
Figure 1
Sequence of patients receiving ketamine in group K. The quality of analgesia was measured using VAS (from 0 to 10) and was defined as ineffective (VAS score >3) or effective (VAS score ≤3).
DOI: https://doi.org/10.4414/smw.2011.13195
Opioids are the agents of choice for the treatment of moderate to severe acute and chronic pain. However, over the past several years a large number of experimental and clinical studies have convincingly confirmed that brief opioid exposure can enhance pain sensitivity presenting as opioid-induced hyperalgesia (OIH) [1–5]. OIH seem to develop more rapidly and more frequently with the administration of potent short-acting opioids such as remifentanil than with longer-acting opioids [6]. A study in healthy human volunteers showed that a skin area with pre-existing mechanical hyperalgesia was significantly enlarged after a 60- to 90-min intravenous remifentanil infusion [7]. Clinically, a relatively large dose of intraoperative remifentanil has been shown to trigger postoperative secondary hyperalgesia and to require higher doses of morphine for adequate analgesia [8]. Based on previous reports that administration of opioids can induce pain inhibitory and pain facilitatory systems, this pain hypersensitivity has been attributed to a relative predominance of pronociceptive mechanisms such as activation of the N-methyl-D-aspartate (NMDA) receptor system.
Ketamine, an uncompetitive NMDA receptor antagonist, is a widely used general anaesthetic due to its analgesic effects. Experimental studies in volunteers and animals have demonstrated that small-dose ketamine can inhibit central sensitisation and prevent OIH [2, 8, 9]. However, the optimal dose of ketamine for prevention of OIH has not been determined. In the present study we therefore used a modified up-and-down method to determine the ED50 and ED95 (effective dose in 50% and 95% of patients respectively) of ketamine for prevention of postoperative hyperalgesia after remifentanil-based anaesthesia in patients undergoing laparoscopic cholecystectomy.
After ethics committee approval (the Affiliated People’s Hospital, Jiangsu University, PR China) and patient written informed consent were obtained, 54 patients (male and female) with American Society of Anesthesiologists physical status I or II were randomised into two groups: group C (control) and group K (ketamine). All patients were scheduled to receive general anaesthesia for laparoscopic cholecystectomy. Noninclusion criteria were as follows: (1) immediate extubation was not planned after surgery; (2) took analgesics regularly or had used opioids within 12 h of surgery; (3) unable to comprehend visual analog scale (VAS); (4) had a history of alcohol or drug abuse, psychiatric disorder, or obesity (>130% of ideal body weight); (5) had chronic inflammatory disease; (6) intraoperative changeover from laparoscopic to open cholecystectomy; (7) age younger than 18 yr; (8) had a condition, such as severe hypertension, acute cardiovascular disorder, or a psychiatric disorder, for which the use ketamine was contraindicated.
During the preoperative anaesthetic evaluation the evening before surgery patients were directed to the use of VAS (0 = no pain, 10 = the worst possible pain). All patients were premedicated with intramuscular atropine 0.5 mg. After the patients had entered the operating room an 18-gauge cannula was placed in the forearm and 10 ml/kg lactated Ringer’s solution was infused. Normal monitoring was used throughout the study, including electrocardiography, noninvasive arterial blood pressure monitoring, pulse oximetry and end-tidal CO2 pressure (PetCO2).
Anaesthesia was induced with midazolam 0.05 mg/kg intravenously (IV), fentanyl 4 μg/kg IV and propofol 1 mg/kg IV, with atracurium 1 mg/kg (intravenous injection) used to facilitate tracheal intubation. After tracheal intubation the patients were ventilated to normocapnia with 50% oxygen. Anaesthesia was maintained with infusion of remifentanil (0.25~0.3 μg·kg–1·min–1) and propofol (3~4 mg·kg–1·h–1). An atracurium infusion was titrated to maintain one twitch in response to a supramaximal train-of-four stimulus at the orbicularis oculi. Atracurium was stopped 15 min before the end of surgery. Insufficient anaesthesia was defined as systolic blood pressure (SBP) exceeding baseline values by 20% for at least 1 min, heart rate exceeding preinduction values by 15%, and/or a bispectral index of 60 or greater. The propofol and remifentanil infusion rates were increased in steps of 1 mg·kg–1·h–1 and 0.05 μg·kg–1·min–1 respectively, when insufficient anaesthesia was suspected. Hypotension was defined as a mean arterial pressure below 60 mm Hg or SBP below 80 mm Hg. The propofol infusion rate was decreased in steps of 1 mg·kg–1·h–1 for bispectral index values that remaining below 60. Additional intravenous fluids were also given as deemed appropriate by the anaesthetist. Similarly, ephedrine or atropine was given as required to treat persistent hypotension or bradycardia. After skin closure, remifentanil and propofol were stopped, after which patients were transferred to the postanaesthesia care unit (PACU). Residual neuromuscular blockade was antagonised with 20~40 μg/kg neostigmine and 10~20 μg/kg atropine. The patient was extubated when spontaneous respiratory rate exceeded 12 breaths/min, the patient responded to verbal commands and PetCO2 was below 45 mm Hg. Patients remained in the PACU for at least 2 h and were given oxygen via a facemask at a rate of 5 L/min after tracheal extubation.
Group K was given ketamine intravenously before skin incision to prevent remifentanil-induced postoperative hyperalgesia. An equal volume of normal saline (NS) was given to the patients in group C. Postoperative pain was assessed using VAS at 10 min after tracheal extubation. Pain assessment was blind. VAS score ≤3 was defined as effective analgesia and VAS score >3 as ineffective [10]. The dose of ketamine received by a patient in group K was determined by the previous patient’s response, using up-and-down sequential allocation technique. The dose adjustment space was 0.2 mg/kg. If a patient’s response was ineffective (VAS score >3), the ketamine dose given to the next patient was increased by 0.2 mg/kg. If the response was effective (VAS score ≤3), the ketamine dose given to the next patient was decreased by the same amount. The first patient received 0.7 mg/kg ketamine [11]. To increase the precision of the final estimator we used a modified up-and-down method based on changing the test interval (dose span) [12, 13]. In this modified method the up-and-down sequence includes two phases: the first phase consists of an original up-and-down sequence on the predetermined equally spaced test levels until three changes of response type are achieved. The second phase is to lower the initial test interval, resuming the up-and-down sequence at the nearest level to the average and continuing the experiment at the next higher or the next lower level according to the response type on the reduced test interval. According to this modified up-and-down method, the initial test interval was reduced to 0.1 mg/kg as second phase test interval. We predefined that 27 patients should be involved in the study.
After postoperative pain was evaluated, patients who reported ineffective analgesia (VAS score >3) were given rescue analgesia and tramadol titration was performed by an anaesthesiologist.
Duration of surgery (defined as the time from the initial skin incision to closure), propofol and remifentanil doses, postoperative nausea and vomiting (PONV), hallucinosis and nystagmus were recorded.
Statistical analysis was performed with the use of SPSS16.0 software (SPSS Inc., Chicago, IL, USA). Data were analysed by Student’s t-test for normally distributed data and the Fisher exact test for incidence data. The ED50, ED95and 95% confidence interval (CI) of ketamine for prevention of postoperative hyperalgesia were obtained by probit analysis.
All 54 patients enrolled completed the study. Demographic data and incidences of adverse effects were similar in the two groups. The VAS score was significantly lower in group K than in group C (P <0.01) (table 1–2).
Probit model was fitted using dosage in mg/kg and dose-response curves based on dose were analysed. ED50 and ED95were estimated by NONMEM version V (UCSF, San Francisco, CA). The quality of the fit was estimated on the basis of improvement in the log likelihood value of NONMEM (an increase of 4 in the log likelihood value consistent with P <0.05 was regarded as significant) and visual evaluation of the fit. This method has been previously used in other studies investigating ED50 and ED95[14, 15]. The ED50and ED95 of ketamine for prevention of postoperative hyperalgesia after remifentanil-based anaesthesia were 0.24 mg/kg (95%CI·0.20~0.30 mg/kg) and 0.33 mg/kg (95%CI·0.28~0.62 mg/kg) respectively (fig. 1–2).
| Table 1: Demographic data and intraoperative outcomes. | ||
| group C (n = 27) | group K (n = 27) | |
| Age (years) | 53.2 ± 7.1 | 51.7 ± 6.7 |
| Weight (kg) | 65.3 ± 10.8 | 66.5 ± 12.0 |
| Duration of surgery (min) | 48.3 ± 7.6 | 47.1 ± 7.2 |
| Remifentanil doses (μg) | 889 ± 179 | 862 ± 184 |
| Propofol doses (mg) | 183 ± 58 | 171 ± 61 |
| Number requiring rescue | 26 | 12 |
| Data are presented as mean ± SD. No significant differences were observed. | ||
| Table 2: VAS score and incidences of adverse effects. | ||
| Group C (n = 27) | Group K (n = 27) | |
| VAS score | 5.5 ± 1.3 | 3.6 ± 1.4** |
| PONV, n (%) | 4 (14.8%) | 3 (11.1%) |
| Hallucinosis, n (%) | 3 (11.1%) | 0 (0%) |
| Nystagmus, n (%) | 1 (3.7%) | 0 (0%) |
| Data are presented as mean ± SD or as percentage. *P<0.05, **P<0.01 vs. group C using Student’s t-test. ▲P <0.05, ▲▲P <0.01 vs. group C using Fisher exact test. | ||
Remifentanil-based anaesthesia is commonly used for laparoscopic cholecystectomy. It provides haemodynamic stability and faster awakening time [16]. However, brief opioid exposure is associated with the onset of OIH. Activation of the NMDA receptor system, acute receptor desensitisation via uncoupling of the receptor from G proteins, increasing calmodulin-dependent protein kinase II, and up-regulation of the cyclic adenosine monophosphate pathway have been suggested as potential mechanisms underlying this phenomenon [17–19]. A key role has been assigned to the endogenous pain facilitatory system involving the NMDA receptor. Ketamine interacts with several receptors and ion channels, but is mainly a noncompetitive NMDA receptor antagonist. NMDA receptor inhibition will, to a certain extent, inhibit acute pain, but in addition NMDA receptor mediated plasticity plays a key role in themaintenance of chronic pain due to central sensitisation. Low doses of ketamine are determined as an intravenous bolus of less than 1 mg·kg–1and/or continuous intravenous infusion at rates below 20 μg·kg–1·min–1 [20]. Joly et al. [8] found intravenous administration of 0.5 mg/kg ketamine followed by intraoperative infusion of 5 μg·kg–1·min–1could prevent larger-dose (0.40 μg·kg–1·min–1) remifentanil-induced secondary hyperalgesia. Zakine et al. [21] discovered that an intravenous bolus of 0.5 mg/kg ketamine followed by an intravenous infusion of 2 μg·kg–1·min–1 for 48 h postoperatively could significantly decrease consumption of morphine. However, Engelhardtet al. [22] reported that bolus dose of 0.5 mg/kg ketamine followed by an intraoperative infusion of 4 μg·kg–1·min–1 could not prevent hyperalgesia resulting from remifentanil-based anaesthesia during paediatric scoliosis surgery. A larger dose of ketamine or extension of the infusion into the postoperative period may inhibit the development of remifentanil-induced hyperalgesia. Luginbuhlet al. [23] reported that low-dose ketamine could not prevent remifentanil-induced hyperalgesia by all types of painful stimuli (e.g., pressure pain), and the results suggested that hyperalgesia induced by opioids and acute opioid tolerance depend on the type of nociceptive stimulus.

Figure 1
Sequence of patients receiving ketamine in group K. The quality of analgesia was measured using VAS (from 0 to 10) and was defined as ineffective (VAS score >3) or effective (VAS score ≤3).
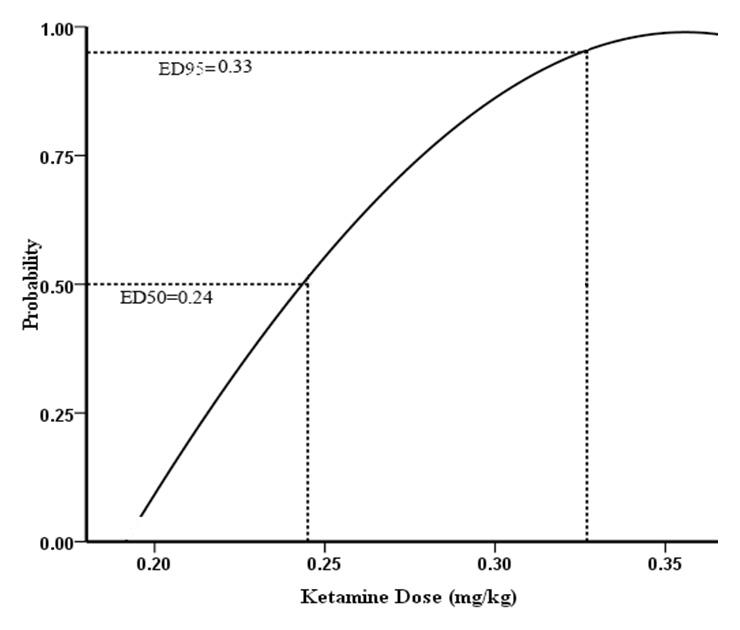
Figure 2
Dose-response curve of ketamine for prevention of postoperative hyperalgesia after remifentanil-based anaesthesia in patients undergoing laparoscopic cholecystectomy.
We used the modified up-and-down method to determine the ED50 and ED95of ketamine for prevention of postoperative hyperalgesia after remifentanil-based anaesthesia in patients undergoing laparoscopic cholecystectomy. This modified method can enhance the precision of the final estimator and decrease the mean squared error under normal tolerance distribution; it has also been shown to be much better than the original method when the initial test interval is relatively broad and the initial dose is away from the final median lethal dose [12]. The ED50 and ED95dosages attained would have general applicability and would be able to guide good clinical practice. According to our stu the ED50and ED95 of ketamine to prevent postoperative hyperalgesia after remifentanil-based anaesthesia were 0.24 mg/kg and 0.33 mg/kg respectively. The incidences of adverse effects were similar in the two groups. As expected, we did not observe severe adverse effects. The incidences of PONV, hallucinosis and nystagmus in group K were much lower than reported by Launo et al. [11]. It may benefit from administering a lower dose of ketamine.
There were some limitations to our study. Firstly, the study drug was administered in an unblinded fashion and hence group allocation was known to the anesthesiologist; this may impact on results. Secondly, only a single assessment of post-operative pain by VAS was made, and it is thus unclear whether the ketamine effect lasts long enough to cover the whole period of hyperalgesia. According to the pharmacokinetics of ketamine, the duration of ketamine is more than 60 min, and thus in our study its effect could cover the time point we have chosen. We use only visual analog scale to address hyperalgesia, because if we choose two tests the pain scores in one test define as effective but the pain scores in the other test define as ineffective. To avoid embarrassment with this situation we used only one test to evaluate pain.
In conclusion, our study showed that the ED50and ED95 of ketamine for prevention of postoperative hyperalgesia after remifentanil-based anaesthesia were 0.24 mg/kg (95%CI·0.20~0.30 mg/kg) and 0.33 mg/kg (95%CI·0.28~0.62 mg/kg) respectively.
1 Colvin LA, Fallon MT. Opioid-induced hyperalgesia: a clinical challenge. Br J Anaesth. 2010;104(2):125–7.
2 Minville V, Fourcade O, Girolami JP, Tack I. Opioid-induced hyperalgesia in a mice model of orthopaedic pain: preventive effect of ketamine. Br J Anaesth. 2010;104(2):231–8.
3 Zissen MH, Zhang G, McKelvy A, Propst JT, Kendig JJ, Sweitzer SM. Tolerance, opioid-induced allodynia and withdrawal associated allodynia in infant and young rats. Neuroscience. 2007;144(1):247–62.
4 Chu LF, Angst MS, Clark D. Opioid-induced hyperalgesia in humans – Molecular mechanisms and clinical considerations. Clin J Pain. 2008;24(6):479–96.
5 Silverman SM. Opioid induced hyperalgesia: clinical implications for the pain practitioner. Pain Physician. 2009;12(3):679–84.
6 De Baerdemaeker LE, Jacobs S, Pattyn P, Mortier EP, Struys MM. Influence of intraoperative opioid on postoperative pain and pulmonary function after laparoscopic gastric banding: remifentanil TCI vs sufentanil TCI in morbid obesity. Br J Anaesth. 2007;99(3):404–11.
7 Angst MS, Koppert W, Pahl I, Clark DJ, Schmelz M. Short-term infusion of the mu-opioid agonist remifentanil in humans causes hyperalgesia during withdrawal. Pain. 2003;106(1–2):49–57.
8 Joly V, Richebe P, Guignard B, Fletcher D, Maurette P, Sessler DI, et al. Remifentanil-induced postoperative hyperalgesia and its prevention with small-dose ketamine. Anesthesiology. 2005;103(1):147–55.
9 Gu X, Wu X, Liu Y, Cui S, Ma Z. Tyrosine phosphorylation of the N-Methyl-D-Aspartate receptor 2B subunit in spinal cord contributes to remifentanil-induced postoperative hyperalgesia: the preventive effect of ketamine. Mol Pain. 2009;5:76.
10 Delage N, Maaliki H, Beloeil H, Benhamou D, Mazoit JX. Median effective dose (ED50) of nefopam and ketoprofen in postoperative patients: a study of interaction using sequential analysis and isobolographic analysis. Anesthesiology. 2005;102(6):1211–6.
11 Launo C, Bassi C, Spagnolo L, Badano S, Ricci C, Lizzi A, et al. Preemptive ketamine during general anesthesia for postoperative analgesia in patients undergoing laparoscopic cholecystectomy. Minerva Anestesiol. 2004;70(10):727–34.
12 Jung H, Choi SC. Sequential method of estimating the LD50 using a modified up-and-down rule. J Biopharm Stat. 1994;4(1):19–30.
13 Albertin A, Casati A, Bergonzi P, Fano G, Torri G. Effects of two target-controlled concentrations (1 and 3 ng/ml) of remifentanil on MAC(BAR) of sevoflurane. Anesthesiology. 2004;100(2):255–9.
14 Butwick AJ, Coleman L, Cohen SE, Riley ET, Carvalho B. Minimum effective bolus dose of oxytocin during elective Caesarean delivery. Br J Anaesth. 2010;104 (3):338–43.
15 Carvalho B, Durbin M, Drover DR, Cohen SE, Ginosar Y, Riley ET. The ED50 and ED95 of intrathecal isobaric bupivacaine with opioids for cesarean delivery. Anesthesiology. 2005;103(3):606–12.
16 Gemma M, Tommasino C, Cozzi S, Narcisi S, Mortini P, Losa M et al. Remifentanil provides hemodynamic stability and faster awakening time in transsphenoidal surgery. Anesth Analg. 2002;94(1):163–8.
17 Koppert W, Schmelz M. The impact of opioid-induced hyperalgesia for postoperative pain. Best Pract Res Clin Anaesthesiol. 2007;21(1):65–83.
18 Bannister K, Dickenson AH. Opioid hyperalgesia. Curr Opin Support Palliat Care. 2010;4(1):1–5.
19 Chen Y, Yang C, Wang ZJ. Ca2+/calmodulin-dependent protein kinase II alpha is required for the initiation and maintenance of opioid-induced hyperalgesia. J Neurosci. 2010;30(1):38–46.
20 Schmid RL, Sandler AN, Katz J. Use and efficacy of low-dose ketamine in the management of acute postoperative pain: a review of current techniques and outcomes. Pain. 1999;82(2):111–25.
21 Zakine J, Samarcq D, Lorne E, Moubarak M, Montravers P, Beloucif S, et al. Postoperative ketamine administration decreases morphine consumption in major abdominal surgery: a prospective, randomized, double-blind, controlled study. Anesth Analg. 2008;106(6):1856–61.
22 Engelhardt T, Zaarour C, Naser B, Pehora C, de Ruiter J, Howard A, et al. Intraoperative low-dose ketamine does not prevent a remifentanil-induced increase in morphine requirement after pediatric scoliosis surgery. Anesth Analg. 2008;107(4):1170–5.
23 Luginbuhl M, Gerber A, Schnider TW, Petersen-Felix S, Arendt-Nielsen L, Curatolo M. Modulation of remifentanil-induced analgesia, hyperalgesia, and tolerance by small-dose ketamine in humans. Anesth Analg. 2003;96(3):726–32.
This work was supported by the Jiangsu Health International Exchange Program (JSH-2011-057). The trial registration number is NCT01301079.