Validation of prognostic factors and survival of patients with multiple myeloma in a real-life autologous stem cell transplantation setting: a Swiss single centre experience
DOI: https://doi.org/10.4414/smw.2011.13203
P
Samaras, M
Blickenstorfer, SR
Haile, D
Siciliano, U
Petrausch, A
Mischo, M
Zweifel, H
Honegger, U
Schanz, G
Stüssi, S
Bauer, A
Knuth, F
Stenner-Liewen
Summary
PRINCIPLES: High-dose chemotherapy with subsequent autologous stem cell transplantation (ASCT) is an important treatment option in younger patients with multiple myeloma (MM). We analysed the outcome of patients treated at our institution outside the clinical trials framework and tried to identify risk factors prognostic for survival.
METHODS: Medical histories of the patients were screened for response, event-free survival (EFS) and overall survival (OS). Pre-transplant variables were analysed to identify possible prognostic risk factors.
RESULTS: Overall, 182 ASCT were performed in 120 patients with MM from 2002 to 2007. Treatment-related mortality (TRM) was 0.5%. Median EFS was 23.1 months (95% confidence interval [CI]: 19.4–28.4) and median OS was 49.8 months (95%CI: 43.7–not reached) in the whole patient population. The median OS in patients who received one ASCT was 46.4 months (95%CI: 35.2–not reached), and 63.7 months (95%CI: 48.9–not reached) in patients who underwent double ASCT.
Patients who already achieved a complete remission (CR) before ASCT had a longer EFS (p = 0.016) than patients without CR. Additionally, patients who achieved a CR after ASCT had a longer EFS (p = 0.0061) and OS (p = 0.0024) than patients without CR. ISS stage <III at first diagnosis strongly correlated with improved EFS (p = 0.0006) and OS (p <0.0001).
CONCLUSIONS: ASCT is a safe and effective treatment mode in eligible patients with MM. TRM was below average at our institution. Achievement of CR after transplantation was the most valuable predictor for improved overall survival.
List of abbreviations
ANC absolute neutrophil count
ASCT autologous stem cell transplantation
BEAM high-dose carmustine, etoposide, cytarabine and melphalan
BMI body mass index
CD34 cluster of differentiation 34
CI confidence interval
CR complete remission
CT computed tomography
Dex dexamethasone
DS Durie Salmon
EFS event free survival
HR hazard ratio
IMIDs immunomodulatory derivatives
ISS international staging system
M2 cyclophosphamide, carmustine, melphalan, prednisone
MM multiple myeloma
MR minor remission
nCR near complete remission
OS overall survival
PD progressive disease
PR partial remission
SD stable disease
Thal thalidomide
TRM treatment related mortality
VAD vincristine, adriamycin, dexamethasone
Vel bortezomib (Velcade©)
VGPR very good partial remission
Introduction
High-dose chemotherapy with autologous stem cell transplantation (ASCT) has been widely established as consolidation or salvage treatment for patients with multiple myeloma (MM) [1–4]. In general the response after first ASCT is being used to direct the subsequent patient treatment; application of two consecutive ASCTs within 2–6 months (so-called double or tandem ASCT) is usually reserved for patients not achieving at least a very good partial remission (VGPR) after the first ASCT, according to results from two randomised prospective trials [5–6]. The indication and timing of high-dose chemotherapy with subsequent ASCT have been matter of debate recently in light of new active drugs, e.g., proteasome inhibitors and immunomodulatory derivatives (IMIDs), which are increasingly incorporated into various first line treatment regimens before ASCT [7–12]; the question whether high-dose chemotherapy can be safely replaced by a non-high dose consolidation treatment will be answered by a recently activated international trial [13]. Furthermore, the impact and composition of maintenance treatment after ASCT and even allogeneic transplantation concepts are currently under discussion and various prospective trials will further define future treatment strategies for younger MM patients [14–17]. Until these data are available and also implemented in daily practice, retrospective analyses – especially of patients who are treated in transplantation centres outside the clinical trials setting – are helpful in demonstrating the current options for patients desirous of knowing their specific risks and benefits at a given institution. We started our autologous stem cell programme in Zürich in 1988 and have performed more than 1000 transplantations since that time, with MM the most frequent indication for ASCT [18]. In terms of quality control we continuously evaluate our programme’s clinical data. We have therefore analysed the clinical course and outcome of our MM patients receiving ASCT between 2002 and 2007 and identified possible risk factors for survival.
Additionally, we have assessed the morbidity of our treatment regimens with regard to length of hospital stay, need for antibiotics and blood product transfusions.
Patients and methods
Patient data assessment
Records from patients with MM who received at least one ASCT at our transplantation centre were analysed retrospectively on the basis of a prospective database. This analysis was approved by our local ethics committee.
The Zürich transplantation centre comprises two hospitals, Zürich University Hospital and Triemli City Hospital, Zürich. Data regarding myeloma stage according to the international staging system (ISS), response to treatment before and after ASCT, event free survival (EFS) and overall survival (OS) were collected. Also, various parameters such as age, gender, body mass index, time from initial diagnosis to ASCT and number of ASCTs per patient were documented if available. As quality control for our transplantation programme we also collected data on the haematological toxicity of the treatment and the supportive care measures performed during the post-transplant period.
Treatment
Individual eligibility for ASCT was discussed in our multidisciplinary autologous transplantation board and determined in accordance with international recommendations. Patients received either one or two ASCTs. The second ASCT was usually performed sequentially 2–6 months after the first ASCT (so-called double or tandem ASCT), or, in a few patients who had initially received single ASCT, later in their disease course in the event of progression. All patients received primary induction chemotherapy as chosen by the attending oncologists. For stem cell mobilisation, either cyclophosphamide on day –12 or vinorelbine on day –8 was given, followed by collection of the stem cells on day 0. Additionally, patients received filgrastim at a dose of 10 μg/kg body weight per day from day –4 on. Aphereses were performed at our transplantation centre until at least 4 x 106 CD34-positive cells per kilogram body weight were collected. Subsequently stem cells were cryopreserved and thawed immediately before retransfusion.
Conditioning chemotherapy consisted of melphalan at a dose of 200 mg/m2, given in two doses at days –3 and –2 or as single dose at day –2 before stem cell transplantation. Patients with reduced performance score, organ dysfunctions, e.g. renal insufficiency or age over 65 years received a reduced dose in the range of 100–140 mg/m2 melphalan. Patients received filgrastim from day +5 on or pegfilgrastim once on day +1 after ASCT to shorten time to engraftment.
Response criteria
Assessment of disease response was chiefly based on serological testing because bone marrow biopsy after transplantation was omitted in most of our patients. Thus, our response assessment varied from the International Myeloma Working Group uniform criteria, which are currently proposed as a new standard for response assessment [19].
Complete Remission (CR) was defined as negative immunofixation and disappearance of monoclonal protein in serum and urine. Near Complete Remission (nCR) was defined as disappearance of monoclonal protein in serum and/or urine but persistence of positive immunofixation. Very Good Partial Remission (VGPR) was defined as reduction of monoclonal protein >90% in serum. Partial Remission (PR) was defined as reduction of paraprotein ≥50% in serum. Patients with Minor Response (MR) showed a paraprotein reduction of between 25–49% respectively. Stable Disease (SD) was defined as every response between minor response and progressive disease (PD). The latter was documented in patients who had an increase in monoclonal protein ≥25% or clinical progression after transplantation. Patients with no data available regarding their response were regarded and coded as non-responders (i.e. SD).
Event free survival (EFS) was defined as time from ASCT to the time of first recurrence after achievement of CR or nCR, to the time of progression in non-CR patients, as verified by serological assessment, bone marrow biopsy or imaging, or to the time of death by any cause. Overall survival (OS) was defined as time from ASCT to the time of death by any cause, as documented in the patient charts.
Statistics
Descriptive statistics (median and range, or counts) were calculated for all variables. Event free and overall survival was calculated from the date of ASCT and censored at the date of last follow-up. Survival curves were computed using the method of Kaplan and Meier, and compared using the logrank test [20]. Univariate regression analysis was performed using Cox proportional hazards regression [21]. P-values <0.05 were considered statistically significant. All analyses were performed in the R programming language [22].
Results
Patient demographics
Between January 2002 and December 2007 a total of 182 ASCTs were performed in 120 MM patients. Sixty-three (53%) patients received single ASCT, and 57 (47%) patients tandem ASCTs during this period. Five (8%) of the single-transplanted patients received a second ASCT later in the disease course after disease progression. The median time from diagnosis to the first ASCT was 6.24 months (range 3.55–283.04 months). Patients predominantly received split dose melphalan for conditioning. Only in six cases (3%) was melphalan given as a single dose at day –2 before ASCT. Three patients (2%) underwent haemodialysis at the time of ASCT, in two patients (2%) underlying amyloidosis of the kidneys had been diagnosed. Cytogenetics were not done routinely at our institution during that time period, pathological findings are reported only in five patients (4%) (three patients with del(13q), one patient with del(13q) and t(4;14), one patient with complex aberrations). Patient characteristics are shown in table 1.
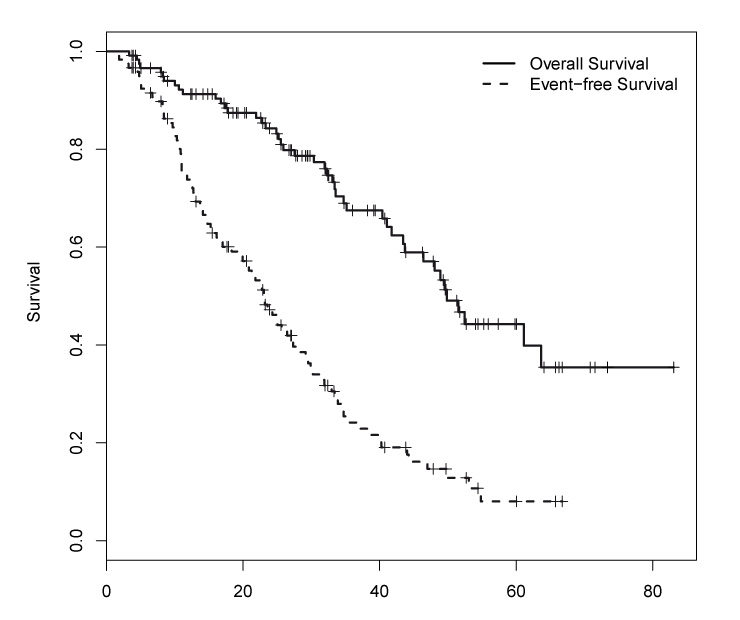
Figure 1
Kaplan–Meier analysis of the event free survival and the overall survival of all patients. Median EFS was 23.1 months (95% CI: 19.4–28.4) and median OS was 49.8 months (95% CI: 43.7 – not reached). ASCT, autologous stem cell transplantation.
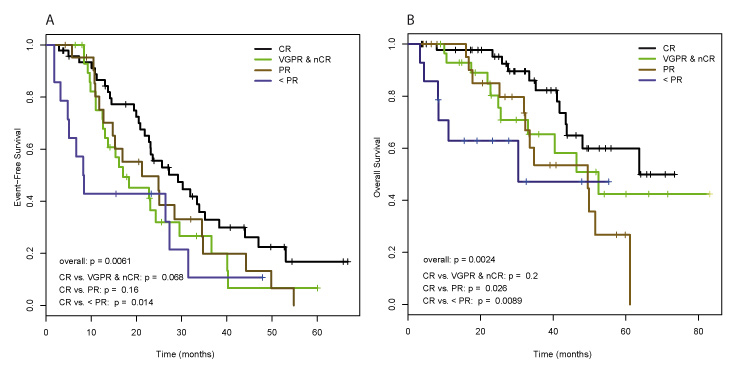
Figure 2
Impact of the response to ASCT treatment on patients’ outcome. (A) Event free survival, and (B) Overall survival. ASCT, autologous stem cell transplantation; CR, complete remission; nCR, near complete remission; VGPR, very good partial remission; PR, partial remission.
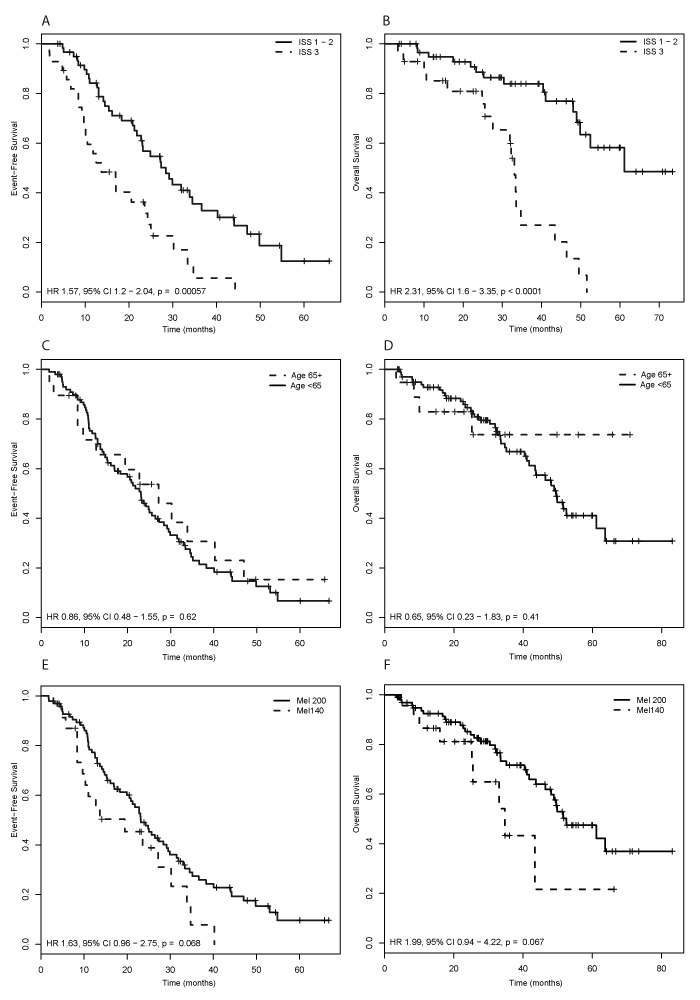
Figure 3
Impact of ISS myeloma stage (A, B), patient age (C, D), and melphalan dose (E, F) on patients’ outcome. (A, C, E) Event free survival, and (B, D, F) Overall survival. ISS, international staging system; Mel, melphalan.
Pre- and post-transplant period
The patients’ peri-transplant outcome is shown in table 2. The conditioning regimens used were standard dosed melphalan (200 mg/m2) (n = 149; 82%), reduced dosed melphalan (100–140 mg/m2) (n = 32; 17%), and a combination of bortezomib, thalidomide, dexamethasone and BEAM (VT-Dexa-BEAM) (n = 1; 1%). Patients subsequently received a minimum of 2 x 106 CD34+ cells per kilogram body weight (median 3.96 x 106 CD34+ cells/ kg; range, 2.01 x 106 – 42.4 x 106). The median duration of grade 4 neutropenia was 6 days (range 3–10 days) and the median time to engraftment was 10 days (range 6–13 days). The median duration of grade 4 thrombocytopenia was 3 days (range 0–15 days). Filgrastim was administered for a median of 7 days (range 1–16). The median length of hospital stay from the day of ASCT was 15 days (range 1–66 days). Patients received a median of 1 (range 0–8) platelet transfusions and a median of 0 (range 0–9) red blood cell transfusions. Fever developed in 108 cases (59%) after ASCT, and antibiotics for therapeutic purposes were given in 147 cases (81%) during the post-transplant period. Bacterial pathogens were isolated in 58 cases (32%). Bacteria isolated were Escherichia coli (n = 20; 11%), coagulase-negative Staphylococcus sp. (n = 14; 8%), non-haemolytic Streptococcus sp. (n = 8; 4%), Clostridium difficile (n = 8; 4%), Pseudomonas aeruginosa (n = 3; 2%), Staphylococcus aureus(n = 2; 1%), Moraxella catarrhalis (n = 2; 1%), and others (n = 12, 6%).
Two patients (1%) died within the first 100 days after ASCT. The first patient died at day +100 after ASCT due to disease progression, the second patient died at day +61 after ASCT due to severe neutropenic enterocolitis and superinfection with cytomegalovirus.
Response and survival
Of the 63 patients who received single ASCT, 57 (90%) had responded (≥PR) to induction chemotherapy. Eighteen (28%) of these patients had either VGPR or nCR, and 6 patients (10%) had already achieved CR status at the time before ASCT. Of the 57 patients who received a true double ASCT, 42 (74%) had at least PR after induction chemotherapy, with 5 (9%) having achieved VGPR or nCR and 2 (4%) having achieved CR before the first ASCT. After high-dose chemotherapy and subsequent ASCT, the 63 single autografted patients had an overall response rate of 82.5%, with 15 patients (24%) having achieved VGPR or nCR and 26 (41%) CR after ASCT. The 57 patients who received double ASCT had an overall response rate of 82.5%. Sixteen patients (28%) had achieved either VGPR or nCR, and 21 (37%) CR after the second ASCT (table 3).
The entire patient population had a median EFS of 23.1 months (95% confidence interval (CI): 19.4–28.4) and the median OS was 49.8 months (95% CI: 43.7 – not reached), respectively (fig. 1). The median EFS in patients who received single ASCT was 21.3 months (95% CI: 16.9–31.5), and 27.2 months (95% CI: 19.9–31.9) in patients who received double ASCT. The corresponding median OS were 46.4 months (95% CI: 35.2 – not reached) and 63.7 months (95% CI: 48.9 – not reached), respectively.
EFS and OS correlated with the response to ASCT. Patients achieving a CR had a significantly prolonged EFS compared to patients with less than PR (HR: 2.38; 95% CI: 1.19–4.75; p = 0.014), and patients achieving a CR also had a significantly prolonged OS compared to patients with PR (HR: 2.5; 95% CI: 1.12–5.61; p = 0.026) or less than PR (HR: 3.77; 95% CI: 1.4–10.19; p = 0.009) (fig. 2a and 2b). The achievement of CR was not significantly better than achieving nCR or VGPR regarding EFS (HR: 1.67; 95% CI: 0.96–2.9; p = 0.07) and OS (HR: 1.72; 95% CI: 0.76–3.89; p = 0.2).
Patients with disease stage I or II according to the International Staging System (ISS) at diagnosis had a better outcome than patients with MM stage III. The median EFS in the former was 28.4 months (95% CI: 22.8–40.2) and 13.7 months (95% CI: 10.1–25.0) in the latter patients (HR: 1.57; 95% CI: 1.2–2.04; p = 0.0006). The corresponding median OS was 61.1 months (95% CI: 49.8 – not reached) for patients with ISS I or II and 33.1 months (95% CI: 27.6–46.4) for patients with ISS III (HR: 2.31; 95% CI: 1.6–3.35; p <0.0001) respectively (fig. 3a and 3b).
Age was not associated with EFS (HR: 0.86; 95% CI: 0.48−1.55; p = 0.62) or OS (HR: 0.65; 95% CI: 0.23−1.83; p = 0.41) (fig. 3c and 3d).
Patients who received standard dose melphalan showed a trend towards improved EFS (HR: 1.63; 95% CI: 0.96−2.75; p = 0.07) and OS (HR: 1.99; 95% CI: 0.94−4.22; p = 0.07) compared to patients who received reduced dose melphalan (fig. 3e and 3f).
Risk factor analysis
By univariate analysis, achievement of a CR before ASCT correlated with better EFS compared with any other response (p = 0.016), without impacting on OS (p = 0.21). Also, CR after ASCT was associated with improved EFS (p = 0.006) and OS (p = 0.0024) compared with any other response. In the subgroup analysis, the achievement of CR after ASCT was associated with better OS especially compared to PR (p = 0.026) and responses less than PR (p = 0.009).
Other parameters significantly associated with a better EFS were paraprotein levels of less than 5 g/dL at diagnosis (p = 0.007), a time of 6–12 months from diagnosis to ASCT compared with earlier transplantation (p = 0.042), and MM stage of less than III according to ISS (p = 0.0006). The latter parameter was also significantly linked with better OS (p <0.0001) (table 4).
|
Table 1: Patient characteristics. |
|
Parameter
|
Patients
N = 120 (100%)
|
|
Age
<65 years – no. (%)
≥65 years – no. (%)
Median – yr
Range – yr |
101 (84)
19 (16)
56.3
28.5–75.2 |
|
Gender
Male – no. (%)
Female – no. (%) |
74 (62)
46 (38) |
|
BMI before ASCT
Median – kg/m²
Range – kg/m² |
25.2
16.76–38.64 |
|
Myeloma type
IgG – no. (%)
IgA – no. (%)
IgM – no. (%)
Bence Jones – no. (%)
Non secretory – no. (%)
Plasma cell leukaemia – no. (%) |
64 (53)
25 (21)
1 (1)
25 (21)
2 (2)
3 (2) |
|
Myeloma stage (DS)
I – no. (%)
II – no. (%)
III – no. (%)
Data missing – no. (%) |
15 (13)
28 (23)
74 (62)
3 (2) |
|
Myeloma stage (ISS)
I – no. (%)
II – no. (%)
III – no. (%)
Data missing – no. (%) |
29 (24)
32 (27)
28 (23)
31 (26) |
|
Number of ASCT received
1 – no. (%)
2 – no. (%) |
58 (53)
62 (47) |
|
Time from diagnosis to 1. ASCT
Median – months
Range – months |
6.24
3.55–283 |
|
β2-Microglobulin levels at diagnosis
<3.5 mg/l – no. (%)
≥3.5 mg/l – no. (%)
Data missing – no. (%)
Median – mg/l
Range – mg/l |
37 (31)
34 (28)
49 (41)
3.38
0.88–82.5 |
|
Paraprotein levels at diagnosis
<5 g/dl – no. (%)
≥5 g/dl – no. (%)
Data missing – no. (%)
Median – g/dl
Range – g/dl |
43 (81)
22 (18)
55 (46)
3.52
0.1–14.3 |
|
Albumin levels at diagnosis
<3.5 g/dl – no. (%)
≥3.5 g/dl – no. (%)
Data missing – no. (%)
Median – g/dl
Range – g/dl |
28 (23)
42 (35)
50 (42)
3.66
2.4–4.94 |
|
Previous chemotherapy regimens
VAD – no. (%)
Seq. VAD and M2 – no. (%)
Seq. melphalan and VAD – no. (%)
Dex monotherapy – no. (%)
ThalDex – no. (%)
Seq. Thal and Vel – no. (%)
Seq. VAD and Thal – no. (%)
Seq. VAD and Vel – no. (%)
≥3 regimens (incl. new drugs) – no. (%) |
89 (74)
8 (7)
1 (1)
1 (1)
10 (8)
3 (2)
2 (2)
1 (1)
5 (4) |
| ASCT, autologous stem cell transplantation; BMI, body mass index; ASCT, autologous stem cell transplantation; DS, Durie-Salmon; ISS, international staging system; VAD, vincristine, adriamycin, dexamethasone; M2, cyclophosphamide, carmustine, melphalan, prednisone; Dex, dexamethasone; Thal, thalidomide; Vel, bortezomib (velcade©). |
|
Table 2: Pre- and post-transplantation variables. |
|
Parameter
|
Cases
(n = 182)
|
|
CD34+ cells reinfused
Median – x106 /kg
Range – x106 /kg |
3.96
2.01–42.4 |
|
Conditioning regimen used
Normal dose melphalan – no. (%)
Reduced dose melphalan – no. (%)
Other regimen – no. (%) |
149 (82)
32 (17)
1 (1) |
|
Time to engraftment ≥500 ANC
Median – days
Range – days |
10
6–13 |
|
Duration of neutropenia grade 4
Median – days
Range – days |
6
3–10 |
|
Duration of thrombocytopenia grade 4
Median – days
Range – days |
3
0–15 |
|
Red blood cell transfusions
Median – no. of transfusions
Range – no. of transfusions |
0
0–9 |
|
Platelet transfusions
Median – no. of transfusions
Range – no. of transfusions |
1
0–8 |
|
Duration of filgrastim treatment
Median – days
Range – days |
7
1–16 |
|
Occurrence of neutropenic fever – no. (%)
Use of antibiotics – no. (%)
|
108 (59)
147 (81) |
|
Hospital stay from day of ASCT
Median – days
Range – days |
15
1–66 |
|
Treatment related mortality – no. (%)
|
1 (0.5) |
| CD34, cluster of differentiation 34; ASCT, autologous stem cell transplantation; ANC, absolute neutrophil count. |
|
Table 3: Response rates before and after ASCT. |
|
Response
|
Status before ASCT in single-transplanted patients (n = 63)
|
Status after ASCT in single transplanted patients (n = 63)
|
| CR – no. (%)
VGPR & nCR – no. (%)
PR – no. (%)
< PR – no. (%) |
6 (10)
18 (28)
33 (52)
6 (10) |
26 (41)
15 (24)
11 (17.5)
11 (17.5) |
|
Response
|
Status before 1. ASCT in double transplanted patients (n = 57)
|
Status after 2. ASCT in double transplanted patients (n = 57)
|
| CR – no. (%)
VGPR & nCR – no. (%)
PR – no. (%)
< PR – no. (%) |
2 (4)
5 (9)
35 (61)
15 (26) |
21 (37)
16 (28)
10 (17.5)
10 (17.5) |
| ASCT, autologous stem cell transplantation; CR, complete remission; VGPR, very good partial remission; nCR, near complete remission; PR, partial remission. |
|
Table 4: Prognostic factors for survival by univariate analysis. |
|
Parameter
|
Event free survival
|
Overall survival
|
|
HR
|
Lower 95 CI
|
Upper 95 CI
|
p-value
|
HR
|
Lower 95 CI
|
Upper 95 CI
|
p-value
|
| Gender male vs female |
1.09 |
0.70 |
1.70 |
0.69 |
0.99 |
0.54 |
1.81 |
0.97 |
| Age ≥65 vs <65 years |
0.86 |
0.48 |
1.55 |
0.62 |
0.65 |
0.23 |
1.83 |
0.41 |
| CR pre-ASCT vs other response |
– |
– |
– |
0.016
|
– |
– |
– |
0.21 |
| CR pre-ASCT vs VGPR & nCR |
0.99 |
0.37 |
2.67 |
1 |
0.63 |
0.14 |
2.82 |
0.55 |
| CR pre-ASCT vs PR |
1.56 |
0.67 |
3.66 |
0.3 |
1.16 |
0.35 |
3.83 |
0.81 |
| CR pre-ASCT vs less than PR |
2.19 |
0.87 |
5.51 |
0.1 |
1.27 |
0.34 |
4.79 |
0.73 |
| CR post-ASCT vs other response |
– |
– |
– |
0.006
|
– |
– |
– |
0.002
|
| CR post-ASCT vs VGPR & nCR |
1.67 |
0.96 |
2.90 |
0.07 |
1.72 |
0.76 |
3.89 |
0.20 |
| CR post-ASCT vs PR |
1.51 |
0.85 |
2.71 |
0.16 |
2.50 |
1.12 |
5.61 |
0.026
|
| CR post-ASCT vs less than PR |
2.38 |
1.19 |
4.75 |
0.01
|
3.77 |
1.40 |
10.19 |
0.009
|
| Melphalan reduced vs standard |
1.63 |
0.96 |
2.75 |
0.07 |
1.99 |
0.94 |
4.22 |
0.07 |
| Paraprotein ≥5 vs <5g/dL |
2.42 |
1.25 |
4.69 |
0.007
|
2.04 |
0.92 |
4.52 |
0.07 |
| ISS 3 vs ISS <3 |
1.57 |
1.20 |
2.04 |
0.001
|
2.31 |
1.60 |
3.35 |
0.000
|
| BMI ≥25 vs <25 |
0.69 |
0.46 |
1.06 |
0.09 |
0.58 |
0.32 |
1.06 |
0.07 |
| One vs ≥ two previous regimens |
0.87 |
0.51 |
1.48 |
0.60 |
0.94 |
0.44 |
2.03 |
0.88 |
| <6 months vs 6–12 months from Dx |
0.61 |
0.37 |
0.98 |
0.042
|
0.65 |
0.34 |
1.27 |
0.21 |
| <6 months vs >12 months from Dx |
0.98 |
0.56 |
1.71 |
0.93 |
1.06 |
0.47 |
2.40 |
0.89 |
| HR, hazard ratio; CI, confidence interval; ASCT, autologous stem cell transplantation; CR, complete remission; nCR, near complete remission; VGPR, very good partial remission; PR, partial remission; ISS, international staging system; BMI, body mass index; Dx, diagnosis. |
Discussion
In the present analysis we observed that ASCTs performed for MM at our centre results in high response rates of over 80% in single and in double transplanted patients. Compared to available data in the literature, the procedure-related mortality during the years 2002 and 2007 was very low with 0.5% [23]. A CR after ASCT and ISS stage of less than III were the best predictors for prolonged survival. Age was not associated with a worse outcome in selected patients eligible for ASCT.
We were able to document a median survival of 49.8 months from the first ASCT in our patients. Separately analysed by the number of ASCTs, the median survival was 46.4 months for single-transplanted patients, and 63.7 months for double-transplanted patients respectively. These results are in accordance with other published outcome analyses [5, 24–26]. In general, only patients who do not achieve at least a VGPR after the first ASCT might benefit from a double ASCT [5–6]. Following this assumption, chiefly patients with an insufficient response proceeded to a second ASCT at our centre.
We showed that patient survival correlates with the response achieved after ASCT. Patients who achieved only a PR or less than PR had a significantly shorter OS compared to patients achieving a CR. On the other hand, CR did not result in a significantly longer OS compared to the patient subgroup achieving nCR or VGPR. This result is remarkable considering that our patient number was rather small and usually only studies with large patient numbers were able to link survival with response status achieved after treatment. A similar association of patient survival with CR after ASCT was reported recently in a subgroup analysis of more than 750 patients treated within the GEM2000 protocol, where the quality of response to induction therapy had indeed a positive impact on the quality of the response to ASCT, but without impacting on survival as impressively as the post-transplant response status [27]. The superiority of VGPR compared with PR after ASCT was demonstrated by an analysis of the IFM-99 trials [28]. We also found a significant impact of CR on patient survival, but we cannot confirm this observation for patients with nCR or VGPR after ASCT, most probably because of small patient numbers. However, CR was not superior to nCR or VGPR in our patient cohort, and in our view this finding supports the general recommendation to omit a second ASCT in patients who achieve at least a VGPR after first ASCT.
We transplanted a very heterogeneous patient cohort with MM at our transplantation centre. Notably, a few selected high-risk patients with co-morbidities such as renal insufficiency or light chain amyloidosis were also treated and a substantial subset of patients (16%) were aged over 65 years. Patients were considered transplantable if they were biologically fit and judged able to tolerate high dose therapy by the attending physician and our regular stem cell transplantation board. The dose of melphalan was individually adjusted according to physical status and organ function. This heterogeneity of the patient collective is reflected in the trend to prolonged EFS and OS in patients receiving standard dose melphalan for conditioning compared to patients with presence of risk factors, whose melphalan dose was reduced. One interesting finding of this analysis is that elderly patients did not have, in general, a worse outcome than younger patients. The fact that EFS was positively influenced by a higher melphalan dose may be partially explained by a simple dose-efficacy correlation, as higher drug doses may achieve a better treatment result [29]. Furthermore, we observed the well known association of initial ISS stage with patient survival. Our findings are in accordance with other reports showing similar data [3, 30–33]. In contrast, the number of previous chemotherapy regimens before ASCT did not affect patient outcome. This finding may be explained by the fact that the majority of the patients received only one regimen for induction, mainly VAD, and that most patients with insufficient response could be “rescued” by a second regimen, either by using combination chemotherapy (M2 protocol) initially or bortezomib and IMIDs during the last years of the observation period. Finally, we did not find a statistically significant impact of time between diagnosis and first ASCT on overall survival.
The main limitation of this analysis is the limited patient number. Additionally, patients were treated outside the framework of a controlled clinical trial and differed considerably regarding number and duration of preceding treatment regimens, induction treatment, and risk factor profile. The data presented here represent a “real-life” transplantation experience, a scenario that is applicable to the majority of patients. These results are free from possible selection bias created by entrance criteria of clinical studies, and the conclusions reached by an analysis such as ours have their impact on daily practice and on communication with patients. The main goal of our efforts remains the continuous improvement in patient care we aim to achieve by monitoring and analysing treatment outcome.
In conclusion, ASCT is a very safe and effective treatment option for eligible patients with MM. Treatment-related mortality is very low. Older patients with good performance status also benefit from an ASCT. Achievement of a CR before ASCT is a predictor for better EFS, and CR status after ASCT remains the best predictor for a prolonged OS and may spare the patients multiple ASCTs.
References
1 Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe francais du myelome. N Engl J Med. 1996;335:91–7.
2 Fermand JP, Ravaud P, Chevret S, Divine M, Leblond V, Belanger C, et al. High-dose therapy and autologous peripheral blood stem cell transplantation in multiple myeloma: Up-front or rescue treatment? Results of a multicenter sequential randomized clinical trial. Blood. 1998;92:3131–6.
3 Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348:1875–83.
4 Barlogie B, Tricot G, Anaissie E, Shaughnessy J, Rasmussen E, van Rhee F, et al. Thalidomide and hematopoietic-cell transplantation for multiple myeloma. N Engl J Med. 2006;354:1021–30.
5 Attal M, Harousseau JL, Facon T, Guilhot F, Doyen C, Fuzibet JG, et al. Single versus double autologous stem-cell transplantation for multiple myeloma. N Engl J Med. 2003;349:2495–502.
6 Cavo M, Tosi P, Zamagni E, Cellini C, Tacchetti P, Patriarca F, et al. Prospective, randomized study of single compared with double autologous stem-cell transplantation for multiple myeloma: Bologna 96 clinical study. J Clin Oncol. 2007;25:2434–41.
7 Cavo M, Zamagni E, Tosi P, Tacchetti P, Cellini C, Cangini D, et al. Superiority of thalidomide and dexamethasone over vincristine-doxorubicindexamethasone (vad) as primary therapy in preparation for autologous transplantation for multiple myeloma. Blood. 2005;106:35–9.
8 Rajkumar SV, Rosinol L, Hussein M, Catalano J, Jedrzejczak W, Lucy L, et al. Multicenter, randomized, double-blind, placebo-controlled study of thalidomide plus dexamethasone compared with dexamethasone as initial therapy for newly diagnosed multiple myeloma. J Clin Oncol. 2008;26:2171–7.
9 Harousseau JL, Attal M, Leleu X, Troncy J, Pegourie B, Stoppa AM, et al. Bortezomib plus dexamethasone as induction treatment prior to autologous stem cell transplantation in patients with newly diagnosed multiple myeloma: Results of an ifm phase ii study. Haematologica. 2006;91:1498–505.
10 Popat R, Oakervee HE, Hallam S, Curry N, Odeh L, Foot N, et al. Bortezomib, doxorubicin and dexamethasone (pad) front-line treatment of multiple myeloma: Updated results after long-term follow-up. Br J Haematol. 2008;141:512–6.
11 Barlogie B, Anaissie E, van Rhee F, Haessler J, Hollmig K, Pineda-Roman M, et al. Incorporating bortezomib into upfront treatment for multiple myeloma: Early results of total therapy 3. Br J Haematol. 2007;138:176–85.
12 Nair B, van Rhee F, Shaughnessy JD, Jr., Anaissie E, Szymonifka J, Hoering A, et al. Superior results of total therapy 3 (2003-33) in gene expression profiling-defined low-risk multiple myeloma confirmed in subsequent trial 2006-66 with vrd maintenance. Blood. 2010;115:4168–73.
13 Blade J, Rosinol L, Cibeira MT, Rovira M, Carreras E. Hematopoietic stem cell transplantation for multiple myeloma beyond 2010. Blood. 2010;115:3655–63.
14 Bruno B, Rotta M, Patriarca F, Mordini N, Allione B, Carnevale-Schianca F, et al. A comparison of allografting with autografting for newly diagnosed myeloma. N Engl J Med. 2007;356:1110–20.
15 Rowan K. Researchers debate best use of stem cell transplants in patients with multiple myeloma. J Natl Cancer Inst. 2009;101:1608–11.
16 Attal M, Harousseau JL, Leyvraz S, Doyen C, Hulin C, Benboubker L, et al. Maintenance therapy with thalidomide improves survival in patients with multiple myeloma. Blood. 2006;108:3289–94.
17 Kroger N, Schwerdtfeger R, Kiehl M, Sayer HG, Renges H, Zabelina T, et al. Autologous stem cell transplantation followed by a dose-reduced allograft induces high complete remission rate in multiple myeloma. Blood. 2002;100:755–60.
18 Jost LM, Honegger HP, Stahel RA. high-dose chemotherapy with autologous bone marrow transplantation: 11 years’ experience in Zurich. Schweiz Med Wochenschr. 2000;130:60–9.
19 Durie BG, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–73.
20 Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Statist Assoc. 1958;53:457–81.
21 Cox DR. Regression models and life tables (with discussion). J R Statist Soc B. 1972;34:187–220.
22 R development core team. R: A language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria. Isbn 3-900051-07-0, url http://www.R-project.Org. 2009
23 Jantunen E, Itala M, Lehtinen T, Kuittinen O, Koivunen E, Leppa S, et al. Early treatment-related mortality in adult autologous stem cell transplant recipients: A nation-wide survey of 1482 transplanted patients. Eur J Haematol. 2006;76:245–50.
24 O’Shea D, Giles C, Terpos E, Perz J, Politou M, Sana V, et al. Predictive factors for survival in myeloma patients who undergo autologous stem cell transplantation: A single-centre experience in 211 patients. Bone Marrow Transplant. 2006;37:731–7.
25 Majolino I, Vignetti M, Meloni G, Vegna ML, Scime R, Tringali S, et al. Autologous transplantation in multiple myeloma: A gitmo retrospective analysis on 290 patients. Gruppo italiano trapianti di midollo osseo. Haematologica. 1999;84:844–52.
26 Alegre A, Diaz-Mediavilla J, San-Miguel J, Martinez R, Garcia Larana J, Sureda A, et al. Autologous peripheral blood stem cell transplantation for multiple myeloma: A report of 259 cases from the Spanish registry. Spanish registry for transplant in mm (grupo espanol de trasplante hematopoyetico-geth) and pethema. Bone Marrow Transplant. 1998;21:133–40.
27 Lahuerta JJ, Mateos MV, Martinez-Lopez J, Rosinol L, Sureda A, de la Rubia J, et al. Influence of pre- and post-transplantation responses on outcome of patients with multiple myeloma: Sequential improvement of response and achievement of complete response are associated with longer survival. J Clin Oncol. 2008;26:5775–82.
28 Harousseau JL, Avet-Loiseau H, Attal M, Charbonnel C, Garban F, Hulin C, et al. Achievement of at least very good partial response is a simple and robust prognostic factor in patients with multiple myeloma treated with high-dose therapy: Long-term analysis of the ifm 99-02 and 99-04 trials. J Clin Oncol. 2009;27:5720–6.
29 Badros A, Barlogie B, Siegel E, Morris C, Desikan R, Zangari M, et al. Autologous stem cell transplantation in elderly multiple myeloma patients over the age of 70 years. Br J Haematol. 2001;114:600–7.
30 Cuzick J, Cooper EH, MacLennan IC. The prognostic value of serum beta 2 microglobulin compared with other presentation features in myelomatosis. Br J Cancer. 1985;52:1–6.
31 Jagannath S, Barlogie B, Dicke K, Alexanian R, Zagars G, Cheson B, et al. Autologous bone marrow transplantation in multiple myeloma: Identification of prognostic factors. Blood. 1990;76:1860–6.
32 Vesole DH, Barlogie B, Jagannath S, Cheson B, Tricot G, Alexanian R, Crowley J. High-dose therapy for refractory multiple myeloma: Improved prognosis with better supportive care and double transplants. Blood. 1994;84:950–6.
33 Harousseau JL, Attal M, Divine M, Marit G, Leblond V, Stoppa AM, et al. Autologous stem cell transplantation after first remission induction treatment in multiple myeloma: A report of the French registry on autologous transplantation in multiple myeloma. Blood. 1995;85:3077–85.


