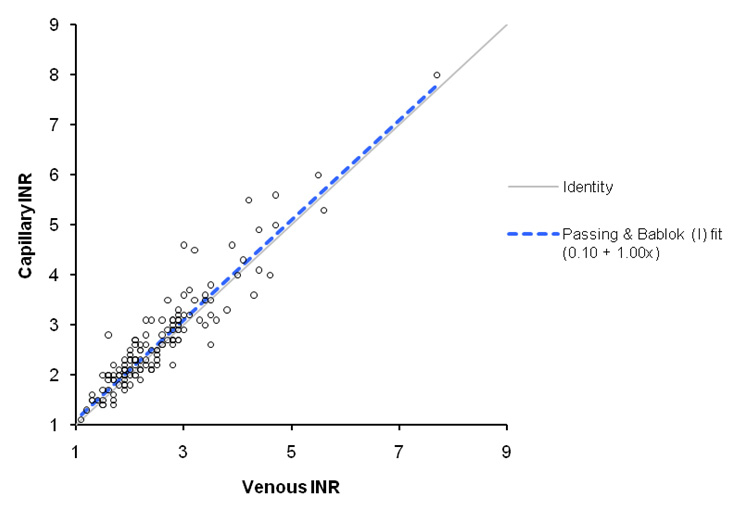
Figure 1
Scatter plot with Passing-Bablock fit.
DOI: https://doi.org/10.4414/smw.2011.13199
Chronic oral anticoagulation is prescribed worldwide to reduce thromboembolic complications in a variety of clinical conditions. The prevalence of oral anticoagulation is thought to be between 0.6 and 0.8% in the general population, and is reported to be increasing [1–3]. Oral anticoagulation with vitamin K antagonists (VKA) is the gold standard in long term anticoagulation [4–5]. VKA have a narrow therapeutic range with loss of efficacy when the dose is too low and a risk of life-threatening bleeding when it is too high. The response to VKA treatment is characterised by wide individual variability due to genetic and environmental components. As a result, regular monitoring of coagulation times, i.e. the international normalised ratio (INR), is mandatory. Conventional management of VKA therapy therefore involves repeated determination of the INR, requiring venous puncture, and subsequent adjustment of the VKA dose by a health professional.
INR self-testing devices have become available and enable patients to determine the INR themselves on a capillary blood sample obtained from their finger. These devices have proven to be reliable in determining the INR in clinical conditions with a high level of safety, precision and accuracy [6–9]. The patient can then either ask a physician or a specialised nurse to determine the dose of VKA on the basis of the capillary INR (INR self-testing, ST), or determine the dose himself with the help of materials such as dose adaptation tables (INR self-management, SM). Both ST and SM require specific patient training, with additional teaching in the case of SM. ST and SM were further validated in trials showing that these methods could achieve results at least equivalent to conventional INR management, with improved patient satisfaction and quality of life [10–13]. A meta-analysis also pointed to a significant reduction in thromboembolic events with SM and ST and a reduction in bleeding episodes with ST, whereas SM was equivalent to conventional management in this regard [14]. Despite these good results, the development of ST and SM remains very limited in many countries. Experience with ST and SM is greatest in Germany. In Switzerland experience of ST and SM is highly favourable, but its development is mainly restricted to the eastern, German-speaking part of the country [15]. Our practice being based in the western, French-speaking part of Switzerland, we intended to provide our patients with the opportunity to benefit from ST and SM, and therefore designed a teaching programme for ST and SM in common private ambulatory practice in Switzerland, with monitoring of clinical outcomes and patient satisfaction. We report here the first four years of our experience with the teaching programme.
We designed a teaching programme for SM and ST. The teaching facility is a small structure run by a single cardiologist with his paramedical staff. The programme was based on current evidence and our experience of clinical practice. The programme includes 4 sessions. The contents of each session are further described in table 1. The total duration of the programme is 5 hours, with the first 4 hours grouped in the same day to further minimize impact on time off work and to decrease travelling to reach the teaching facility.
| Table 1:Teaching programme. | |||
| Session | Duration | Speaker | Content |
| 1 | 90 min | Specialised nurse | Initial anticoagulation-related knowledge evaluation. Anticoagulation principles. Reference booklet. |
| 2 | 60 min | Medical assistant | Practical use of the CoaguChek. Simultaneous laboratory and capillary INR. |
| 3 | 90 min | Medical doctor (cardiologist) | Anticoagulation principles, common complications, dose adjustment. Pitfalls, frequently asked questions. Final anticoagulation-related knowledge evaluation. |
| 4 (at 3 months) | 60 min | Specialised nurse / medical doctor | Review of the initial experience, questions. |
Patients were referred by general practitioners and cardiologists in private practice in the community or from the Haemostasis Unit of Geneva University Hospital. Patient information was obtained by means of a specific questionnaire completed by the referring physician. An initial 20-minute evaluation of each patient was made prior to the day of teaching to verify that they met all inclusion and no exclusion criteria for the teaching programme. Inclusion criteria were the need for long term anticoagulation and an interest in improving autonomy. Exclusion criteria were inability to understand the principles of oral anticoagulation or technical inability to perform the test, such as poor visual acuity, severe tremor and poor coordination. Another exclusion criterion was inability to pay for the device, which is not covered by standard health insurance plans in Switzerland.
Anticoagulation-related knowledge was evaluated with the same questionnaire before and after teaching. A sample of the questionnaire forms Annex 1. At the end of the teaching programme the cardiologist in charge decided for each patient whether he/she was able to perform SM or not. This decision was taken on the basis of the patient’s performance during the interactive teaching programme and required feedback from the different speakers involved. For patients judged capable of both SM and ST, their personal preference was respected. If the patient was unable to perform SM it was determined whether he was capable of performing ST. Patients unable to perform ST were excluded. Patients able to begin with ST or SM were provided with a prescription for the device and a reference booklet. The referring doctor was given a report and instructions.
The VKA used was acenocoumarol. We used the CoaguChek S portable coagulometer (Roche Diagnostics, Basel, Switzerland), and its most recent version, the CoaguChek XS, as it became available. The CoaguChek XS presents improvements over the previous version, such as the use of a thromboplastin with a lower ISI, a test strip that includes an internal quality control and can be stored at room temperature, and reduced device size.
In the SM group patients followed an algorithm to determine dose changes and testing frequency. For this purpose we used the algorithm developed by Menendez-Jandula et al. for SM in patients on acenocoumarol [11]. The referring doctor and the teaching centre remained available for questions and troubleshooting. In addition, a 24/7 hotline was made available to the patients with the on call physician for haemostasis at Geneva University Hospital. Patient information was forwarded to the hotline service after teaching to allow for proper management of calls. The first dose was determined by the referring physician using previous experience of the patient with VKA when available. Patients in the self-testing group first called their referring physician for instructions on dosing and testing frequency according to the physician’s judgment. They initially called after each INR test, and later only if the INR was out of the target range. Three months after the teaching session a fourth session was held with an open discussion to answer possible questions. The INR obtained with the CoaguChek was correlated to a venous INR performed in an accredited laboratory when starting the experiment, at 3 months and every 6 months thereafter. A venous INR was also obtained in the event of unexpected results with the CoaguChek. The venous INRs were determined using a Sysmex CA-1500 automated system (Sysmex Europe GmbH, Hamburg, Germany) and an Innovin thromboplastin (Siemens Healthcare Diagnostics, Deerfield, IL). At one year patients were asked to complete a questionnaire on their experience with CoaguChek and send a copy of their anticoagulation booklet. The booklet contains information on daily VKA doses, INR results and venous INR results, interruptions of treatment for procedures or adverse events, adverse events and calls to a physician for patients in the self management group. Further data on adverse events were obtained from the referring physicians. The information was reviewed and analysed. The INRs derived from the CoaguChek S and XS were then compared to laboratory INRs by linear regression analysis (Passing-Bablock) and determination of a correlation coefficient.
The first four years of our experience with the teaching programme were analysed. During this period, 169 patients were referred for teaching. After an initial evaluation, 90 patients were included in the teaching programme (53%). The selection was conducted according to inclusion and exclusion criteria. After completion of the teaching programme 80 patients (89%) were assigned to self-management and 10 (11%) to self-testing. No patient had to be excluded after teaching. The first 49 patients were equipped with a CoaguChek S, and the remaining 41 patients were with the latest version of the device, CoaguChek XS, as it became available.

Figure 1
Scatter plot with Passing-Bablock fit.
The 1 year questionnaire was returned by 54 patients (60%). The data on adverse events, satisfaction with the teaching programme and satisfaction with self-management or self-testing are therefore based on these 54 patients. A full report of INR values at 1 year could be obtained in 35 of the patients who returned the questionnaire (65%), totalling 35 patient-years of follow-up. The analysis of INR percentage in the target range was conducted on these data. The patient demographics and other characteristics are summarised in table 2.
Table 3 summarises testing frequency and the proportion of INRs in the target range (target ± 0.5). The proportion of INRs in the ‘extended’ target range (target ± 0.7) is also given. The INR testing interval is reported separately for the first month and for the overall programme, since INR testing frequency was substantially higher during the first month and stabilised at a lower level thereafter.
The adverse events analysis was performed on a follow-up of 54 patient-years. There were 9 adverse events, 2 thrombotic and 7 haemorrhagic. 7 of these adverse events could be treated in the ambulatory setting (6 minor bleedings, 1 deep venous thrombosis), whereas 2 required hospitalisation and were considered major adverse clinical events (MACE). The first hospitalisation occurred for a cerebro-vascular accident, the second for a haemorrhage that required transfusion. Both MACE occurred despite a venous INR in the target range. The rate of MACE was 3.7 per 100 patient-years. There was no death from any cause.
In the self-management group, 50% of the patients called their physician for help with INR management at least once during the 12-month follow-up. 16% of the patients experienced a technical problem with the device. All problems could be solved. Most cases were user-related and solved by repeating the step-by-step instructions for use. Some patients experienced problems when trying to use the device at an external temperature outside the recommended range. In one case the device failed and had to be replaced. The 24/7 hotline was never called.
The average performance on the knowledge test was 9.1 points out of a maximum of 14 before teaching and 12.8 points after teaching. On a scale of 1 to 4, 4 being the highest, the mean satisfaction with the teaching programme was 3.6. 91% of the patients rated the number of sessions as appropriate. Patient satisfaction with SM and ST was high, with most patients reporting decreased stress when travelling, less stress associated with venous puncture, greater autonomy and an on the whole time-saving experience. Patient satisfaction with the teaching programme was also high. These results are further detailed in table 4.
The correlation analysis was based on 141 paired INRs, 78 for the CoaguChek S and 63 for the CoaguChek XS. The overall R-squared correlation coefficient was 0.871. A Passing-Bablock regression analysis is presented in figure 1. Accuracy was higher with the CoaguChek XS than with the CoaguChek S (R-squared correlation coefficient 0.897 versus 0.871, p <0.025).
| Table 2:Patient demographics (n = 54). | |||
| SM | ST | Total | |
| N | 48 | 6 | 54 |
| Sex (M:F) | 29:19 | 3:3 | 32:22 |
| Age (mean ± SD) | 57.6 ± 13 | 64.6 ± 12 | 58.6 ± 13 |
| Indication for VKA | |||
| Rhythmic (AF / flutter) | 16 | 2 | 18 (33%) |
| Mechanical valve | 17 | 1 | 18 (33%) |
| Years 1–3 Year 4 | 6 11 | 1 0 | 7 (20%) 11 (58%) |
| Recurrent DVT / PE, CVA | 15 | 3 | 18 (33%) |
| Duration of prior VKA therapy mean (median) (days) | 1643 (682) | 390 (180) | 1515 (600) |
| AF: atrial fibrillation; DVT: deep venous thrombosis; PE: pulmonary embolism; CVA: cerebro-vascular accident | |||
| Table 3:INR testing frequency and results. | |||
| SM | ST | Total | |
| N (patients) | 30 | 5 | 35 |
| INR testing interval (days) Median (IQR) | |||
| Month 1 | 6 (3.8–7.5) | 7.5 (6.4–8.1) | 6 (3.8–7.5) |
| Global (month 1–12) | 7.5 (7.2–14.4) | 7.5 (5.6–12.7) | 7.5 (7.2–14.4) |
| INRs in the therapeutic range (%) | |||
| Strict: INR 2.0–3.0 or 2.5–3.5 (n = 9) Mean (SD) Median (IQR) | 60.0 (10.4) 59.8 (50.1–68.6) | 64.4 (12.7) 70.5 (60.7–72) | 60.6 (10.7) 61.5 (50.3–70.4) |
| Extended (strict ± 0.2) Mean (SD) Median (IQR) | 74.2 (10.8) 74.4 (69.2–81.1) | 80.94 (15.7) 88 (78.6–90) | 75.2 (11.6) 75.9 (69.2–83.7) |
| SD: standard deviation; IQR: interquartile range | |||
| Table 4:Patient satisfaction. | |||
| SM | ST | Total | |
| N (patients) | 48 | 6 | 54 |
| Overall satisfaction with the teaching programme (scale of 4) | 3.6 | 3.4 | 3.6 |
| “The number of sessions is appropriate” | 91.6% | 83.3% | 91% |
| “After the teaching, I feel reassured regarding adverse events” | 98% | 100% | 98.2% |
| “The CoaguChek device is easy to use” | 95.9% | 100% | 96% |
| “The stress associated with venous puncture is reduced” | 71.4% | 83.3% | 72.7% |
| “The frequency of medical visits is reduced” | 95.9% | 66.7% | 92.7% |
| “I feel reassured when travelling” | 87.8 | 100% | 89.1% |
INR self-testing (ST) and INR self-management (SM) have been expanding as an alternative to the conventional management of anticoagulation. Initial investment is necessary with both ST and SM. First, the patient needs to be provided with a testing device. In our experience the cost of the device and testing strips had to be borne by the patient, because standard health insurance plans in Switzerland do not cover these costs. This accounts for most of the impressive 47% dropout rate between referral and inclusion. The coverage varies between countries, from no coverage to partial coverage, age-group specific coverage (i.e., paediatric patients in France) or full coverage (Germany). The trend is clearly towards better coverage, making ST and SM a choice available to more patients.
Another initial investment is the teaching that ST and SM require. Our goal was to design a teaching programme that could provide good patient satisfaction and reproduce the good results that were reported in major ST and SM trials [10–13]. Our programme is consistent with available recommendations for SM teaching [16–17]. Patient selection is of crucial importance. We used the selection criteria described earlier and experience of the teaching programme helped us to better predict what patients would benefit most from SM. The patients that turned out to be unable to perform SM after teaching but able to perform ST were all early patients. We also became more selective with overtly anxious patients, since they can be destabilised by the increased autonomy and responsibility associated with SM and tend to repeat testing inappropriately in a desire for reassurance. This initial selection appears to be effective in minimising that problem, the overall testing frequency in our experience being slightly lower than the recommended testing frequency.
The high patient satisfaction with the teaching programme possibly reflects the special attention paid to providing time for discussion and individual questions. The 4th session at 3 months involves a second visit to the testing facility but was found to be useful by patients and trainers as an opportunity to review the many questions that arise only after an initial period of practical experience. The knowledge test results improved after teaching, but the teaching programme is best evaluated by efficacy and safety criteria, in this case the percentage of INRs in the therapeutic range and the rate of adverse events. The overall percentage of INR in the therapeutic range, derived from a meta-analysis of SM and ST studies, was 61.6% for SM and 55.1% with conventional management of the INR in anticoagulation clinics [14]. In the more recent THINRS study, time within the target INR range was 62.4% in the conventional management group and 66.2% in the ST group [13]. The overall percentage of INRs in the therapeutic range of 60.6% in our experience is therefore within the expected range on the basis of available evidence. The observed rate of MACE of 3.7 per 100 patient-years is also within the expected range on oral anticoagulation [14]. The rate of MACE on oral anticoagulation reported in the ACOA study ranged from 2.2 per 100 patient-years in the SM group to 7.3 in the conventional management group. [11]
As expected, patient satisfaction with SM and ST was high, with most patients reporting a time-saving, stress-reducing experience providing them with more autonomy. The individual comments allowed us to gain more insight into the patient experience. Many, for instance, appreciate being able to determine the INR easily when they have a doubt or need to be reassured. This may happen in the case of a minor bleed after inadvertently modifying the dose or after eating a meal they suspect to be unusually rich in vitamin K. These settings are not strict medical indications for extra monitoring of the INR, but for many patients the immediate availability of the test may improve the relationship they have to their disease and its treatment.
Though the 24/7 hotline seemed important to us when starting the programme as an additional safeguard, the absence of calls in four years prompted us not to maintain it. This may facilitate implementation of other similar teaching programmes, as the obligation to ensure 24/7 support may be viewed as a heavy burden on health practitioners. The correlation between laboratory and CoaguChek INRs in our experience was close to that reported in validation studies for the device [8], with significantly higher laboratory to CoaguChek correlation with the newer version of the device, the CoaguChek XS. These results are consistent with available data [18]. The CoaguChek XS is therefore progressively replacing the CoaguChek S in our practice.
There are limitations to this study. First, the lack of a control group obliges us to refer to benchmark trials to put the results in perspective. It must be borne in mind that our objective was not to validate ST and SM against conventional management, as there are now strong data addressing this question [10–12, 14, 19], but to evaluate the implementation of a new ST and SM programme. Further, the INR analysis is performed as a percentage of INRs in the therapeutic range, which is imperfectly correlated with the actual time spent in the therapeutic range. The return rate of the questionnaire was 60%, which is not unusual for a questionnaire but implies that 40% of the patients were not included in the analysis, possibly significantly modifying the results. More reliable patients may have been selected by the questionnaire. Similarly, a complete record of INR values was available only for a subset of patients. MACE are not concerned, as additional information was obtained from primary care physicians. Finally, the study was limited to the first year of ST and SM, and does not assess the sustainability of the results after the first year. Additional training or spotcheck supervision may be required for some patients in order to maintain the same level of performance over time. This would need to be investigated in a future study.
The future of long term oral anticoagulation is bright, with new molecules such as dabigatran and rivaroxaban being now close to market approval in some of the classical indications for VKA treatment [20–22]. These new oral anticoagulants offer the substantial advantage of requiring no routine monitoring of coagulation tests or plasma concentrations. It is therefore legitimate to anticipate that they may progressively supplant VKA in most of their major indications, reducing the need for INR ST and SM. This will certainly take time, however, as this step by step approach in broadening indications has not reached some of the high-risk patient groups, such as mechanical valve patients. These patients tend to be good candidates for SM as mechanical valves are typically implanted in younger patients more likely to match the inclusion criteria for SM. The increase in the proportion of mechanical valve patients in our experience from 20% in the first 3 years to 58% in the fourth year may be explained by steadily growing confidence in the programme and by optimised patient selection. We remain confident that there is a future for ST and SM, and welcome every opportunity to improve clinical outcomes and quality of life in patients on chronic oral anticoagulation.
In conclusion, the INR self-testing and self-management programme described could be implemented and led to a high level of patient satisfaction. Based on the data collected during the experience, these good results could be obtained without a price in terms of safety and clinical efficacy, and militate in favour of greater expansion for ST and SM.
Sample questions from the questionnaire on anticoagulation-related knowledge.
1 Filippi A, Sessa E, Trifiro G, Mazzaglia G, Pecchioli S, Caputi AP, et al. Oral anticoagulant therapy in Italy: prescribing prevalence and clinical reasons. Pharmacol Res. 2004;50(6):601–3.
2 Nilsson GH, Bjorholt I, Johnsson H. Anticoagulant treatment in primary health care in Sweden – prevalence, incidence and treatment diagnosis: a retrospective study on electronic patient records in a registered population. BMC Fam Pract. 2003;4:3.
3 Holm T, Lassen JF. How many patients are on oral anticoagulant therapy in Denmark? Methods to estimate the number. Ugeskr Laeger. 2003;165(18):1871–5.
4 Hirsh J. Oral anticoagulant drugs. N Engl J Med. 1991;324(26):1865–75.
5 Hirsh J, Dalen JE, Anderson DR, Poller L, Bussey H, Ansell J, et al. Oral anticoagulants: mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest. 1998;114(5Suppl):445S–69S.
6 Kapiotis S, Quehenberger P, Speiser W. Evaluation of the new method Coaguchek for the determination of prothrombin time from capillary blood: comparison with Thrombotest on KC-1. Thromb Res. 1995;77(6):563–7.
7 Machin SJ, Mackie IJ, Chitolie A, Lawrie AS. Near patient testing (NPT) in haemostasis – a synoptic review. Clin Lab Haematol. 1996;18(2):69–74.
8 Bereznicki LR, Jackson SL, Peterson GM, Jeffrey EC, Marsden KA, Jupe DM. Accuracy and clinical utility of the CoaguChek XS portable international normalised ratio monitor in a pilot study of warfarin home-monitoring. J Clin Pathol. 2007;60(3):311–4.
9 Hentrich DP, Fritschi J, Müller PR, Wuillemin WA. INR comparison between the CoaguChek S and a standard laboratory method among patients with self-management of oral anticoagulation. Thrombosis research. 2007;119(4):489–95.
10 Cromheecke ME, Levi M, Colly LP, de Mol BJ, Prins MH, Hutten BA, et al. Oral anticoagulation self-management and management by a specialist anticoagulation clinic: a randomised cross-over comparison. Lancet. 2000;356(9224):97–102.
11 Menendez-Jandula B, Souto JC, Oliver A, Montserrat I, Quintana M, Gich I, et al. Comparing self-management of oral anticoagulant therapy with clinic management: a randomized trial. Ann Intern Med. 2005;142(1):1–10.
12 Christensen TD, Maegaard M, Sorensen HT, Hjortdal VE, Hasenkam JM. Self-management versus conventional management of oral anticoagulant therapy: A randomized, controlled trial. Eur J Intern Med. 2006;17(4):260–6.
13 Matchar DB, Jacobson A, Dolor R, Edson R, Uyeda L, Phibbs CS, et al. Effect of home testing of international normalized ratio on clinical events. N Engl J Med. 2010;363(17):1608–20.
14 Heneghan C, Alonso-Coello P, Garcia-Alamino JM, Perera R, Meats E, Glasziou P. Self-monitoring of oral anticoagulation: a systematic review and meta-analysis. Lancet. 2006;367(9508):404–11.
15 Fritschi J, Raddatz-Muller P, Schmid P, Wuillemin WA. Patient self-management of long-term oral anticoagulation in Switzerland. Swiss Med Wkly. 2007;137(17–18):252–8.
16 Fitzmaurice DA, Machin SJ. Recommendations for patients undertaking self management of oral anticoagulation. Bmj. 2001;323(7319):985–9.
17 Ansell J, Jacobson A, Levy J, Voller H, Hasenkam JM. Guidelines for implementation of patient self-testing and patient self-management of oral anticoagulation. International consensus guidelines prepared by International Self-Monitoring Association for Oral Anticoagulation. Int J Cardiol. 2005;99(1):37–45.
18 Sobieraj-Teague M, Daniel D, Farrelly B, Coghlan D, Gallus A. Accuracy and clinical usefulness of the CoaguChek S and XS Point of Care devices when starting warfarin in a hospital outreach setting. Thrombosis research. 2009;123(6):909–13.
19 Matchar DB JA, Dolor R, Edson R, Uyeda L, Phibbs CS, Vertrees JE, Shih MC, Holodniy M, Lavori P; THINRS Executive Committee and Site Investigators. Effect of home testing of international normalized ratio on clinical events. N Engl J Med. 2010;363(17):1608–20.
20 Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–51.
21 Garcia D, Libby E, Crowther MA. The new oral anticoagulants. Blood. 2009 Oct 30.
22 Rivaroxaban-once daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation: rationale and design of the ROCKET AF study. Am Heart J. 2010;159(3):340–7 e1.
No financial support and no other potential conflict of interest relevant to this article was reported.