Figure 1
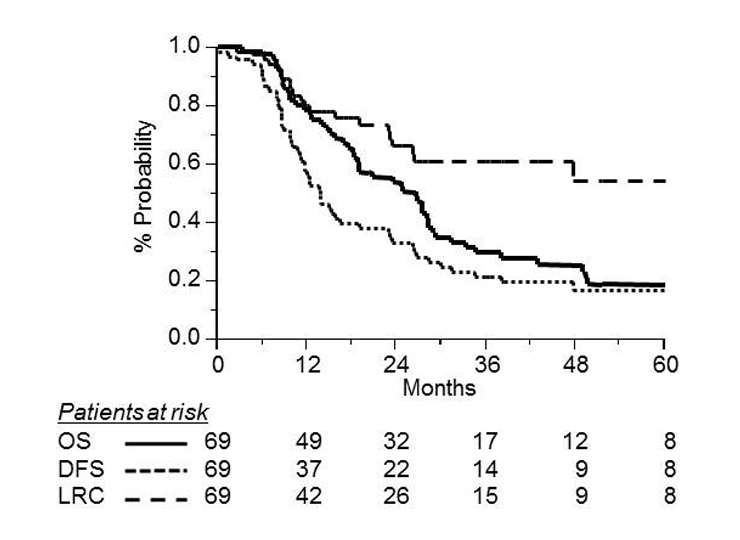
Kaplan-Meier curves for overall survival (OS, solid line; DFS, dotted line; and LRC, dashed line) in 69 patients with limited stage small-cell lung cancer.
DOI: https://doi.org/10.4414/smw.2011.13205
Treatment strategies for small-cell lung cancer (SCLC) have gradually evolved over the past few decades. In the past, chemotherapy (CT) alone was considered the standard therapy for patients with SCLC. However, the overall outcome of patients has remained poor due to both local and systemic relapses [1]. Chest radiotherapy (RT) in combination with CT has become a generally accepted treatment for limited stage SCLC (LDSCLC), since it has been shown to be superior to CT alone [2–3]. Moreover, the routine use of prophylactic cranial irradiation (PCI) has contributed to a reduction in the rate of brain recurrences, and to the improvement in overall survival [4]. During the same period, strategies assessing more dose-intensive RT through hyper-fractionation and/or concomitant platinum-based CT resulted in a greater survival benefit [5, 6].
Although it is clear that chest RT improves both local control and survival [2–3], several important questions including RT dose, RT fractionation, RT volume, timing of CT, and RT/CT sequence of administration remain controversial and unanswered. The local failure rate in the chest remains unacceptably high with moderate RT doses. In the study by Turrisi et al. [5], local failure at 5 years reached 75% with daily RT compared to 42% with twice daily RT. In our institutions, RT doses have been increased progressively since the late 1990s. This study aimed to evaluate the outcome and patterns of failure in patients with LDSCLC treated with radiation of at least 50 Gy in two institutions.
A retrospective study of 69 consecutive patients with LDSCLC, treated between 1997 and 2006, was performed. There were 23 (33%) women and 46 (67%) men. The median age was 60 years (range, 36–78 years). Most patients had one or more symptoms. Dyspnea was the most common presenting symptom accounting for 35% (n = 24) of presentations, followed by cough 17% (n = 12), chest pain 19% (n = 13), and superior vena cava obstruction 4% (n = 3). Four patients (6%) were referred for incidental abnormal findings on chest radiographs, and seven patients (10%) were found to have paraneoplastic syndromes, either inappropriate anti-diuretic hormone syndrome or Lambert Eaton syndrome.
Concurrent malignancy was found in 2 patients (3%): one with in-operable rectal cancer treated with palliative stenting, and the other with early breast cancer treated with standard conservative treatment.
Upon referral, minimal diagnostic workup consisted of a physical examination, bronchoscopy, complete blood count, blood biochemistry and imaging studies. Besides chest radiography, computed tomography of the chest and upper abdomen, brain computed tomography or magnetic resonance imaging (MRI), radionucleide bone scan and, more recently, positron emission tomography (PET) were done. A total of 23% of the patients underwent bone-marrow aspiration and nine patients (13%) underwent mediastinoscopy (n = 8) or mediastinostomy (n = 1). All patients had biopsy-proven SCLC.
Patients were classified as limited-stage disease when their tumour was confined to one hemithorax with or without hilar, mediastinal or supraclavicular nodes, and treatable within a single radiotherapy volume. All of the patients had LDSCLC according to the 2002 UICC/AJCC classification system for lung cancer [7, 8]. For patients treated before 2002, staging was updated from the clinical description to fit the new classification. There were 7 (10%) T1, 25 (36%) T2, 15 (22%) T3, and 22 (32%) T4 tumours. The N-classification included 2 (3%) patients with Nx, 8 (12%) patients with N0, 5 (7%) with N1, 37 (53%) with N2, and 17 (25%) with N3 disease. Patient characteristics are presented in table 1.
| Table 1: Patient characteristics in 69 patients with small cell lung cancer. | ||
| Characteristics | ||
| Median age (yrs) | 60 | 36–78 |
| Median amount of smoking (packs per year) | 50 | 20–120 |
| N | % | |
| Gender | ||
| Male | 46 | 66 |
| Female | 23 | 33 |
| Performance status (WHO) | ||
| 0 | 51 | 75 |
| 1 | 16 | 23 |
| 2 | 1 | 1 |
| Not known | 1 | 1 |
| T-classification (UICC) | ||
| T1 | 7 | 10 |
| T2 | 25 | 36 |
| T3 | 15 | 22 |
| T4 | 22 | 32 |
| N-classification (UICC) | ||
| Nx | 2 | 3 |
| N0 | 8 | 12 |
| N1 | 5 | 7 |
| N2 | 37 | 53 |
| N3 | 17 | 25 |
| Chemotherapy | ||
| Sequential | 47 | 68 |
| Concomitant | 22 | 32 |
| Thoracic radiation | ||
| Median dose (Gy) | 60 | 20–66 |
| Median dose/fraction (Gy) | 1.8 | 1.5–2 |
| PCI use | ||
| Yes | 47 | 68 |
| No | 22 | 32 |
| Abbreviation: UICC: Union Internationale Contre le Cancer; WHO: World Health Organisation; PCI: prophylactic cranial irradiation | ||
Varied chemotherapy regimens were given because patients were referred from different oncologists. Patients were treated with etoposide (100 mg/m² on days 1–3), and either cisplatin (100 mg/m² on day 1; n = 25) or carboplatin (AUC 5; n = 16). In 4 patients, carboplatin was substituted for cisplatin because of clinically significant ototoxicity or sensory neural damage. Etoposide and cisplatin (EP) or carboplatin (CP) were combined with adriamycin (n = 4) or paclitaxel (n = 1).
Ten patients received the ICE regimen, which consisted of ifosfamide (5 g/m², day 1), carboplatin (300 mg/², day 1) and etoposide (180 mg/m², day 1–2). Nine other patients were treated with induction chemotherapy using epirubicin and paclitaxel followed by intensification with a ifosfamide (2.5 g/m2 day 1–4), carboplatin (AUC 5, day 1–4) and etoposide (300 mg/m2, day 1–4) regimen, with mesna followed by autologous stem cell infusion. At least 4 cycles (median, 6) of chemotherapy were given, each cycle separated by 21–28 days.
A total of 22 patients (32%) received RT concurrently with chemotherapy, and 47 (68%) received RT sequentially to chemotherapy. The sequential RT was used when tumours were so large that concurrent chemo-radiotherapy was considered to carry a high risk of severe radiation pneumonitis. Consolidating thoracic 3-dimensional conformal RT was given to all patients. Megavoltage equipment was used with energies varying from 6 to 18 MV. RT was delivered to the primary tumour, ipsilateral hilum, and mediastinal lymph nodes to a dose of 40 Gy in daily fractions of 1.8–2 Gy using mainly parallel opposed antero-posterior and postero-anterior fields (n = 59), or 3 fields (n = 8). The supraclavicular region was included only when involved. Oblique off-cord fields, including the site of primary tumour and involved lymph nodes, were used for the remaining treatment course. The dose of RT delivered to the lung was limited to 35% of the volume receiving no more than 20 Gy. The spinal cord dose was limited to 45 Gy. The majority of patients were treated with a daily fractionation of 1.8–2 Gy. The treatment was administered twice daily in 5 patients (1.5 Gy per fraction, with 6 hours or more between fractions). The median total dose was 60 Gy (range, 20–66 Gy). The median overall treatment time was 42 days (range, 22–64).
Following treatment, 47 patients (68%) also received a prophylactic cranial irradiation (PCI) delivered by lateral opposing fields using 6–18 MV photons. This treatment was proposed to all patients without any progressive disease. Five patients refused the treatment, one had progressive disease and in 16 patients the reason for not doing PCI was unknown. The median total dose was 30 Gy (range, 26–38) in daily 1.8–2 Gy fractions.
The initial tumour response was based on CT scans following completion of the treatment. Complete response (CR) was defined as the complete disappearance of all objective evidence. Near complete response (near-CR) was defined as a greater than 90% reduction in the maximum diameter of the tumour with persistent scanographic abnormalities. Partial response (PR) was defined as regression of 50% or more of the tumour,, and progressive disease (PD) was defined as an increase of at least 25% of the tumour. Distribution of the treatment characteristics by treatment arm, sequential RT versus concomitant RT, are presented in table 2. After completion of the treatment, patients were seen for response and toxicity testing every 3 months for the first 2 years, every 6 months until 5 years, and yearly thereafter. A computed tomography scan of the chest was obtained every 6 months for 2 years, and yearly for the following 3 years.
| Table 2: Distribution of treatment characteristics by treatment arm-sequential (S; n = 47) versus concomitant(C; n = 22) CT/RT. | ||
| Characteristics | S (%) | C (%) |
| Type of chemotherapy | ||
| EP/Carboplatin-Etoposide | 25 (53) | 20 (90) |
| ICE | 19 (41) | |
| Others | 3 (6) | 2 (10) |
| PCI use | ||
| Yes | 35 (75) | 12 (54) |
| No | 12 (25) | 10 (46) |
| Response to treatment* | ||
| CR | 10 (21) | 9 (41) |
| nCR | 13 (28) | 4 (18) |
| PR | 23 (49) | 7 (32) |
| Abbreviation: CT/RT: chemo-radiotherapy; EP: etoposide-cisplatin; ICE: ifosfamide, carboplatin, etoposide; PCI: prophylactic cranial irradiation; CR: complete response; nCR: near complete response; PR: partial response;*data missing in 3 patients. | ||
Mean values were compared by the Student’s t-test. Proportions were compared using the chi-square test for values greater than 5, and Fisher’s exact test for those less than or equal to 5. Kaplan-Meier product-limit estimates were used to evaluate overall survival (OS), disease-free survival (DFS), and loco-regional control (LRC) (9). Univariate analyses included all variables thought to be influencing the outcome (age, gender, World Health Organisation (WHO) performance status (PS), T- and N-classification, timing of RT, PCI use, treatment response, and thoracic RT dose). Multivariate analysis (Cox Model) included WHO PS, timing of RT, PCI use, treatment response, and thoracic RT dose. Time to any event was measured from the date of pathological diagnosis. The events were death (all causes of death included) for OS, death or any relapse for DFS, and loco-regional relapse for LRC (patients who died without local or loco-regional relapse were censored at the time of death), respectively. Confidence intervals (CI) were calculated from standard errors. Differences between groups were assessed using the log-rank test [10]. The Bonferroni method was used to adjust the individual p-values in order to obtain overall significance levels depending on the number of parameters tested (p-adjusted equals individual p-value times number of parameters tested) [11].
With a median follow-up of 36 months (range, 6–107 months), 16 (23%) of the 69 patients remained alive without disease. Causes of death included SCLC in 48, and treatment-related toxicity in 2 patients.

Figure 1
Kaplan-Meier curves for overall survival (OS, solid line; DFS, dotted line; and LRC, dashed line) in 69 patients with limited stage small-cell lung cancer.
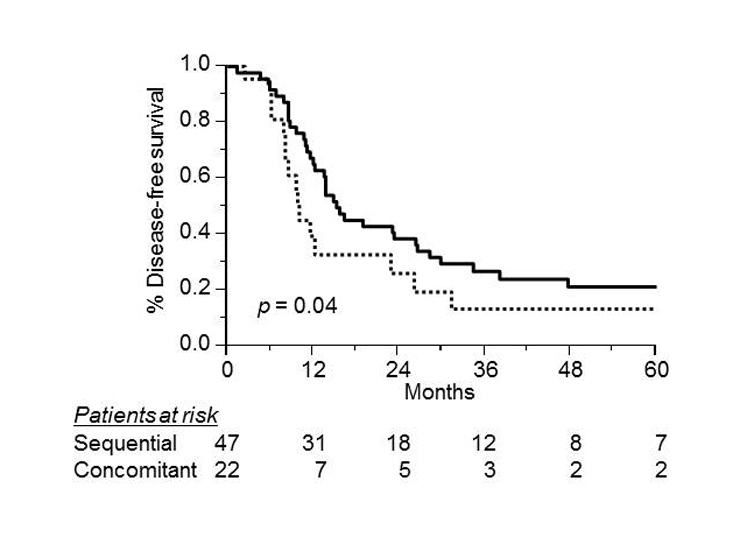
Figure 2
Disease-free survival according to sequential (solid line, n = 47) versus concomitant (dotted line, n = 22) chemotherapy in 69 patients with limited stage small-cell lung cancer (log rank test, p = 0.02).

Figure 3
Loco-regional control rate according to sequential (solid line, n = 47) versus concomitant (dotted line, n = 22) chemotherapy in 69 patients with limited stage small-cell lung cancer (log rank test, p = 0.04).
Among all patients, 19 (29%), 30 (45%), and 17 (26%) patients achieved a CR, a near-CR and a PR, respectively. Three patients progressed during treatment. Of the 69 patients, 22 had chest failure (31%), and 36 patients (52%) developed distant metastases at one or more sites. Brain relapse was identified in 17 patients (24%), of whom 9 had received PCI. Twelve (17%) patients relapsed only in the brain, including 5 patients after PCI. Other sites of metastases were the liver (n = 8), bone (n = 7), adrenals (n = 7), and retroperitoneal lymph nodes (n = 4).
The median OS for all patients was 24 months. The 3-year OS, DFS and LRC rates for all 69 patients were 29% (95% CI, 18–40%), 23% (95% CI, 13–33%), and 60% (95% CI, 47–73%), respectively (fig. 1).
We examined the effect of various factors on OS in univariate analyses (table 3), including age, gender, WHO PS, T- and N-classification, timing of RT, PCI use, treatment response, and thoracic RT dose. Response to treatment was the only significant factor (p = 0.04) for overall survival. Patients with CR had better outcomes than patients with PR (35% vs 16%). Although not statistically significant, sequential chemo-radiotherapy appeared to be superior to concomitant chemo-radiotherapy. At 3 years, patients in the sequential group had an OS rating of 32%, whereas patients in the concomitant group had a 22% rating (p = 0.21). Likewise, a trend toward improved survival was observed for patients with a good PS (p = 0.06) and in patients receiving PCI (p = 0.07). The 3-year OS was 35% (95% CI, 21–49%) for patients who underwent PCI compared with 17% (95% CI, 0–34%) for those not receiving PCI. The multivariate analysis showed that improved survival was associated with a complete response to treatment (relative risk (RR = 3.39; p = 0.04).
In the univariate analysis, better DFS was significantly associated with the timing of RT, performance status 0, PCI group and complete response to treatment, but no effect was seen with age, gender, thoracic RT dose, or T and N classification (table 3). The use of sequential CT/RT correlated significantly with DFS. The 3-year DFS rate was 27% (95% CI, 15–39%) for those receiving sequential CT/RT, and 13% (95% CI, 0–28%) for those receiving the concomitant RT (p = 0.04; fig. 2). A better DFS was significantly associated with PS 0 (p = 0.004), complete response to treatment (p = 0.03), and PCI group (p = 0.03). The 3-year DFS was 27% (95% CI, 14–40%) for patients who underwent PCI compared with 14% (95% CI, 0–27%) for those not receiving PCI. Among the 16 patients who remained alive without disease, 13 received PCI and 11 of these were in the sequential group. In the multivariate analysis, improved DFS was only associated with PS 0 group (RR = 2.44; p = 0.008).
In the univariate analysis (table 3), among the variables tested, the timing of RT significantly influenced the 3-year LRC (p = 0.02). At 3 years, the local control rate was 68% (95% CI, 53–83%) for the sequential group and 42% (95% CI, 15–69%) for the concurrent group (fig. 3). The response to treatment also significantly influenced the 3-year LRC (43% for PR vs 70% for CR; p = 0.04). A subgroup analysis was performed to elucidate the differences in outcomes between patients treated with a median dose of ≥60 Gy and those treated with a dose of <60 Gy. No statistically significant differences were found in local control, between the two groups (p = 0.78). The 3-year LRC was 60% (95% CI, 39–81%) for patients treated with <60 Gy versus 61% (95% CI, 44–78%) for those receiving ≥60 Gy. In the multivariate analysis, improved LRC was associated with sequential RT/CT (RR = 1.74; p = 0.02), and complete response (RR = 1.76; p = 0.01)
All patients but one received their planned course of RT. This patient with concomitant rectal cancer refused further chest radiation after 20 Gy of a planned 60 Gy. He was treated with supportive care alone. Toxicity was scored according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 3.0 [12]. It was assessable in 61 patients (88%). Details regarding toxicity are listed in table 4. The most common grade 3 or worse toxicity was myelosuppression, followed by nausea, vomiting, pulmonary toxicity, and esophagitis. In general, ICE CT tended to cause more febrile neutropenia and thrombocytopenia than the EP regimen, the latter causing more nephrotoxicity and neurotoxicity. Of the 69 patients, 2 (3%) died of treatment-related toxicity (Grade 5). One death was due to acute herpetic encephalitis secondary to late pancytopenia, and the other was related to acute pneumonia. Six cases of radiation pneumonitis (one grade 3) were observed, as well as 2 cases of mild esophagitis (grade 2).
| Table 3: Univariate analyses (log-rank test) on disease-free survival, overall survival and loco-regional control. | |||||||||||||
| N | 3-year DFS (%) | %95 CI (%) | p-value | p-value* | 3-year OS (%) | %95 CI (%) | p-value | p-value* | 3-year LRC (%) | %95 CI (%) | p-value | p-value* | |
| All patients | 69 | 23 | 13–33 | - | - | 29 | 18–40 | - | - | 60 | 47–73 | - | - |
| Age (yrs) | |||||||||||||
| <61 | 36 | 22 | 8–34 | 0.97 | NS | 25 | 11–39 | 0.80 | NS | 53 | 33–83 | 0.23 | NS |
| ≥61 | 33 | 24 | 11–37 | 33 | 16–50 | 68 | 47–89 | ||||||
| PS | |||||||||||||
| 0 | 51 | 27 | 16–38 | 0.004 | S | 31 | 20–42 | 0.06 | NS | 60 | 40–80 | 0.6 | NS |
| 1-2 | 18 | 12 | 0–27 | 25 | 4–46 | 49 | 32–66 | ||||||
| Gender | |||||||||||||
| Female | 23 | 32 | 8–30 | 0.30 | NS | 35 | 15–55 | 0.5 | NS | 73 | 53–93 | 0.34 | NS |
| Male | 46 | 19 | 7–31 | 26 | 12–40 | 54 | 36–72 | ||||||
| Clinical T-classification | |||||||||||||
| T1 | 7 | 57 | 21–93 | 0.47 | NS | 57 | 22–92 | 0.86 | NS | 100 | - | 0.17 | NS |
| T2 | 25 | 23 | 3–43 | 24 | 4–44 | 64 | 41–87 | ||||||
| T3 | 15 | 14 | 5–23 | 32 | 7–57 | 33 | 6–60 | ||||||
| T4 | 22 | 18 | 2–34 | 22 | 4–40 | 65 | 42–88 | ||||||
| Clinical N-classification** | |||||||||||||
| N0 | 8 | 38 | 4–72 | 0.41 | NS | 37 | 5–69 | 0.63 | NS | 70 | 35–100 | 0.07 | NS |
| N1 | 5 | 60 | 18–100 | 60 | 20–100 | 100 | |||||||
| N2 | 37 | 16 | 5–27 | 28 | 16–44 | 38 | 18–58 | ||||||
| N3 | 17 | 21 | 2–40 | 20 | 0–40 | 93 | 79–100 | ||||||
| Thoracic RT dose (Gy) | |||||||||||||
| <60 | 33 | 21 | 7–35 | 0.46 | NS | 29 | 13–42 | 0.52 | NS | 60 | 39–81 | 0.80 | NS |
| ≥60 | 36 | 25 | 11–39 | 30 | 11–47 | 62 | 42–82 | ||||||
| Chemotherapy | |||||||||||||
| Sequential | 47 | 27 | 16–38 | 0.04 | NS | 32 | 18–50 | 0.21 | NS | 68 | 53–83 | 0.02 | NS |
| Concomitant | 22 | 13 | 0–28 | 22 | 2–42 | 42 | 15–69 | ||||||
| Response to treatment*** | |||||||||||||
| PR | 30 | 11 | 0–22 | 0.04 | NS | 16 | 2–30 | 0.04 | NS | 43 | 20–66 | 0.04 | NS |
| CR-nCR | 36 | 29 | 14–44 | 35 | 19–51 | 70 | 52–88 | ||||||
| PCI | |||||||||||||
| Yes | 47 | 27 | 14–40 | 0.03 | NS | 35 | 21–49 | 0.07 | NS | 67 | 51–83 | 0.05 | NS |
| No | 22 | 14 | 0–27 | 17 | 0–34 | 45 | 20–70 | ||||||
| * Bonferroni correction;NS: not significant; S: significant; RT: radiotherapy; PCI: prophylactic cranial irradiation; DFS: disease-free survival; OS: overall survival; LRC: locoregional control;** data missing in 2 patients ; *** data missing in 3 patients; PR: partial response; CR: complete response; nCR: near complete response. | |||||||||||||
| Table 4: Acute and late toxicity in *patients with small cell lung cancer treated with chemoradiation (CTCAE v3.0 classification). | |||||
| Toxicity | Grade 0–2 | Grade 3 | Grade 4 | Total | % of total |
| Acute | |||||
| Febrile neutopenia | - | 17 | 4 | 21 | 34 |
| Leukopenia | 5 | 3 | 1 | 9 | 15 |
| Anaemia | 12 | 5 | 1 | 18 | 29 |
| Thrombocytopenia | 1 | 1 | 5 | 7 | 11 |
| Dysphagia | 5 | 5 | 8 | ||
| Neuropathy | 6 | 1 | 3 | 10 | 16 |
| Nephropathy | 6 | 1 | 7 | 11 | |
| Nausea-vomiting | 12 | 4 | 16 | 26 | |
| Ototoxicity | 2 | 2 | 3 | ||
| Late | |||||
| Pneumonitis | 5 | 1 | 6 | 9 | |
| Esophagitis | 2 | 2 | 3 | ||
| Leucoencephalopathy | 2 | 2 | 3 | ||
| Memory impairment | 2 | 2 | 3 | ||
| *no toxicity data were available in 8 patients; CTCAE: Common Terminology Criteria for Adverse Events | |||||
Optimising the management of patients with LDSCLC continues to be a challenge. To date, the optimal timing of chest RT is still controversial. Some studies and meta-analyses have shown that the early administration of RT, compared to late radiation, may improve significantly the outcome [13–17] while others have not [1, 18–20]. However, these studies are difficult to compare given the heterogeneity in the therapeutic modalities: RT varied in terms of total dose [1, 5, 16] and fractionation schemes: conventional [1, 18] versus twice-daily regimen [5, 16–17, 21]. Likewise, the drugs used in CT, and the dose intensity varied within previous studies [5, 14].
At present, there is only one prospective study, specifically addressing the role of concomitant versus sequential CT/RT in LDSCLC (17). In this study from the Japanese Clinical Oncology Group, 231 patients received 4 cycles of cisplatin plus etoposide, and were randomly assigned to either sequential or concurrent twice daily 45-Gy thoracic RT. Although the median survival time for patients receiving concurrent therapy was improved compared with patients receiving sequential treatment (27.2 vs 19.7 months), there was no statistically significant difference (p = 0.10). In many centres, patients are still receiving sequential CT/RT because of initial tumour bulk requiring large fields. In the present study, the DFS (p = 0.04) but not the OS (p = 0.21) was significantly better in the group receiving sequential CT/RT. At 3 years, the LRC was 68% for the sequential group, and 42% for the concurrent group (p = 0.01). However, our findings should be carefully interpreted due to the small sample size and retrospective setting. One explanation for this finding may be related to the heterogeneity of CT regimens. In this study, 41% of the patients in the sequential group received the ICE regimen, while no patient received the ICE regimen in the concomitant group. The better DFS in the sequential group can also be explained by the fact that PCI was delivered more frequently for the sequential group than the concomitant group (table 2).
Chemotherapeutic regimens have evolved from cyclophosphamide, doxorubicin, vincristine (CAV) regimens (22) to cisplatin–based combinations because of their survival advantage in limited stage (23), as well as better tolerance in combination with chest RT. Since the mid 1980s, the cisplatin and etoposide regimen was considered as the standard treatment in these patients [6, 21]. This combination yielded a response rate ranging from 60–90% [6, 24]. The addition of ifosfamide to EP or CP regimens, called VIP or ICE, have been tested against the standard EP with modest survival benefits [25–27]. In order to increase the therapeutic ratio, some investigators have attempted more intensive chemotherapy, which appears to be very toxic [28]. Moreover, when associated with early concurrent chest RT, VIP failed to show survival benefits due to higher treatment-related mortality.
Other agents and chemotherapeutic combinations are also active. Preliminary data from phase I/II randomised trials testing the addition of paclitaxel to EP show an improvement in response, and acceptable toxicity in the taxane-containing arm [29]. However, when this combination was tested in phase III trials, it was associated with excessive toxicity and mortality [30].
Nevertheless, paclitaxel was tested in combination with radiation therapy. A phase II study of 38 patients was conducted to assess the feasibility of a combination of paclitaxel, carboplatin and etoposide, with concurrent involved field-RT; this demonstrated high response rates and safety. A 5-year OS of 27% was observed with a very low recurrence in the chest despite a total dose of 45 Gy given once daily [31]. Surprisingly, the rate of brain metastases as the first site of failure was high (13 out of 19 patients) in this study.
When addressing the issue of the optimal dose to control local disease, no study has provided a firm conclusion regarding this issue. Since SCLC has been considered an RT-sensitive disease, modest doses have been used in the range of 40–50 Gy [1, 14]. A dose-response relationship was suggested by one phase III trial, and two studies from the Massachusetts General Hospital [32–34]. Doses of 40, 50 and 57 Gy resulted in a local failure rate of 49%, 37%, and 22% respectively [33]. Turrisi et al. [4] reported dose intensification for patients with LDSCLC through hyper-fractionated and accelerated RT. In this study, a dose of 45 Gy twice-daily RT in 3 weeks was compared to 45 Gy daily fractionated RT starting with the first cycle of cisplatin plus etoposide CT for the 2 arms. Improved overall survival was observed in the twice-daily regimen, with a 5-year overall survival rate of 26% versus 16% for the once-daily regimen. The local control rate was 64% for the twice-daily arm versus 48% for the once-daily schedule. However, a higher rate of grade 3 or 4 esophagitis was observed with the twice-daily schedule (27% vs 11%). Conversely, Bonner et al. [35] compared a split-course hyper-fractionated RT to conventional RT, and did not find any survival benefit with RT starting at the fourth or fifth cycle of CT. Moreover, a split-course regimen was used in the hyper-fractionated group, which is considered to probably have an unfavourable biological effect. Thus, the benefit found by the Eastern Cooperative Oncology Group [35] seems to be related to the dose intensity of hyper-fractionation and CT rather than hyper-fractionation alone. However, when deciding which dose and fractionation to deliver, clinicians are reluctant to use the twice-daily regimen, since it is not only associated with high toxicity but is also inconvenient for daily RT practice. Dose escalation of daily fractionated RT is another method of dose intensification. A conventional fractionation schema is chosen with higher total dose, at least biologically equivalent to the 45 Gy. In 1998, Choi et al. [36] reported a dose-escalation phase I radiotherapy trial. Patients were either treated with conventional thoracic irradiation or twice-daily fractionation. The maximum-tolerated dose of radiation was determined to be 70 Gy (in 35 fractions of 2 Gy) for daily RT. Likewise, Bogart et al. [37] reported results of a phase II, dose escalation, multi-institutional, prospective trial. In this study, the total dose was 70 Gy delivered once-daily with concurrent carboplatin/etoposide CT following induction CT with paclitaxel and topotecan. Such dose escalation produced acceptable toxicity with 16% reported grade 3 toxicity. Moreover, the 2-year overall survival and failure-free survival rates were 48% and 31%, respectively. In our present study, the median radiation dose was 60 Gy, and there was no difference in terms of outcome between patients receiving doses of 60 Gy or more and patients receiving 60 Gy or less.
In LDSCLC, a meta-analysis of studies assessing the use of PCI revealed an absolute survival benefit of 5.4% at 3-years favouring PCI in patients achieving a complete remission [4]. The 3-year survival rate was 15.3% in the control group versus 20.7% in the treatment group. However, a number of patients still refuse this treatment because of the risk of neuro-cognitive function alteration. In the present series, 47 patients (68%) received PCI. Improved DFS was observed in patients receiving PCI (p = 0.03). Moreover, a trend toward improved survival was observed for patients who underwent PCI (p = 0.07). The 3-year OS was 35% for patients who underwent PCI compared with 17% for those not receiving PCI. Furthermore, PCI was recently recommended even for patients with extensive SCLC [38], where a clear advantage of PCI was found with respect to the incidence of symptomatic metastases. A survival advantage was also found.
In summary, in this study no RT dose-response relationship was observed. Complete response to treatment seems to be the most important factor for survival and local control. A better DFS was significantly associated with PCI group.
1 Perry MC, Eaton WL, Propert KJ, et al. Chemotherapy alone or chemotherapy with chest radiation therapy in limited small-cell carcinoma of the lung. N Engl J Med. 1987;316:912–8.
2 Pignon JP, Arriagada R, Ihde DC, et al. Meta-analysis of thoracic radiotherapy for small-cell lung cancer: unanswered questions. Squamous cell carcinoma of the anal margin. N Engl J Med. 1992;327:1618–24.
3 Warde P, Payne D. Does thoracic irradiation improve survival and local control in limited small-cell carcinoma of the lung? A meta-analysis. J Clin Oncol. 1992;10:890–5.
4 Auperin A, Arriagada R, Pignon JP, Le Péchoux C, Gregor A, Stephens R, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. N Engl J Med. 1999;341:476–84.
5 Turrisi AT, Kyungmann K, Blum R ,Sause WT, Livingstone RB, Komaki R, et al. Twice-daily compared with once daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med. 1999;340:265–71.
6 Evans WK, Shepherd FA, Feld, et al. VP-16 and cisplatin as first-line therapy for small-cell lung cancer. J Clin Oncol. 1985;3:1471–7.
7 American Joint Committee on Cancer: AJCC cancer staging manual. New York: Springer; 2002.
8 International Union Against Cancer: TNM classification of malignant tumours. New York: Wiley-Liss, Inc.; 1998.p.17–47.
9 Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81.
10 Peto P, Pike MC, Armitage P, et al. Design and analysis of randomised clinical trials requiring prolonged observation of each patient: Part II. Br J Cancer. 1977;35:1–39.
11 Beck-Bornholdt HP, Dubben HH. Potential pitfalls in the use of p-values and in interpretation of significance levels. Radiother Oncol. 1994;33:171–6.
12 Trotti A, Colevas AD, Setser A, et al. CTCAE v.3: Development of a comprehensive Grading System for the adverse events of cancer treatment. Semin Radiat Oncol. 2003;13:176–81.
13 Fried DB, Morris DE, Poole C, et al. Systematic review evaluating the timing of thoracic radiotherapy in combined-modality therapy for limited-stage small-cell lung cancer. J Clin Oncol. 2004;23:4785–93.
14 Murray N, Coy P, Pater JL, et al. Importance of timing for thoracic irradiation in the combined modality treatment of limited stage small-cell lung cancer. J Clin Oncol. 1993;11:336–44.
15 De Ruysscher D, Pijls-Johannesma M, Van-steenkiste J, et al. Systematic review and meta-analysis of randomised, controlled trials of the timing of chest radiotherapy in patients with limited stage small-cell lung cancer. Ann Oncol. 2006:17;543–52.
16 Jeremic B, Shibamato Y, Acimovic L, et al. Initial versus delayed accelerated hyperfractionated radiation therapy and concurrent chemotherapy in limited small-cell lung cancer: A randomized study. J Clin Oncol. 1997;15:893–900.
17 Takada M, Fukuoka M, Kawahara M, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with cisplatin and etoposide for limited-stage small – cell lung cancer: results of the Japan Clinical Oncology Group Study 9104. J Clin Oncol. 2002;20:3054–60.
18 Gregor A, Drings P, Burghouts J, et al. Randomized trial of alternating versus sequential radiotherapy/chemotherapy in limited-disease patients with small-cell lung cancer: A European Organization for Research and Treatment of Cancer Lung Cancer Cooperative Group Study. J Clin Oncol. 1997;15:2840–9.
19 Spiro SG, James LE, Rudd RM, et al. Early compared with late radiotherapy in combined-modality treatment for limited-disease small-cell lung: A London Lung Cancer Group multicenter randomized clinical trial and meta-analysis. J Clin Oncol. 2006;24:3823–30.
20 Perry MC, Herndon JE III, Eaton WL, et al. Thoracic radiation therapy added to chemotherapy for small cell lung cancer: An update of Cancer and Leukemia Group B study 8083. J Clin Oncol. 1998,16:2466–7.
21 Skarlos DV, Samantas E, Briassoulis E, et al. Randomized comparison of early versus late hyperfractionated thoracic irradiation concurrent with chemotherapy in limited-disease small-cell lung cancer: A randomized phase II study of the Hellenic Cooperative oncology group(HeCOG). Annal Oncol. 2001;12:1231–8.
22 Albain KS, Crowly JJ, LeBlanc M, et al. Determinants of improved outcome in small-cell lung cancer: An analysis of the 2,850-patient Southwest Oncology Group data base. J Clin Oncol. 1990;8:1563–74.
23 Sundstrom S, Bremnes RM, Kaasa S, et al. Cisplatin and etoposide regimen is superior to cyclophosphamide, epirubicin, and vincristine regimen in small-cell lung cancer: results from a randomized phase III trial with 5 year’s follow-up. J Clin Oncol. 2002;20:4665–72.
24 Wolf M, Havemann K, Holle R, et al. Cisplatin/etoposide combination versus ifosfamide-etoposide combination in small-cell lung cancer: A multicenter German randomized trial. J Clin Oncol. 1987;5:1880–9.
24a Loehrer PJ Sr, Ansari R, Gonin R, et al. Cisplatin plus etoposide with and without ifosfamide in extensive small-cell lung cancer: A Hoosier oncology group study. J Clin Oncol. 1995;13:2594–9.
25 Thatcher N. Ifosfamide, carboplatin, etoposide (ICE) regimen in small cell lung cancer. Lung Cancer. 1993;9:51–4.
26 Fetscher S, Brugger W, Engelhardt R, et al. Dose-intense therapy with etoposide, ifosfamide, cisplatin, and epirubicin (VIP-E) in 100 consecutive patients with limited-and extensive-disease small-cell lung cancer. Ann Oncol. 1997;8:49–56.
27 Leyvraz S, Pampallona S, Martinelli G, et al. A threefold dose intensity treatment with ifosfamide, carboplatin, and etoposide for patients with small cell lung cancer: A randomized trial. JNCI. 2008;100:533–41.
28 Glisson BS, Kurie JM, Perez-Soler R, et al. Cisplatin, etoposide and paclitaxel in the treatment of patients with extensive small cell lung carcinoma. J Clin Oncol. 1999;17:2309–13.
29 Mavroudis D, Papadakis E, Georgoulias V, et al. A multicenter randomized clinical trial comparing paclitaxel-cisplatin-etoposide versus cisplatin-etoposide as first-line treatment in patients with small-cell lung cancer. Ann Oncol. 2001;12:463–70.
30 Baas P, Belderbos JSA, Senan S, et al. Concurrent chemotherapy (carboplatin, placlitaxel, etoposide) and involved-field radiotherapy in limited stage small cell lung cancer: a Dutch multicenter phase II study. BJC. 2006;94:625–30.
31 Choi NC, Carey RW. Importance of radiation dose in achieving improved loco-regional tumor control in limited stage small-cell lung carcinoma. Int J Radiat Oncol Biol Phys. 1989;17:307–10.
32 Roof KS, Fidias P, Lynch TJ, et al. Radiation dose escalation in limited-stage small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2003;57:701–8.
33 Coy P, Hodson I, Payne DG, et al. The effect of dose of thoracic irradiation on recurrence in patients with limited stage small cell lung cancer. Initial results of a Canadian multicenter randomized trial. Int J Radiat Oncol Biol Phys. 1988;14:219–26.
34 Bonner JA, Sloan JA, Shanahan TG, et al. Phase III comparison of twice-daily split-course irradiation versus once-daily irradiation for patients with limited stage small-cell lung carcinoma. J Clin Oncol. 1999;17:2681–91.
35 Choi N, Herndon J, Rosenman J, et al. Phase I study to determine the maximum-tolerated dose of radiation in standard daily and hyperfractionated-accelerated twice daily radiation schedules with concurrent chemotherapy for limited-stage small-cell lung cancer. J Clin Oncol. 1998;16:3528–36.
36 Bogart JA, Herndon JE, Lyss A, et al. 70 Gy thoracic radiotherapy is feasible concurrent with chemotherapy for limited-stage small-cell lung cancer: Analysis of Cancer and Leukaemia Group B study 39808. Int J Radiat Oncol Biol Phys. 2004;59:460–8.
37 Slotman B, Faivre-Finn C, Kramer G, et al. Prophylactic cranial irradiation in extensive small cell lung cancer. N Engl J Med. 2007;357:664–70.
Funding / potential competing interests: No financial support and no other potential conflict of interest relevant to this article were reported.
Authors’ contribution: *Kaouthar Khanfir and Mohamed Elhfid contributed equally to this work; **Mahmut Ozsahin and Abderrahim Zouhair contributed equally as a senior author to this work
Presented at the 49th Annual Meeting of the American Society for Therapeutic Radiology and Oncology, Los Angeles, CA, October 28 and November 1, 2007.