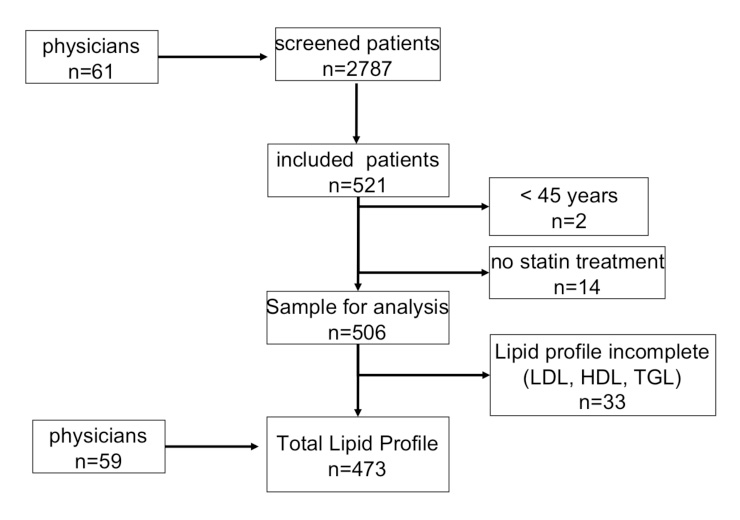
Figure 1
Flow chart of the Focus on Comprehensive Lipid Management Survey in Swiss Patients.
DOI: https://doi.org/10.4414/smw.2011.13200
Primary and secondary prevention studies have shown that the use of statins is associated with significant reductions in cardiovascular morbidity and mortality [1–5, 6–8].
A recently published meta-analysis [9] analysing the results of 76 randomised control trials involving 170’255 participants showed that statin therapy was associated with a substantial reduction of all-cause mortality (Relative Risk (RR) 0.90), cardiovascular disease (CVD) mortality (RR 0.80), fatal and non-fatal myocardial infarction (MI) (RR 0.82 and 0.74 respectively), revascularisation (RR 0.76), and a composite of fatal and non-fatal strokes (RR 0.86).
However, around 70% of cardiovascular events occur despite treatment with statins [10, 11].
This residual cardiovascular risk may be partially explained by un-modifiable cardiovascular risk factors such as age, gender and a family history of cardiovascular diseases, as well as by an insufficient treatment of risk factors other than dyslipidemia such as smoking, arterial hypertension, diabetes and a sedentary lifestyle.
Moreover, therapy with statins is not sufficient for a comprehensive lipid management.
Many epidemiologic studies have documented the strong inverse correlation between high-density lipoprotein-cholesterol (HDL-C) levels and cardiovascular morbidity and mortality.
A post hoc analysis of the recently completed study Treating to New Targets (TNT) [12] showed that low levels of HDL-C and raised triglyceride (TG) levels are strongly linked to a significantly increased risk of coronary heart disease (CHD) even in patients who achieve the current low-density lipoprotein cholesterol (LDL-C) targets. A recent analysis of The PROspective CArdiovascular Munster (PROCAM), the REsiduAl risk LIpids and Standard Therapies (REALIST) surveys presented at the annual meeting of the European Society of Cardiology showed that when all risks factors were matched, the odds of experiencing a MI were increased five-fold for men with a LDL-C at the target level (less than or equal to 100 mg/dL), presenting a low level of HDL-C (<45 mg/dL) and an elevated level of TG (>150 mg/dL) [13]. Moreover, the Interheart study identified that the imbalance between atherogenic and atheroprotective lipoproteins was the most powerful potentially modifiable risk factor for CVD [14].
At the present time, less data are available on the effect of increasing HDL on cardiovascular risk.
In a meta-analysis analysing the data from the Coronary Primary Prevention Trial, the Multiple Risk Factor Intervention Trial, the Lipid Research Clinics Prevalence Mortality Follow-up Study and the Framingham Heart Study, for every 1-mg/dL (0.026 mmol/L) increase in plasma HDL cholesterol in the populations studied, there was a decrease in CHD risk of approximately 2% in men and 3% in women independent of other risk factors, including plasma LDL cholesterol [15].
Similarly a re-analysis of the Armed Forces Regression Study [16] suggested that in a population of patients with stable atherosclerosis, the greater the percentage increase in HDL achieved, the greater the cardio-protective benefit.
Moreover, a very recently published study showed, in the Framingham population, that a low HDL-C level was associated with a 40% increase in the incidence of heart failure independently of its association with myocardial infarction [17].
Beside HDL-C, some epidemiological studies have suggested that hypertriglyceridemia is also associated with increased cardiovascular morbidity and mortality.
A meta-analysis of 17 population-based prospective studies involving 46’413 men and 10’864 women indicated that TG levels were associated with a significant increase of 32% and 76%, respectively, in cardiovascular risk based on univariate analysis. This association remained significant even after adjustment for HDL-C [18].
Concerning the effect of therapy, treatment of elevated TG in clinical trials has been shown to reduce cardiovascular events, cardiac deaths and total mortality [19, 20].
However, the association between TG and cardiovascular disease is more modest compared to one of the other risk factors and the role of TG in cardiovascular prevention is controversial.
In particular, it is unclear whether hypertriglyceridemia should be considered an independent predictor of coronary events or an indicator of the presence of a cluster of cardiovascular factors such as the metabolic syndrome [11].
Moreover, it is of note that lipids, and in particular HDL and TG, have a different impact on cardiovascular health in males and females [21, 22].
The Focus on Comprehensive Lipid Management Survey in Swiss Patients (FOCUS) aimed to evaluate the control of lipid profile and the cardiovascular risk in patients aged ≥45 years and under treatment with a statin for at least three months before inclusion.
The FOCUS Study took place in Switzerland from July 2008 to June 2009.
This cross-sectional epidemiological survey was conducted by 61 cardiologists, endocrinologists, internists and primary care physicians in Switzerland.
Physicians were asked to screen outpatients older than 45 years, who had been on statin therapy for at least 3 months. A clinical history (including gender, age, history of atherothrombotic events, risk factors and family history) as well as a clinical examination and the recording of the latest lipid values on statin therapy were required in all patients. Laboratory data were abstracted from patients’ charts, under the actual statin therapy and were not older than 12 months.
Patients with a history of cancer, hepatic or renal diseases and endocrinological disease other than diabetes were excluded from the study.
The local ethical committee approved the study. Participants signed an informed consent form, were informed about the results and, if necessary, they were advised to modify their lifestyle/medical therapy.
Total cholesterol (TC), HDL-C, LDL-C and TG were measured in venous or capillary blood. If LDL-C was not available and triglycerides were lower than 4.5 mmol/L, the LDL-C value was calculated using the Friedewald formula (LDL-C=TC - HDL-C – (Triglycerides/2.2)).
According to the recommendations of the National Cholesterol Educational Program for fasting testing conditions [23], we defined “High LDL-C” as LDL-C ≥2.6 mmol/L for patients at high risk, ≥3.4 mmol/L for patients at intermediate risk and ≥4.1 mmol/L for low risk patients. “Low HDL-C” was defined as HDL-C <1.3 mmol/L in women and < 1 mmol/L in men, and “elevated triglycerides” were defined as ≥1.7 mmol/L for patients with diabetes and/or metabolic syndrome and as ≥5.0 mmol/L for the other patients.
Criteria for the diagnosis of diabetes mellitus were an established disease of diabetes or casual plasma glucose greater than 11 mmol/l.
Criteria for the diagnosis of arterial hypertension were a previous or current diagnosis, defined according to the current guidelines [24, 25].
Current smokers were identified by the questions “Have you ever smoked and did you stop more than 1 year ago? Do you currently smoke every day, some days, or not at all?” as recommended by the National Centre for Health Statistics for estimates of smoking prevalence.
The body mass index was calculated by dividing a person’s weight in kilograms by their height in meters squared. Being overweight and obesity were defined as a body mass index greater than 25 and 30, respectively. Waist circumference was measured at a level midway between the lower rib margin and the iliac crest with the tape all around the body in a horizontal position.
Subjects with a metabolic syndrome were defined according to the International Diabetes Federation (IDF) definition [26] as patients presenting 3 or more of the following factors:
– Waist circumference >88 cm in females and <102 in males
– HDL-C <1.3 mmol/L in women and <1 mmol/L in men
– TG ≥1.7 mmol/L
– Systolic blood pressure ≥130 mm Hg
– Diastolic blood pressure ≥85 mm Hg
– Plasma glucose ≥6.1 mmol/L
The 10-year risk for a cardiovascular event was calculated according to the algorithms based on the data of the PROCAM Study [27] and was adapted for the Swiss population by the AGLA (Arbeitsgruppe Lipide und Atherosklerose of the Swiss society of cardiology; http://www.agla.ch ). According to the European guidelines [28, 29], patients were divided into “low risk “(10-year risk <10%), “intermediate risk” (10-year risk 10–20%) and a “high risk” (10-year risk >20%).
Continuous variables are expressed as mean ± standard deviation. Categorical variables are expressed as frequencies and percentages. Comparisons between categorical variables were performed using 2-sided Pearson chi-square statistic. A probability of less than 0.05 was considered statistically significant. Statistical analysis was performed using SPSS™ software version 15.0.
Overall 2’787 patients were screened, and 506 patients were recruited for the study by 59 physicians. As a complete lipid profile was not available for 33 patients, the final analysis was done with 473 patients (fig. 1).

Figure 1
Flow chart of the Focus on Comprehensive Lipid Management Survey in Swiss Patients.
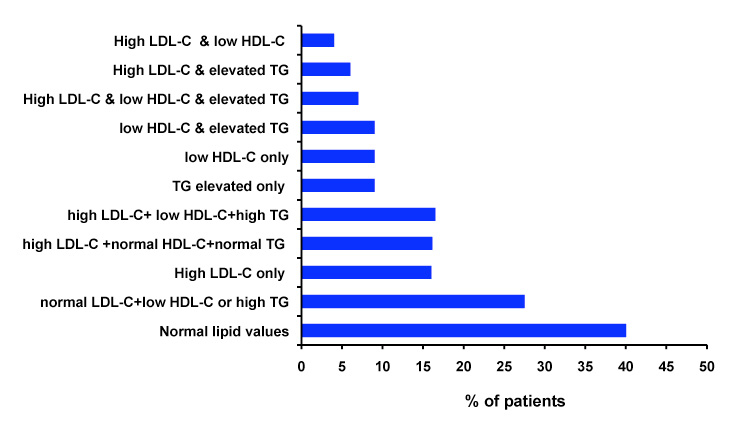
Figure 2
Abnormalities of the lipid profile found in the 473 patients included in the final analysis. LDL-C: low density lipoprotein-cholesterol, TG: triglycerides, HDL-C: high density lipoprotein-cholesterol. High LDL-C was defined as LDL-C >2.6 mmol/L high risk patients, LDL-C ≥4.1 mmol/L in patients with low cardiovascular risk; low HDL-C was defined as HDL-C <1.3 mmol/L in women or <1 mmol/L in men; high TG were defined as TG ≥1.7 mmol/L in high risk patients and ≥5.0 mmol/L in patients with low or intermediate risk.
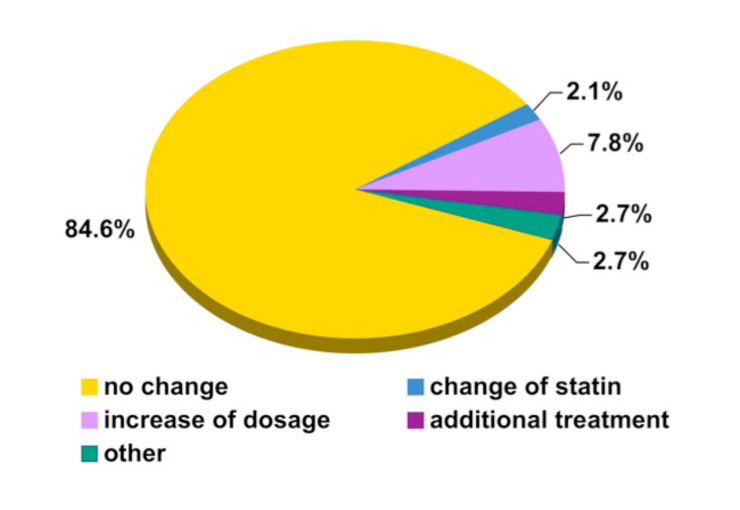
Figure 3
Change of lipid modifying treatment based on the laboratory results from the 473 patients included in the final analysis.
The clinical characteristics of the patients included in the study overall and divided according to gender and low and high cardiovascular risk are shown in table 1. The lipid lowering drugs used at baseline are shown in table 2.
The patients included in the final analysis were recruited by 59 physicians working in Switzerland (45.9% in the German part and 54.1% in the French/Italian part).
A total of 40.7% of the patients were treated by endocrinologists or diabetes specialists, 33.9% by cardiologists, 18.6% by internal medicine specialists, 5.1% by specialists in angiology and 1.7% by nephrologists.
The FOCUS study population was characterised by a high prevalence of cardiovascular risk factors (table 3). A family history of cardiovascular disease was reported in 146 patients (30.9%), 70 were current smokers and 133 patients were previous smokers (14.8% and 28.1% respectively); a diagnosis of dyslipidemia was reported in 443 patients (93.7%, 9.7% defined as “familiar dyslipidemia”), arterial hypertension was reported in 357 patients (75.5%), and a diagnosis of diabetes mellitus in 257 patients (54.3%). A metabolic syndrome was diagnosed according to the IDF criteria in 145 patients (30.7%).
According to this high prevalence of risk factors, we found that 86.5% of the population had a 10-year cardiovascular risk higher than 20%.
A total of 45% of the patients had a previous diagnosis of coronary artery disease, 7.6% of heart failure, 12.1% of a cerebrovascular disease, and 12.1% had a previous diagnosis of peripheral artery disease.
As required by the inclusion criteria, all the patients were treated with a statin.
The mean lipid values by gender and the cardiovascular risk class are shown in table 1.
The alterations were similar in patients with a high cardiovascular risk and patients with CHD, while patients with diabetes were more frequently characterised by high TG level (table 4).
It is of note that under statin therapy only 40% of the patients included in the FOCUS Study had a lipid profile within the recommended limits.
The abnormalities of the lipid profile found in the FOCUS population are shown in figure 2. It is of note that 27.5% of the patients presented a normal LDL-C associated with low HDL-C or high TG, high LDL-C with normal HDL-C and triglycerides was found in 16.1%, and the combination of high LDL-C, low HDL-C and high TG was found in 16.5%.
A low HDL was more frequently found in patients younger than 65 years (33.2% vs 24.8%, p = 0.044), and in patients with diabetes mellitus (32.7% vs 24.1%, p = 0.039) or with a metabolic syndrome (61.4 vs 14.3%, p <0.001).
High TG were more frequent in patients with diabetes mellitus (46.7% vs 12.5%, p <0.001) or with a metabolic syndrome (78.6 vs 10.1%, p <0.001).
The physicians were asked to change the therapy if necessary and to define which parameters were important in the decision for changing.
Even though around 60% of the patients did not reach one or more lipid-targets (as defined in the method section according to the current guidelines), the therapy was changed in only 15.4% of the patients (fig. 3).
The treating specialists modified the lipid lowering therapy as follows: they changed the statin (2.1%), increased the doses of the statin (7.8%), prescribed an additional drug (2.7%) and they started another intervention (2.8%) (fig. 3).
A total of 81.4% of the physicians changed their therapeutic strategies because LDL-C was higher than the therapeutic goal, 67% because HDL-C was low, 66.6% changed the therapy from looking at total cholesterol and 59% because triglycerides were elevated. Just a third of physicians (36.2%) considered the TC/HDL-C ratio as relevant.
The values given to the different parameters differed notably according to the patient groups and to the speciality of the physicians.
In patients with diabetes mellitus, LDL-C (87.5% vs. 74.1%, p = 0.001), HDL-C (76.3% vs. 56%, p <0.001) and TG (68.9% vs. 47.2%, p <0.001) were considered more important in order to modify the therapy, compared to non-diabetic patients.
On the contrary, HDL-C and TG levels were considered more relevant in the absence of CHD (HDL-C 76.9% vs. 54.9%, p <0.001; TG 66.5% vs. 49.8%, p = 0.001), while LDL-C was considered similarly in patients with or without CHD (77.5% vs. 84.6%, respectively, p = 0.096).
Plasma glucose (62.6% vs. 38.9%, p <0.001) and HbA1c (70.4% vs. 14.4%, p <0.001) were considered to be relevant more in diabetic patients than in non-diabetic patients.
Lipids play a different role as a cardiovascular risk factor according to gender. LDL-C seems to be a less important risk factor in pre-menopausal women; HDL-C is a better predictor of risk in women than in men, and TG are an independent predictor of CAD risk in post-menopausal women [22].
Therefore we analysed the data of the FOCUS population according to gender.
In this study, 180 female and 293 male were included. A total of 162 female (90%) were in post-menopausal status.
Coronary artery disease was significantly more prevalent in men compared to women (56.7% vs. 26.1%, p <0.001).
The prevalence of hypertension, diabetes, metabolic syndrome and dyslipidemia was similar in male and female patients.
A lipid profile within the normal limits was found in 38.9% of male patients and in 41.7% of females.
A LDL-C at a goal level associated with other abnormalities in the lipid profile (low HDL-C and/or high TG) was found in 27% of males and in 28.3% of the female patients.
Even the mean HDL was higher in females (1.45 ± 0.41) than in males (1.23 ± 0.36) (p = 0.019).
The prevalence of high triglycerides was similar in both genders (28.3% in females vs. 32.8% in males, p = ns).
| Table 1:Baseline characteristics of the study population. | |||||
| Clinical characteristics | All | Gender | Cardiovascular risk | ||
| Female | Male | <10% | <20% | ||
| Gender (female/male) | 180/293 | ||||
| Age (years) | 66.3 ± 9.41 | 67.3 ± 9.98 | 65.8 ± 9.01 | 65.2 ± 11.5 | 66.5 ± 9.13 |
| BMI (kg/m2) | 28.5 ± 4.96 | 28.6 ± 5.5 | 28.4 ± 4.6 | 26.5 ± 4.1 | 28.7 ± 5.0 |
| Systolic blood pressure (mm Hg) | 138.8 ± 16.7 | 139.7 ± 16.2 | 138.4 ± 17.1 | 133.8 ± 12.6 | 139.3 ± 17.1 |
| Diastolic blood pressure (mm Hg) | 81.4 ± 9.7 | 82.8 ± 8.5 | 80.6 ± 10.3 | 82.4 ± 8.8 | 81.2 ± 9.8 |
| Total cholesterol (mmol/L) | 4.52 ± 1.04 | 4.73 ± 0.98 | 4.39 ± 1.05 | 4.75 ± 0.82 | 4.48 ± 1.06 |
| LDL cholesterol (mmol/L) | 2.45 ± 0.9 | 2.56 ± 0.86 | 2.37 ± 0.92 | 2.65 ± 0.7 | 2.41 ± 0.9 |
| HDL cholesterol (mmol/L) | 1.31 ± 0.4 | 1.45 ± 0.41 | 1.23 ± 0.36 | 1.47 ± 0.3 | 1.29 ± 0.4 |
| Triglycerides (mmol/L) | 1.71 ± 0.99 | 1.58 ± 0.78 | 1.79 ± 1.1 | 1.42 ± 0.71 | 1.75 ± 1.02 |
| Plasma glucose (mmol/L) | 6.7 ± 2.18 | 6.56 ± 1.93 | 6.79 ± 2.31 | 5.33 ± 0.9 | 6.9 ± 2.24 |
| HbA1c (%, in diabetics only) | 6.97 ± 1.34 | 6.94 ± 1.34 | 6.99 ± 1.35 | 5.5 ± 0.63 | 7.09 ± 1.32 |
| LDL: low density lipoprotein, HDL: high density lipoprotein | |||||
| Table 2:Lipid lowering drugs at baseline. | ||
| Drugs | Number of patients (%) | Mean doses |
| Atorvastin | 201 (42.5) | 30 mg |
| Fluvastatin | 17 (3.6) | 53 mg |
| Pravastatin | 89 (18.8) | 32 mg |
| Rosuvastatin | 25 (5.3) | 12 mg |
| Simvastatin | 141 (29.8) | 30 mg |
| Any statin + ezetimibe | 27 (5.7%) | |
| Any statin + fibrate | 4 (0.8%) | |
| Any statin + niacin | 1 (0.2%) | |
| Table 3:Prevalence of cardiovascular risk factors in the study population. | |
| Positive family history for CV disease | 146/473 |
| Post-menopausal status | 162/180 |
| Diabetes mellitus | 257/473 |
| Dyslipidemia | 443/473 |
| Hypertension | 357/473 |
| Tobacco consumption Current smokers Former smokers | 70/473 133/473 |
| Sedentary lifestyle | 222/473 |
| Metabolic syndrome | 145/473 |
| Previous CHD | 213/473 |
| Table 4:Lipid profile alterations found in the study population with high cardiovascular risk. | |
| Lipid profile (patients with cardiovascular risk >20%) | Absolute number, percentage |
| No lipid alterations | 136/409, 33.3% |
| LDL-C >2.6 mmol/L | 151/409, 36.9% |
| Low HDL-C | 126/409, 30.8% |
| High triglycerides | 147/409, 35.9% |
| Lipid profile (patients with history of CHD) | |
| No lipid alterations | 75/213, 35.2% |
| LDL-C >2.6 mmol/L | 74/213, 34.7% |
| Low HDL-C | 66/213, 31.0% |
| High triglycerides | 72/213, 33.8% |
| Lipid profile (patients with diabetes) | |
| No lipid alterations | 75/257, 29.2% |
| LDL-C >2.6 mmol/L | 86/257, 33.5% |
| Low HDL-C | 84/257, 32.7% |
| High triglycerides | 120/257, 46.7% |
| LDL-C: low density lipoprotein-cholesterol, TG: triglycerides, HDL-C: high density lipoprotein-cholesterol; CHD: coronary heart disease.High LDL-C was defined as LDL-C >2.6 mmol/L high risk patients, LDL-C ≥4.1 mmol/L in patients with low cardiovascular risk; low HDL-C was defined as HDL-C <1.3 mmol/L in women or <1 mmol/L in men; high triglycerides were defined as triglycerides ≥1.7 mmol/L in high risk patients and ≥5.0 mmol/L in patients with low or intermediate risk. Cardiovascular risk was defined according to the European guidelines [28, 29]. We divided patients into “low risk “(10-year risk <10%), “intermediate risk” (10-year risk 10–20%) and “high risk” (10-year risk >20%). | |
Switzerland is a country characterised by a low risk for cardiovascular morbidity and mortality, and from 1970 to 2004 the mortality rate for ischemic heart disease diminished from 76.4/ to 51.3/100’000 persons [30].
Nevertheless, cardiovascular disorders are still the leading cause of death. In 2007, in Switzerland 10’107 of 29’544 deaths in men and 12’506 of 31’545 deaths in women were caused by cardiovascular disease [31].
Well-organised and focused primary and secondary prevention therefore have the potential to change the fate of thousands of people in Switzerland.
Reducing LDL-Cholesterol reduces cardiovascular risk in patients with or without cardiovascular disease (CVD) and epidemiological data have suggested that increasing HDL and reducing triglycerides could be beneficial in terms of reducing cardiovascular risk. Checking and, if necessary, treating lipid profiles is one of the most important strategies for an effective prevention of CVD [29].
The use of statins is a cornerstone in the secondary prevention of CVD and it is associated with a substantial reduction in cardiovascular morbidity and mortality [23, 29].
Moreover, a recent meta analysis of a trial enrolling patients with cardiovascular risk factors but without established CVD, showed that the use of statins was associated with a significantly improved chance of survival and large reductions in the risk of major cardiovascular events [32].
Nevertheless, patients treated with statins still have a consistent “residual risk” [10, 11].
Even if part of this residual risk may be explained by non-modifiable risk factors, we have the possibility to reduce it by an effective and comprehensive treatment of all the components of the lipid profile and by a simultaneous treatment of the different cardiovascular risk factors.
In the FOCUS study, we observed that only 40% of the patients had a normal lipid profile even if they were all treated by a statin. Moreover, many patients with a LDL-C level below the target for primary or secondary prevention were characterised by a low HDL-C, and/or high TG.
Two main data from the FOCUS study should be highlighted:
Firstly, a change in therapy only occurred in a quarter of the patients with an abnormal lipid profile and secondly, alterations of the lipid profile other than LDL-C levels were less often taken into consideration by the physicians in order to decide a therapeutic change. HDL-C and TG were considered to be relevant for changing the therapy in the presence of diabetes mellitus or metabolic syndrome as well as in patients without CHD or with a low cardiovascular risk.
The parameters taken into account by the physicians for changing the therapy did not differ by age, gender or geographic area.
Last but not least, it is of note that if a change in therapy was done, it was mainly an increase in the statin doses even though this strategy was shown to be associated with small incremental reductions in the cholesterol level. In fact, doubling the dose of a statin lowers LDL-cholesterol by an additional 6% [33].
When investigating gender related differences, we found that, in the population included in the FOCUS study, the prevalence of CHD was significantly higher in male patients compared to females. Moreover, females presented a higher HDL compared to males but a significant higher percentage of them were classified as low HDL-C probably due to the higher cut-off. Nevertheless, the importance of HDL-C level was equal among males and females.
The FOCUS study included a selected population characterised by previous treatment with a statin. This inclusion criterion may be responsible for the high prevalence of dyslipidemia and high cardiovascular risk observed in the enrolled population. Moreover, the fact that the majority of recruiting physicians were endocrinologists or diabetes specialists may explain the high prevalence of diabetes mellitus and metabolic syndrome seen in the study population.
Therefore the data collected in the FOCUS study should not be generalised to an unselected Swiss population.
Evidence linking statins to cardiovascular risk protection and reduced mortality is clear and consistent across all adult age groups and it extends to individuals with cardiovascular disease and with cardiovascular risk factors.
Reducing the residual cardiovascular risk in patients treated with statins remains an exciting clinical challenge.
Comprehensive lipid management and a global reduction of the whole cardiovascular risk should be considered as winning strategies to improve the prognosis of statin-treated patients.
1 Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, MacFarlane PW, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995; 333(20):1301–7.
2 Downs JR, Clearfield M, Weis S, Whitney E, Shapiro DR, Beere PA, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;279(20):1615–22.
3 Sever PS, Dahlof B, Poulter NR, Wedel H, Beevers G, Caulfield M, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial – Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361(9364):1149–58.
4 Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994;344(8934):1383–9.
5 Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335(14):1001–9.
6 Shepherd J, Blauw GJ, Murphy MB, Bollen EL, Buckley BM, Cobbe SM, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360(9346):1623–30.
7 Kizer JR, Madias C, Wilner B, Vaughan CJ, Mushlin AI, Trushin P, et al. Relation of different measures of low-density lipoprotein cholesterol to risk of coronary artery disease and death in a meta-regression analysis of large-scale trials of statin therapy. Am J Cardiol. 2010;105(9):1289–96.
8 Mora S, Glynn RJ, Hsia J, MacFadyen JG, Genest J, Ridker PM. Statins for the primary prevention of cardiovascular events in women with elevated high-sensitivity C-reactive protein or dyslipidemia: results from the Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) and meta-analysis of women from primary prevention trials. Circulation. 2010;121(9):1069–77.
9 Mills EJ, Rachlis B, Wu P, Devereaux PJ, Arora P, and Perri D. Primary prevention of cardiovascular mortality and events with statin treatments: a network meta-analysis involving more than 65,000 patients. J Am Coll Cardiol. 2008;52(22):1769–81.
10 Shah PK, Kaul S, Nilsson J, and Cercek B. Exploiting the vascular protective effects of high-density lipoprotein and its apolipoproteins: an idea whose time for testing is coming, part I. Circulation. 2001;104(19):2376–83.
11 Gotto AM, Jr. High-density lipoprotein cholesterol and triglycerides as therapeutic targets for preventing and treating coronary artery disease. Am Heart J. 2002;144(6 Suppl):S33–42.
12 Barter P, Gotto AM, LaRosa JC, Maroni J, Szarek M, Grundy SM, et al. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med. 2007;357(13):1301-10.
13 Assmann G and Sacks F, http://www.escardio.org . 2009.
14 Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):937–52.
15 Gordon DJ, Probstfield JL, Garrison RJ, Neaton JD, Castelli WP, Knoke JD, et al. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 1989;79(1):8–15.
16 Devendra GP, Whitney EJ, and Krasuski RA. Impact of increases in high-density lipoprotein cholesterol on cardiovascular outcomes during the armed forces regression study. J Cardiovasc Pharmacol Ther. 15(4):380–3.
17 Velagaleti RS, Massaro J, Vasan RS, Robins SJ, Kannel WB, and Levy D. Relations of Lipid Concentrations to Heart Failure Incidence. The Framingham Heart Study. Circulation. 2009.
18 Hokanson JE and Austin MA. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: a meta-analysis of population-based prospective studies. J Cardiovasc Risk. 1996;3(2):213–9.
19 McBride P. Triglycerides and risk for coronary artery disease. Curr Atheroscler Rep. 2008;10(5):386–90.
20 Miller M, Cannon CP, Murphy SA, Qin J, Ray KK, and Braunwald E. Impact of triglyceride levels beyond low-density lipoprotein cholesterol after acute coronary syndrome in the PROVE IT-TIMI 22 trial. J Am Coll Cardiol. 2008;51(7):724–30.
21 Tan YY, Gast GC, and van der Schouw YT. Gender differences in risk factors for coronary heart disease. Maturitas. 65(2):149–60.
22 LaRosa JC. Lipids and cardiovascular disease: Do the findings and therapy apply equally to men and women? Women’s Health Issues. 2005;2(2):102–13.
23 Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–421.
24 Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, et al. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2007;25(6):1105–87.
25 Mancia G, Laurent S, Agabiti-Rosei E, Ambrosioni E, Burnier M, Caulfield MJ, et al. Reappraisal of European guidelines on hypertension management: a European Society of Hypertension Task Force document. J Hypertens. 2009.
26 Alberti KG, Zimmet P, and Shaw J. The metabolic syndrome – a new worldwide definition. Lancet. 2005;366(9491):1059–62.
27 Assmann G, Cullen P, and Schulte H. Simple scoring scheme for calculating the risk of acute coronary events based on the 10-year follow-up of the prospective cardiovascular Munster (PROCAM) study. Circulation. 2002;105(3):310–5.
28 De Backer G, Ambrosioni E, Borch-Johnsen K, Brotons C, Cifkova R, Dallongeville J, et al. European guidelines on cardiovascular disease prevention in clinical practice. Third Joint Task Force of European and Other Societies on Cardiovascular Disease Prevention in Clinical Practice. Eur Heart J. 2003;24(17):1601–10.
29 Graham I, Atar D, Borch-Johnsen K, Boysen G, Burell G, Cifkova R, et al. European guidelines on cardiovascular disease prevention in clinical practice: executive summary. Eur Heart J. 2007;28(19):2375–414.
30 Statistik Bf, Von Generation zu Generation: Entwicklung der Todesursachen 1970 bis 2004. 2008, BFS: Neuchâtel.
31 Statistik Bf, Statistik der Todesursachen 2007. 2009, BFS: Neuchâtel.
32 Brugts JJ, Yetgin T, Hoeks SE, Gotto AM, Shepherd J, Westendorp RG, et al. The benefits of statins in people without established cardiovascular disease but with cardiovascular risk factors: meta-analysis of randomised controlled trials. BMJ. 2009;338:b2376.
33 Jones P, Kafonek S, Laurora I, and Hunninghake D. Comparative dose efficacy study of atorvastatin versus simvastatin, pravastatin, lovastatin, and fluvastatin in patients with hypercholesterolemia (the CURVES study). Am J Cardiol. 1998;81(5):582–7.
The FOCUS study was supported by MSD, Switzerland.
Isabella Sudano and Georg Noll declare no conflict of interest. Lorenzo Hess received honoraria for data management and statistical analysis from MSD. Diana Arnet is currently employed by MSD, Switzerland and owns Merck stock.