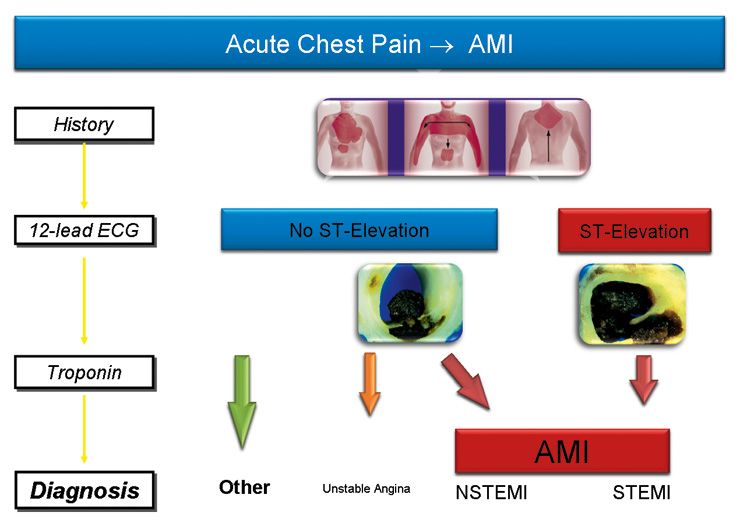
Figure 1
12-lead ECG and measurement of cardiac troponin complement clinical assessment in the diagnosis of acute myocardial infarction (AMI).
DOI: https://doi.org/10.4414/smw.2011.13202

Figure 1
12-lead ECG and measurement of cardiac troponin complement clinical assessment in the diagnosis of acute myocardial infarction (AMI).
Acute myocardial infarction (AMI) is the major cause of death and disability worldwide with an ongoing increase in incidence. The risk of death is highest within the first few hours from AMI onset [5–7]. Approximately 15 to 20 thousand patients per million inhabitants per year present to the emergency department (ED) with acute chest pain or other symptoms suggestive of AMI in Europe and the United States each [5–7]. Rapid identification of AMI is critical to initiate effective evidence-based medical treatment and management [6–8]. Fortunately the majority of patients presenting with acute chest pain to the ED do NOT have AMI, but often benign disorders such as musculoskeletal pain instead.

Figure 2
Rule in of acute myocardial infarction (AMI) can be at presentation (0h) in patients with unequivocal ST-elevations, at 1h in patients with elevations in cardiac troponin (cTn) in the measurement performed at presentation (turnaround time is around 1h in most hospitals) and at 7h if the first cTn is normal and the elevation in cTn becomes apparent only at the second measurement performed after 6 hours. Rule out requires a normal 2nd cTn level and therefore takes 7 hours. The clinical consequences of the delayed rule in and rule out are profound.
The 12-lead ECG and cTn are the diagnostic cornerstones and complement clinical assessments (fig. 1 + 2) [6–8]. In most patients with ST-elevation AMI, clinical assessment and the ECG provide a straight forward diagnosis and allow the initiation of revascularisation within minutes. However, ST-elevation AMI represents only about 5% of consecutive patients presenting with acute chest pain [9]. Therefore, in many other patients, in fact the vast majority, the physician is left with considerable uncertainty following the clinical assessment and the initial ECG. The ECG by itself is often insufficient to diagnose an AMI since ST deviation may be observed in other conditions, such as early repolarisation patterns, acute pericarditis, left ventricular hypertrophy, left bundle brunch block, hyperkalemia and the Brugada syndrome [6–8, 10, 11]. Therefore, cTns have become a prominent role in the diagnosis of AMI. cTns, sensitive and specific biochemical markers of cardiomyocyte necrosis [6–8, 12–17], are very helpful in clinical practice to identify patients with acute coronary syndromes at high risk, and to select those patients who will benefit from early coronary angiography and, whenever possible, percutaneous coronary intervention [6–8, 12–17]. In addition, fully automated standard cTn assays, including the current fourth-generation Roche TnT, are superior to all other biomarkers that have been clinically available in the diagnosis of AMI, such as CK-MB and myoglobin, and are therefore considered the preferred marker in the diagnosis of AMI [13, 15, 18, 19]. It is important to highlight that cTn indicates and quantifies cardiomyocyte necrosis irrespective of its cause.
The major limitation of contemporary cTn assays is a sensitivity deficit in the first few hours of AMI due to a delayed increase of circulating levels. With these tests, circulating levels become detectable in peripheral blood only after 3 to 4 hours [6–8, 20]. The diagnosis of AMI consequently requires prolonged monitoring over 6 to 12 hours and serial blood sampling (fig. 2). Delayed “rule in” may increase morbidity and potentially mortality in AMI [6–8], whereas delayed “rule out” contributes to overcrowding in the ED with the associated costs [21].
Figure 3 highlights the main difference between contemporary and high-sensitive cTns assays. High-sensitive cTn assays have two differentiating features from contemporary cTn assays: 1) detection of cTn in healthy persons and 2) a precise definition of what is “normal” (= the 99th percentile) [8, 9, 22]. This feature is of key importance as a cTn value above the 99th percentile of a normal reference population is a “conditio sine qua non” for the diagnosis of AMI.
“Sensitive” and “high-sensitive” are used by the manufacturer to describe their assays with increased sensitivity [23–25]. Although there is no consensus regarding when the term “sensitive” and when the term “high-sensitive” should be applied in the description of cTn assays, it is important to note that there are substantial analytical differences among the new assays. Some allow the detection of cTn in about 50% of a normal reference population, whereas others allow detection in up to 90% of a normal reference population (fig. 3). One reasonable option is to use “sensitive” for the former and “high-sensitive” for the later [26]. It is still unclear whether these analytical differences impact on their clinical performance. Evidence from one large multicentre study suggested that this might not be the case for the most important indication: the diagnosis of AMI [27].
Two large prospective multicentre studies showed that sensitive and high-sensitive cTn assays have a higher diagnostic accuracy compared to contemporary cTn assays at presentation, in the diagnosis of AMI (fig. 4). The benefit observed for the sensitive and high-sensitive cTn assays was most pronounced in patients presenting soon after chest pain onset [27]. These findings were confirmed in a second multicentre study [28]. Improvements in the early diagnosis of AMI in the “early presenters” is of paramount importance as it offers the opportunity to expand the concept of “time is muscle” from patients with ST-elevation AMI to all AMI patients [6–8]. More rapid “rule in” may reduce morbidity by allowing earlier revascularisation, earlier transfer to the coronary care unit, and earlier initiation of evidence-based AMI treatment [6–8]. In addition, the sensitive and high-sensitive cTn assays may allow the reliable “rule out” of AMI within a much shorter period as with standard cTn assays. Used in conjunction with clinical assessment and the ECG, sensitive and high-sensitive cTn assays may allow a significant reduction in the percentage of patients with diagnostic uncertainty who require continuous ECG monitoring and serial blood sampling for 6 to 9 hours. The cost-savings associated with this increase in early diagnostic accuracy might be substantial [21]. Ongoing studies by our group and others will define the best algorithms on how to apply sensitive and high-sensitive cTn assays to most rapidly rule out and rule in AMI.
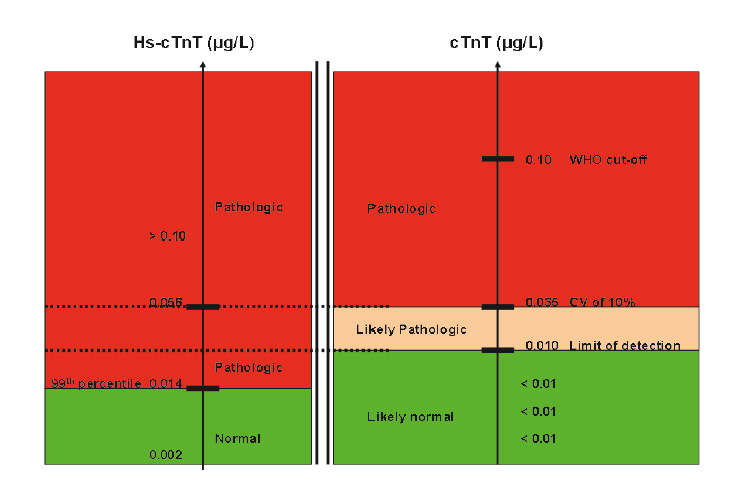
Figure 3
Interpretation of cTn concentrations in clinical practice, and changes offered by the consideration of high-sensitive cTn. The main difference between contemporary cTnT (right, 4th generation Roche cTnT) and high-sensitive cTnT (left) is that the later allows a precise definition of the normal range. For cTnT, two different cut-off levels have been used. Levels of <0.01 ug/l are undetectable and are considered “normal”. Levels between 0.01 and 0.035 ug/l are “likely pathological” but associated with high imprecision, and levels above 0.035 ug/l are pathological. The high-sensitive cTnT assay detects some patients with previously undetectable cTnT levels to have high-sensitive cTnT levels above the 99th percentile and measures with high precision in the “likely pathological” range of the conventional cTnT.
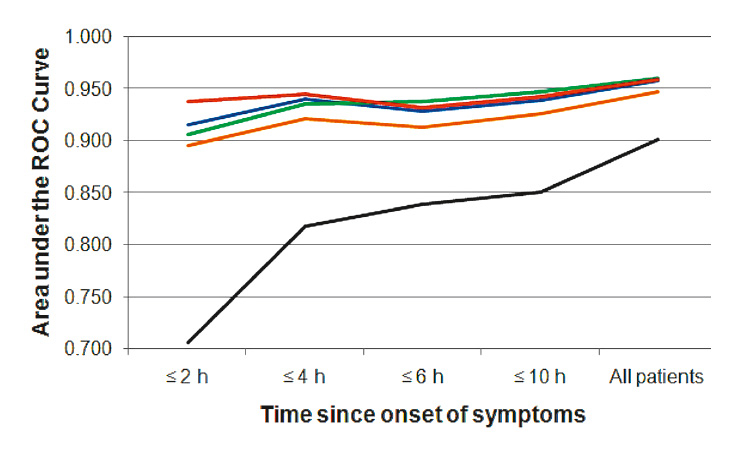
Figure 4
Diagnostic accuracy at presentation as quantified by the area under the receiver operating characteristic (ROC) curves for a contemporary cardiac troponin (cTn) assay (black, 4th generation cTnT) and four sensitive or high-sensitive cTn assays (red – Siemens cTnI Ultra; blue – Abbott cTnI Architect; green – Roche high-sensitivity cTnT; orange – Roche cTnI) in the diagnosis of acute myocardial infarction according to chest pain onset.
With the board decision of the universal definition of AMI in 2007, different subtypes of AMI have been introduced for the first time. AMI is no longer restricted to the concept of acute coronary plaque rupture resulting in decreased oxygen supply (type I) but also in conditions with elevated oxygen demand (type II, e.g. sepsis, hypertensive crisis, tachycardic atrial fibrillation) in the absence of a dominant coronary atherosclerosis. With the clinical launch of high-sensitive cTn assays, AMI will be diagnosed more often, especially Type II AMI with elevated oxygen demand. These patients do not have an acute plaque rupture and vascular thrombosis and it still has to be shown whether these patients do profit from acute implementation of aggressive dual platelet inhibition and anticoagulants.
The high-sensitive cTnT assay was the first high-sensitive cTn assay available for widespread clinical application. Following the publication of the data showing superior performance in the early diagnosis of AMI, many institutions throughout Europe have replaced the contemporary cTnT assay with the high-sensitive cTnT assay. While this transition is technically easy as both assays run on the same platform and the high-sensitive cTnT assay is not more expensive than the cTnT assay, the challenges faced on the clinical side have been substantial and largely underestimated. Many institutions have switched assays with little educational effort to prepare their clinicians on how to best apply the high-sensitive cTnT test results.
Additionally, many institutions in Europe were ill-prepared and many institutions in the United States are ill-prepared for the transition to the high-sensitive cTn assays, although some were using already a sensitive cTn assay. However, many institutions did not apply the cut-off level suggested by current guidelines (the 99th percentile) [8, 9, 22], but rather decided to use higher cut-off levels with a higher specificity for AMI. This made life easy for cardiologists, but harmed patients with early AMI or other causes of cardiomyocyte necrosis which remained undetected.
For years, the clinical application of cTn results was rather simple. An elevated cTn level was considered equivalent to the diagnosis of an acute coronary syndrome and justified the immediate initiation of the respective management: antiplatelet and anticoagulation therapy, transfer to the coronary care unit, and a cardiology consultation for possible early coronary angiography.
The clinical introduction of high-sensitive cTn assays is a trade-off. They allow the detection of AMI in the first few hours and also the detection of non-ischaemic causes of cardiomyocyte necrosis associated with multiple diseases, but challenges the clinician to differentiate them.
We are just beginning to understand the potential associated with the use of high-sensitive cTn assays in multiple other indications. This tool allows quantification of cardiomyocyte necrosis in the stable phase of established cardiac disease like coronary artery disease (CAD) or heart failure, and even in the general population to identify those patients with either silent or clinically underestimated disease and therefore with a high risk of death [1–3].
We would like to use stable CAD as an example to discuss the potential clinical use of high-sensitive cTn assays in risk stratification. In most patients with stable CAD, cTn levels in peripheral blood are below the limit of detection for conventional assays. The distribution and determinants of very low circulating cTn, as well as their association with cardiovascular events, in such patients were previously unknown. Using the new Roche high-sensitive cTnT assay, the concentration of cTnT in plasma samples from patients with stable CAD and preserved left ventricular ejection fraction function was measured [29]. High-sensitive cTnT levels were analysed in relation to the incidence of cardiovascular events during follow-up. High-sensitive cTnT levels were detectable in more than 90% of patients and above the 99th percentile in 11% of patients. After adjustment for other independent prognostic indicators, there was a strong and graded increase in the cumulative incidence of cardiovascular death and of heart failure. Increased risk associated with higher levels of cTnT was evident well below the limit of detection of conventional cTnT assays and below the 99th percentile of values in a healthy population. The next step now is to prove that treatment modifications are available to offer to patients identified to be at increased risk, which will ultimately improve patient outcome.
High-sensitive cTn assays allow the precise quantification of cardiomyocyte necrosis. As AMI is not the only cause of myocyte necrosis, it is key to consider the absolute level as well as the change in cTn within 1–3 hours as important criteria in the differential diagnosis of the cause of cardiomyocyte necrosis (fig. 5): the higher the absolute value of high-sensitive cTn at ED presentation in patients with suspected AMI, the higher the probability that it is AMI [1–3]. The differential diagnosis of a small amount of myocardial injury and therefore mild elevation of cTn is broad and includes acute and chronic disorders. CTn has to be interpreted as a quantitative variable. The term “troponin-positive” should therefore be avoided. “Detectable” levels will become the norm and have to be clearly differentiated from “elevated” levels.

Figure 5
The differential diagnosis of high-sensitive cardiac troponin T (hs-cTnT) levels is highly dependent on the absolute level.
Secondly, the larger the rise in high-sensitive cTn within the first few hours in the ED, the higher the probability that it is AMI. Thereby, serial changes documented by a second measurement help to differentiate acute cardiac disorders (showing a rise and/or fall) from chronic cardiac disease which usually exhibit constant cTn levels. It is a matter of debate whether absolute or relative changes best separate acute from chronic cTn elevations, as well as AMI from other causes of cTn elevation. Preliminary data suggest that an absolute change of 30% in the cTn level at the 99th percentile within 6 hours might be reasonable criteria for the rise and/or fall required for the diagnosis of AMI. However it is important to highlight that a detailed clinical assessment remains mandatory to differentiate AMI from the other potential causes of myocardial injury.
High-sensitive cTn assays improve the detection of cardiomyocyte necrosis in patients with acute systemic critical illness affecting the heart such as severe sepsis and septic shock [30]. The most appropriate management of critically-ill patients identified to have myocardial injury/necrosis is still unknown.
Ongoing studies will define the best algorithms on how to apply the data from high-sensitive cTn assays in clinical practice. Rule out and rule in algorithms as well as the timing of the second measurement will have to be fine-tuned for each specific cTn assay. Until data from large studies have validated more rapid rule out algorithms, it is strongly suggested to continue to stick to the current guidelines and repeat the measurements after 6 hours [7, 8, 31]. It is important to highlight that the diagnostic performance of sensitive cTn assays in the diagnosis of AMI outside the setting of chest pain patients presenting to the ED is unknown.
The more widespread application of high-sensitive cTn tests and the application of the 99th percentile as the decision limit for AMI will lead to a substantial increase in the detection of patients with slightly elevated levels of cTn. In some of them, AMI will be the diagnosis, whereas in many others the mechanisms of myocardial injury will not be AMI. Future studies need to define the most appropriate therapeutic measures in response to the detection of myocardial injury in many of these settings.
High-sensitive cTn assays provide a new noninvasive window to the heart. We should not be afraid of having a closer look. Accepting that we do not fully understand all of what we see through this novel window is a crucial step to direct future research in the right direction, in order to improve the management of patients with cardiovascular disorders.
1 Thygesen K, Mair J, Katus H, Plebani M, Venge P, Collinson P, et al. Recommendations for the use of cardiac troponin measurement in acute cardiac care. Eur Heart J. 31(18):2197–204.
2 deFilippi CR, de Lemos JA, Christenson RH, Gottdiener JS, Kop WJ, Zhan M, et al. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA. 304(22):2494–502.
3 de Lemos JA, Drazner MH, Omland T, Ayers CR, Khera A, Rohatgi A, et al. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 304(22):2503–12.
4 Mueller C, Muller B, Perruchoud AP. Biomarkers: past, present, and future. Swiss Med Wkly. 2008;138(15-16):225–9.
5 Nawar EW, Niska RW, Xu J. National Hospital Ambulatory Medical Care Survey: 2005 Emergency Department Summary: Advance Data from Vital and Health Statistics. Centers for Disease Control and Prevention 2007.
6 Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE, Jr., et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2007;116(7):e148–304.
7 Bassand JP, Hamm CW, Ardissino D, Boersma E, Budaj A, Fernandez-Aviles F, et al. Guidelines for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes. Eur Heart J. 2007;28(13):1598–660.
8 Thygesen K, Alpert JS, White HD, Jaffe AS, Apple FS, Galvani M, et al. Universal definition of myocardial infarction. Circulation. 2007;116(22):2634–53.
9 Apple FS, Wu AH, Jaffe AS. European Society of Cardiology and American College of Cardiology guidelines for redefinition of myocardial infarction: how to use existing assays clinically and for clinical trials. Am Heart J. 2002;144(6):981–6.
10 Pope JH, Aufderheide TP, Ruthazer R, Woolard RH, Feldman JA, Beshansky JR, et al. Missed diagnoses of acute cardiac ischemia in the emergency department. N Engl J Med. 2000;342(16):1163–70.
11 Wang K, Asinger RW, Marriott HJ. ST-segment elevation in conditions other than acute myocardial infarction. N Engl J Med. 2003;349(22):2128–35.
12 Morrow DA, Cannon CP, Rifai N, Frey MJ, Vicari R, Lakkis N, et al. Ability of minor elevations of troponins I and T to predict benefit from an early invasive strategy in patients with unstable angina and non-ST elevation myocardial infarction: results from a randomized trial. JAMA. 2001;286(19):2405–12.
13 Morrow DA, Antman EM, Tanasijevic M, Rifai N, de Lemos JA, McCabe CH, et al. Cardiac troponin I for stratification of early outcomes and the efficacy of enoxaparin in unstable angina: a TIMI-11B substudy. J Am Coll Cardiol. 2000;36(6):1812–7.
14 Mueller C, Neumann FJ, Perruchoud AP, Zeller T, Buettner HJ. Prognostic value of quantitative troponin T measurements in unstable angina/non-ST-segment elevation acute myocardial infarction treated early and predominantly with percutaneous coronary intervention. Am J Med. 2004;117(12):897–902.
15 Hochholzer W, Buettner HJ, Trenk D, Laule K, Christ M, Neumann FJ, et al. New definition of myocardial infarction: impact on long-term mortality. Am J Med. 2008;121(5):399–405.
16 Hamm CW, Heeschen C, Goldmann B, Vahanian A, Adgey J, Miguel CM, et al. Benefit of abciximab in patients with refractory unstable angina in relation to serum troponin T levels. c7E3 Fab Antiplatelet Therapy in Unstable Refractory Angina (CAPTURE) Study Investigators. N Engl J Med. 1999;340(21):1623–9.
17 Kastrati A, Mehilli J, Neumann FJ, Dotzer F, ten Berg J, Bollwein H, et al. Abciximab in patients with acute coronary syndromes undergoing percutaneous coronary intervention after clopidogrel pretreatment: the ISAR-REACT 2 randomized trial. JAMA. 2006;295(13):1531–8.
18 Eggers KM, Oldgren J, Nordenskjold A, Lindahl B. Diagnostic value of serial measurement of cardiac markers in patients with chest pain: limited value of adding myoglobin to troponin I for exclusion of myocardial infarction. Am Heart J. 2004;148(4):574–81.
19 McCann CJ, Glover BM, Menown IB, Moore MJ, McEneny J, Owens CG, et al. Novel biomarkers in early diagnosis of acute myocardial infarction compared with cardiac troponin T. Eur Heart J. 2008.
20 Macrae AR, Kavsak PA, Lustig V, Bhargava R, Vandersluis R, Palomaki GE, et al. Assessing the requirement for the 6-hour interval between specimens in the American Heart Association Classification of Myocardial Infarction in Epidemiology and Clinical Research Studies. Clin Chem. 2006;52(5):812–8.
21 Forberg JL, Henriksen LS, Edenbrandt L, Ekelund U. Direct hospital costs of chest pain patients attending the emergency department: a retrospective study. BMC Emerg Med. 2006;6:6.
22 Apple FS, Jesse RL, Newby LK, Wu AH, Christenson RH. National Academy of Clinical Biochemistry and IFCC Committee for Standardization of Markers of Cardiac Damage Laboratory Medicine Practice Guidelines: Analytical issues for biochemical markers of acute coronary syndromes. Circulation. 2007;115(13):e352–5.
23 Mingels A, Jacobs L, Michielsen E, Swaanenburg J, Wodzig W, van Dieijen-Visser M. Reference Population and Marathon Runner Sera Assessed by Highly Sensitive Cardiac Troponin T and Commercial Cardiac Troponin T and I Assays. Clin Chem. 2008.
24 Melanson SE, Morrow DA, Jarolim P. Earlier detection of myocardial injury in a preliminary evaluation using a new troponin I assay with improved sensitivity. Am J Clin Pathol. 2007;128(2):282–6.
25 Apple FS, Smith SW, Pearce LA, Ler R, Murakami MM. Use of the Centaur TnI-Ultra Assay for Detection of Myocardial Infarction and Adverse Events in Patients Presenting With Symptoms Suggestive of Acute Coronary Syndrome. Clin Chem. 2008;54(4):723–8.
26 Apple FS. A new season for cardiac troponin assays: it’s time to keep a scorecard. Clin Chem. 2009;55(7):1303–6.
27 Reichlin T, Hochholzer W, Bassetti S, Steuer S, Stelzig C, Hartwiger S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009;361(9):858–67.
28 Keller T, Zeller T, Peetz D, Tzikas S, Roth A, Czyz E, et al. Sensitive troponin I assay in early diagnosis of acute myocardial infarction. N Engl J Med. 2009;361(9):868–77.
29 Omland T, de Lemos JA, Sabatine MS, Christophi CA, Rice MM, Jablonski KA, et al. A sensitive cardiac troponin T assay in stable coronary artery disease. N Engl J Med. 2009;361(26):2538–47.
30 Kelley WE, Januzzi JL, Christenson RH. Increases of cardiac troponin in conditions other than acute coronary syndrome and heart failure. Clin Chem. 2009;55(12):2098–112.
31 Morrow DA, Cannon CP, Jesse RL, Newby LK, Ravkilde J, Storrow AB, et al. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: Clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Circulation. 2007;115(13):e356–75.
Funding / potential competing interests: We disclose that Dr Twerenbold has received speaker honoraria from Brahms. Dr Reichlin has received research grants from the Swiss Heart Foundation, the Professor Max Cloëtta Foundation, the University of Basel and the Department of Internal Medicine, University Hospital Basel as well as speaker honoraria from Brahms and Roche. Dr Mueller has received research support from the Swiss National Science Foundation (PP00B-102853), the Swiss Heart Foundation, Abbott, Biosite, Brahms, Nanosphere, Roche, Siemens, and the Department of Internal Medicine, University Hospital Basel, as well as speaker honoraria from Abbott, Biosite, Brahms, Roche, and Siemens.