simple daily oral supplementation.
Hypovitaminosis D was highly prevalent in our population of rheumatology patients (86%) and certainly under recognised. Most patients (79%) had 25-OH vitamin D levels values within the range of what we defined as being an insufficiency, but with still 7% having levels defined as a true deficiency. As the testing was done in autumn with the residual effect of the summer sunshine on vitamin D (storage) levels [28–30], we could have expected better results. Possible explanations are the lack of sufficient sunshine in Switzerland, even in summer, or sun avoidance of older patients (osteoporosis) or patients with photosensitivity (inflammatory diseases). However, patients were recruited within a region (Lake Geneva coast) with one of the highest yearly relative sunshine durations, while true cases of photosensitivity with sun avoidance were exceptional in the present population, and our results are probably valid for most Western Europe countries.
Age, sex and BMI did not predict hypovitaminosis D in our population. Oral supplementation was the only variable that did influence the prevalence of hypovitaminosis to some extent. Supplementation with 400 to 800 IU of vitamin D3 was relatively frequent (38%) in our population, and the proportion of patients with normal results was significantly higher in those taking supplementation, with 70% of patients with normal results already being prescribed oral daily supplementation of vitamin D3 at the time of the dosage. However, only a quarter of patients on oral daily supplementation of calcium and vitamin D3 had a normal value of 25-OH vitamin D; a fact probably mainly related to poor adherence [31], although insufficient supplementation is another option [32].
Vitamin D is of course essential for bone and neuromuscular health. However, it also has pleiotropic effects on other tissues and functions. As it’s true physiopathological importance remains unclear, [1], the high prevalence of hypovitaminosis D in our younger population of inflammatory diseases is of concern as vitamin D levels have been shown to be correlated with disease activity scores in early inflammatory synovitis, rheumatoid arthritis and SLE [23–25, 33]. The vitamin D receptor is highly concentrated in T cells and vitamin D analogues have been shown to markedly suppress animal models of autoimmune diseases [34]. Vitamin D probably exerts immunomodulatory effects. There are no specific guidelines for the management of vitamin D levels in patients with inflammatory diseases, nor randomised clinical trials on the effects of supplementation on disease activity. Nevertheless as rheumatoid arthritis is by itself an independent risk factor for osteoporotic fracture it appears reasonable to aim for at least the same level in this population as in patients with osteoporosis [35]. Finally, with such prevalence of hypovitaminosis D, clinical studies are definitively needed to evaluate the impact of supplementation in the various rheumatologic populations, in particular with regard to disease activity.
Sufficient levels of 25-OH vitamin D are historically defined as a level sufficient to prevent an increase of parathyroid hormone (PTH) levels, and the reference of 31 µg/l (78 nmol/l) is often suggested despite the fact that this cut-off is certainly different for each individual [16, 36]. The International Osteoporosis Foundation (IOF) also suggests reaching vitamin D levels of 30 µg/l (but no more) to reduce the risk of fall and fracture, and it is usually accepted that this level can be reached with a daily intake of 800–1000 IU of vitamin D3 [37, 38]. However, our results demonstrated that more than 70% of the patients were below this threshold despite daily oral supplementation. Therefore, it appears to be very important to motivate our patients regularly to improve treatment adherence, but also to check for vitamin D insufficiency in these patients as there is emerging evidence that usual preparations of vitamin D3 and calcium may not be effective at normalising vitamin D in everyone [39].
1 Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–81.
2 DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80(6 Suppl):1689S–96S.
3 de Torrente de la Jara G, Pecoud A, Favrat B. Female asylum seekers with musculoskeletal pain: the importance of diagnosis and treatment of hypovitaminosis D. BMC Fam Pract. 2006;7:4.
4 Plotnikoff GA, Quigley JM. Prevalence of severe hypovitaminosis D in patients with persistent, nonspecific musculoskeletal pain. Mayo Clin Proc. 2003;78(12):1463–70.
5 Bischoff-Ferrari HA, et al. Higher 25-hydroxyvitamin D concentrations are associated with better lower-extremity function in both active and inactive persons aged > or = 60 y. Am J Clin Nutr. 2004;80(3):752–8.
6 Bischoff-Ferrari HA, et al. Effect of Vitamin D on falls: a meta-analysis. JAMA. 2004;291(16):1999–2006.
7 Trivedi DP, Doll R, Khaw KT. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ. 2003;326(7387):469.
8 Chapuy MC, et al. Vitamin D3 and calcium to prevent hip fractures in the elderly women. N Engl J Med. 1992;327(23):1637–42.
9 Janssen HC, Samson MM, Verhaar HJ. Vitamin D deficiency, muscle function, and falls in elderly people. Am J Clin Nutr. 2002;75(4):611–5.
10 Hypponen E, et al. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet. 2001;358(9292):1500–3.
11 Penckofer S, et al. Vitamin D and diabetes: let the sunshine in. Diabetes Educ. 2008;34(6):939–40, 942, 944 passim.
12 Giovannucci E, et al. 25-hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med. 2008;168(11):1174–80.
13 Holick MF. Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr. 2004;79(3):362–71.
14 Stene LC, Joner G. Use of cod liver oil during the first year of life is associated with lower risk of childhood-onset type 1 diabetes: a large, population-based, case-control study. Am J Clin Nutr. 2003;78(6):1128–34.
15 Sanders KM, et al. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA. 2010;303(18):1815–22.
16 Chapuy MC, et al. Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos Int. 1997;7(5):439–43.
17 Holick MF, Matsuoka LY, Wortsman J. Age, vitamin D, and solar ultraviolet. Lancet. 1989;2(8671):1104–5.
18 Boulat O, et al. Clinical chemistry variables in normal elderly and healthy ambulatory populations: comparison with reference values. Clin Chim Acta. 1998;272(2):127–35.
19 Holick MF. Environmental factors that influence the cutaneous production of vitamin D. Am J Clin Nutr. 1995;61(3 Suppl):638S–645S.
20 Mouyis M, et al. Hypovitaminosis D among rheumatology outpatients in clinical practice. Rheumatology (Oxford), 2008;47(9):1348–51.
21 Chiu G. Vitamin D deficiency among patients attending a central New Zealand rheumatology outpatient clinic. N Z Med J. 2005;118(1225):U1727.
22 Cutolo M, et al. Circannual vitamin d serum levels and disease activity in rheumatoid arthritis: Northern versus Southern Europe. Clin Exp Rheumatol. 2006;24(6):702–4.
23 Cutolo M, et al. Vitamin D in rheumatoid arthritis. Autoimmun Rev. 2007;7(1):59–64.
24 Cutolo M, Otsa K. Review: vitamin D, immunity and lupus. Lupus. 2008;17(1):6–10.
25 Merlino LA, et al. Vitamin D intake is inversely associated with rheumatoid arthritis: results from the Iowa Women’s Health Study. Arthritis Rheum. 2004;50(1):72–7.
26 Mithal A, et al. Global vitamin D status and determinants of hypovitaminosis D. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2009;20(11):1807–20.
27 Rosen CJ. Clinical practice. Vitamin D insufficiency. N Engl J Med. 2011;364(3):248–54.
28 Bhattoa HP, et al. Prevalence and seasonal variation of hypovitaminosis D and its relationship to bone metabolism in community dwelling postmenopausal Hungarian women. Osteoporos Int. 2004;15(6):447–51.
29 Kull M Jr, et al. Seasonal variance of 25-(OH) vitamin D in the general population of Estonia, a Northern European country. BMC Public Health. 2009;9:22.
30 Rizzoli R, et al. Risk factors for vitamin D inadequacy among women with osteoporosis: an international epidemiological study. Int J Clin Pract. 2006;60(8):1013–9.
31 Tang BM, et al. Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: a meta-analysis. Lancet. 2007;370(9588):657–66.
32 Lips P, et al. The prevalence of vitamin D inadequacy amongst women with osteoporosis: an international epidemiological investigation. J Intern Med. 2006;260(3):245–54.
33 Patel S, et al. Association between serum vitamin D metabolite levels and disease activity in patients with early inflammatory polyarthritis. Arthritis and rheumatism. 2007;56(7):2143–9.
34 Cantorna MT, Mahon BD. Mounting evidence for vitamin D as an environmental factor affecting autoimmune disease prevalence. Experimental biology and medicine. 2004;229(11):1136–42.
35 Kanis JA, et al. Assessment of fracture risk. Osteoporos Int. 2005;16(6):581–9.
36 Dawson-Hughes B, Harris SS, Dallal GE. Plasma calcidiol, season, and serum parathyroid hormone concentrations in healthy elderly men and women. Am J Clin Nutr. 1997;65(1):67–71.
37 Pearce SH, Cheetham TD. Diagnosis and management of vitamin D deficiency. BMJ. 2010;340:b5664.
38 Audran M, Briot K. Critical reappraisal of vitamin D deficiency. Joint Bone Spine. 2010:77(2):115–9.
39 Ryan PJ. Vitamin D therapy in clinical practice. One dose does not fit all. Int J Clin Pract. 2007;61(11):1894–9.
No financial support and no other potential conflict of interest relevant to this article were reported.