
Figure 1
The unstable Biodyn sandal with a regular shaped shoe sole.
DOI: https://doi.org/10.4414/smw.2011.13182
A well-functioning control of posture represents an important prerequisite for the successful performance of many everyday (e.g., stair climbing) and sports-related activities (e.g., gymnastics). However, the ability to control posture is a dynamic process across the lifespan, with children and seniors showing the most pronounced deficits due to maturation and biologic aging [1]. Furthermore, age-related deteriorations in postural control start relatively early during adulthood and can already be found in middle-aged adults. Era et al. [2] assessed static postural control (i.e., sway velocity) on a force platform in a randomly selected sample of subjects aged 30 years and over. They observed that differences in balance performance were already apparent among young (30- to 39-year olds) and middle-aged adults (40- to 49-year olds) and became even more pronounced after the age of 60 years. Given that impaired postural control represents a major intrinsic sports injury and fall-risk factor [3–6], it is not surprising that injury and fall-rates are high in children, middle-aged and particularly older adults [7–9]. However, it has frequently been reported that balance training has the potential to reduce fall incidence rates. For example, Barnett et al. [10] observed that six months of balance training reduced the rate of falls measured over a 12-month follow-up period. Recently, Clemson et al. [11] showed a significant reduction in fall rate after six months of balance training embedded in daily life activities.
When conducting balance training programs, different exercises, materials, and tools are used that induce postural instability. The neuromuscular system compensates for this instability by an increased activity of lower limb muscles which results in greater joint stiffness [12]. Recently, it was shown that both the performance of balance exercises (e.g., monopedal stance on a Swiss ball), as well as wearing unstable shoe constructions with rounded soles result in increased activity of muscles encompassing the ankle joint during standing [13, 14]. One example for an unstable shoe construction is the shoe design developed by Masai Barefoot Technology (MBT). From a mechanical point of view, instability is provided by the rounded sole in the anterior-posterior direction and by a cushioned heel sensor in the medio-lateral direction. From a functional perspective, this design attempts to simulate an unstable surface, thereby requiring continual activation of muscles encompassing the ankle joint [15]. Thus, sensory input to the neuromuscular system is increased, which affects the efferent output. As a consequence, this could represent sufficient training stimuli in counteracting balance deficits, particularly in sedentary populations. Higher activity of lower extremity muscles was also observed when walking in an unstable shoe construction with rounded soles, compared to walking in a regular shoe [14, 16]. Furthermore, Korsten et al. [17] studied the effects of traditional balance training compared to the prolonged and regular use of unstable shoe constructions with rounded soles on measures of postural control during monopedal stance in healthy young adults. After four weeks of training/accommodation, significant decreases in postural sway in the anterior-posterior and medio-lateral direction were observed for both experimental groups [17]. In addition, Landry et al. [15] observed that an increased activity in selected smaller intrinsic foot muscles while standing in an unstable shoe with rounded soles compared to a stable control shoe persisted even after a six week accommodation period. Studies investigating the acute and/or long-term effects of unstable shoe constructions on the activity of lower extremity muscles during different task conditions have primarily used the MBT shoe which is characterised by its rounded sole [14, 16, 17]. In contrast to this concept, a recently developed functional shoe construction has a regularly shaped sole with the unstable element integrated into the sole of the shoe. Thus, this shoe construction appears from the outside to be a regular shoe. Yet, it is unresolved whether the integrated unstable element has an effect on muscle activity during standing and walking. This is an important prerequisite for functional footwear that makes it suitable for use in an everyday, therapeutic and/or exercise specific environment.
Therefore, the objective of this study was to compare measures of postural control and muscle activity during standing and locomotion for women using a new sandal construction with an unstable element integrated into the sole of the shoe, a conventional stable control sandal, and a barefoot condition. It was expected that both postural instability as well as higher muscle activities during standing and walking could be observed when wearing the unstable sandal.
A total of 22 women between the ages of 30 to 50 years provided written informed consent to participate in the study after the experimental procedures had been explained (table 1). The subjects represented staff members recruited by flyers from the Faculty of Medicine of the University of Basel. Initially, 30 women were recruited for the study. Three did not meet the inclusion criteria and 5 were not able to attend during the provided time table. The included participants were healthy with no previous lower extremity trauma and no history of serious muscular, neurological, cardiovascular, metabolic or inflammatory diseases. None of the subjects had an athletic background and all of them performed less than five hours of sports activities per week. The study was approved by the ethics committee of the University of Basel and all experiments were conducted according to the latest revision of the Declaration of Helsinki.

Figure 1
The unstable Biodyn sandal with a regular shaped shoe sole.
The unstable shoe condition tested in this study was the Biodyn sandal (model no. 4500) from Biodyn Human Footwear Technology, Germany (mass: 278 g). The tested sandal was characterised by a two-part-function sole, which was regularly shaped and not rounded. The first part consists of a soft element (polyurethane foam) that is integrated into the rear part (heel) of the shoe sole. The second part represents a freely moving pivot point (thermoplastic polyurethane; diameter 122 mm) underneath the arch of the foot, enabling three-dimensional movement of the foot while walking (fig. 1). Due to the regularly shaped sole, prior instruction sessions are not necessary before wearing these sandals. The tested control sandal (mass: 245 g) was a standard commercially available sandal that came with a similar hook-and-pile fastener technique as the Biodyn sandal.
All measurements were conducted in our human biomechanics laboratory. Test circumstances (e.g., room illumination, temperature, noise) were in accordance with recommendations for posturographic testing [18]. Prior to testing, all subjects underwent a 12 minute warm up consisting of six minutes of bipedal and monopedal balance exercises as well as six minutes of walking on a treadmill at their preferred gait speed. Notably, two minute walks were conducted for each test condition (i.e., barefoot, control and Biodyn sandal). Biomechanic tests included measurements of static and dynamic postural control. Static postural control (centre of pressure (COP) displacements) was tested during bipedal and monopedal stance on a balance platform (eyes open, firm ground). Dynamic postural control was assessed while walking on a treadmill at the subjects’ preferred gait speed. During all tests, muscle activity was analysed by means of electromyography. Static postural control was always tested before dynamic postural control to keep the effects of neuromuscular fatigue minimal. During all tests, three test conditions were applied: barefoot, regular/control sandal, Biodyn sandal. The sequence of test conditions was randomised for each test (i.e., static/dynamic balance) and subject.
Static postural control was assessed by means of a balance platform (GKS 1000®, Mittweida, Germany). The balance platform consists of four uni-axial sensors measuring displacements of the COP in the medio-lateral and anterior-posterior directions. Under static conditions, the balance platform was firmly fixed on the floor. Subjects performed the tests during bipedal and monopedal stance (i.e., dominant leg). The dominant leg was determined according to the lateral preference inventory [19]. During testing, knees were bent at 30°, hands rested on hips and the gaze was fixated on a cross on the nearby wall. Subjects were instructed to remain as stable as possible and to refrain from any voluntary movements during the trials. Prior to testing, subjects performed two practice trials under bipedal and monopedal conditions. Thereafter, three test trials were conducted. Data was acquired for 30 s at a sampling rate of 40 Hz [18]. The COP C90 area, which represents the surface area covered by the trajectory of the COP with a 90% confidence interval (COP C90 area in cm2), was computed. The best trial (least surface area covered) was used for further analysis. The intraclass correlation coefficient (ICC) was calculated for COP C90 area with an ICC of 0.87.
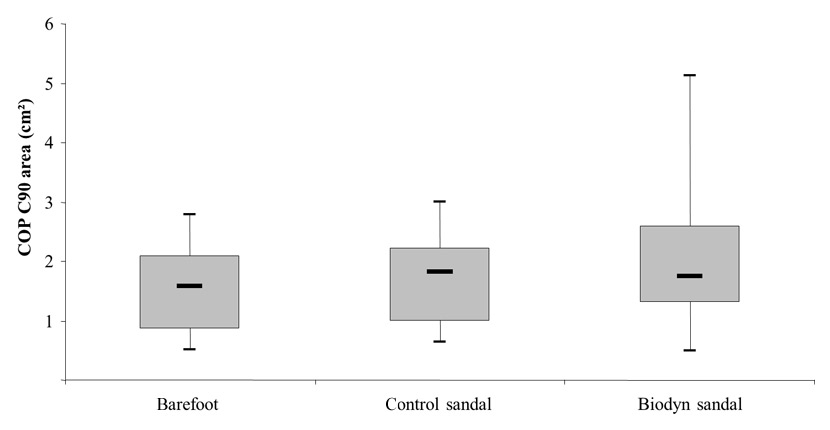
Figure 2
Box plots comparing centre of pressure (COP) C90 area (i.e., surface area covered by the trajectory of the COP with a 90% confidence interval) between testing conditions obtained during bipedal stance.
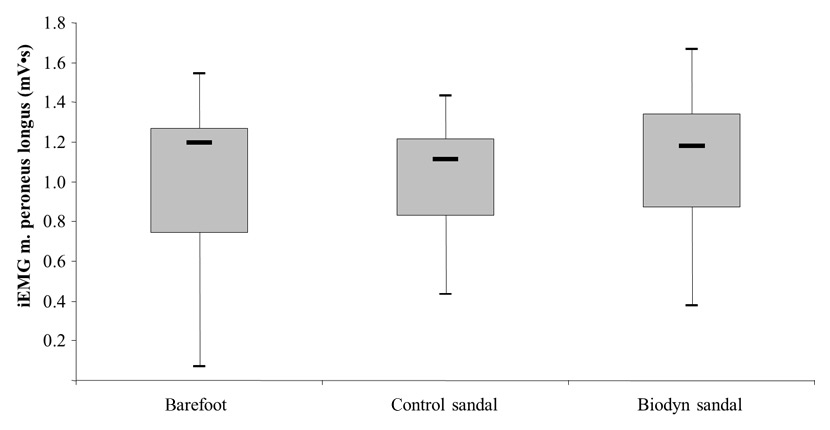
Figure 3
Box plots comparing muscle activity (iEMG) between testing conditions, of the m. peroneus longus obtained during bipedal stance.
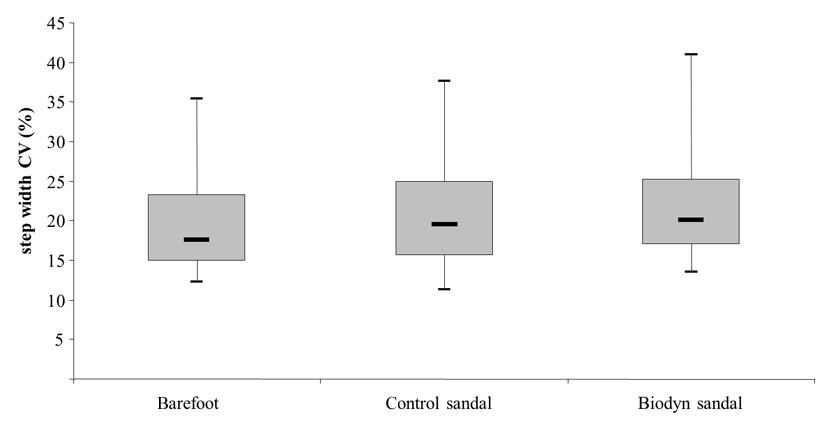
Figure 4
Box plots comparing the coefficient of variation (CV) in step width between testing conditions obtained during walking at a preferred gait speed.
Prior to the gait analysis on the treadmill, habitual gait speed was assessed from the interval between passing two photoelectric barriers (TAG Heuer HL 2-31®, La Chaux-de-Fonds, Switzerland) which were located 12 m apart on an even walkway. Participants walked at self-selected speeds, initiating and terminating each walk a minimum of 1 m before and after the 12-m walkway to allow sufficient distance to accelerate to and decelerate from a steady state of ambulation across the walkway. Three trials were performed and the mean speed of the three trials was used for the gait analysis on a pressure sensitive treadmill (Zebris FDM-T Treadmill System®, Isny, Germany). The basic FDM-T system consists of a treadmill ergometer with an integrated, calibrated measuring sensor. The movement of the treadmill is compensated so that completely stable gait and roll-off patterns can be analysed. The running/walking area of the treadmill has a size of 150 x 50 cm and comprises of 5378 pressure/force sensors. Data was acquired for 400 steps at a sampling rate of 120 Hz. According to a study conducted by Owings and Grabiner [20], 400 steps are necessary to detect valid data of step width, length, and time and the variability of the respective parameters. A step is defined as the sequence of events between contact of one foot and the next contact of the opposite foot. Consequently, a stride is the sequence of events between contact of one foot and the next contact of the same foot [21]. Data analysis was started by calculating means and standard deviations (SD) of step width, stride length and stride time. Step width was defined as the perpendicular distance (cm) from the centre of one foot to the line of progression of the centre of the other foot. Stride length was defined as the linear distance (cm) between successive heel contacts of the same foot. Additionally, stride time was defined as the time (s) between the first contacts of two consecutive footfalls of the same foot. Stride-to-stride variability were calculated for the above mentioned parameters according to the following formula [(SD/Mean)*100] and used as outcome measures [22]. ICC-values between trials were calculated and ranged from 0.81 to 0.98 for the different gait parameters.
Bipolar surface electrodes (Blue Sensor, Ambu, Balerup, Denmark; diameter 10 mm, centre to centre distance 25 mm) were placed over m. tibialis anterior (TA), m. soleus (SO), m. gastrocnemius medialis (GM), and m. peroneus longus (PL) of the dominant leg. Electrodes were positioned on the muscle according to the European recommendations for surface electromyography [23]. The longitudinal axes of the electrodes were in line with the direction of the underlying muscle fibres. The reference electrode was attached to the shin bone. Inter-electrode resistance was kept below 5 kΩ by shaving, slightly roughening, degreasing and disinfecting the skin. Pulling artefacts were avoided by properly fixing the electrode cables with tape to the skin. EMG signals were sampled at 1000 Hz, amplified and high-pass filtered at 10 Hz, and were carefully monitored for artefacts and noise (e.g., visual inspection of the zero line). EMG data were quantified by integrating the full-wave rectified EMG-signals (iEMG). For static postural control, the best trial (least surface area covered) was selected for the bipedal and monopedal condition and muscle activity was analysed over the time interval of 30 s. In terms of dynamic postural control, electrical switches implemented into inlays of the shoe (underneath the heel and the ball of the dominant foot) were used as a trigger for determining the stance phase (corresponds to load acceptance phase or heel down) and the swing phase (corresponds to push-off phase or toe-off) of a regular gait cycle. Thus, we were able to quantify mean muscle activity during the swing and stance phase of the dominant leg over 400 steps.
An a priori power analysis [24] with an assumed Type I error of 0.05 and a Type II error rate of 0.05 (95% statistical power) was calculated for activity of lower extremity muscles (m. peroneus longus) when standing with the MBT shoe [17]. The analysis revealed that 20 participants would be sufficient for finding statistically significant differences between test conditions. The model was fed with the following values: mean ± SD of difference 1.2 ± 1.6; resulting effect size .75. Data assessment for normal distribution (using Kolmogorov-Smirnov test) and variance homogeneity (using Levene’s test) revealed that not all parameters were normally distributed or variances were inhomogeneous. As a consequence, we decided to use the non-parametric Wilcoxon signed-rank test (Z-value) for related measurements on a single sample for detecting differences between test conditions (Biodyn sandal vs. control sandal and barefoot condition). P-values were Bonferroni adjusted. The significance level was set at p< .05. If not reported otherwise, data are presented using box-and-whisker plots showing the smallest observation (sample minimum), the lower quartile (25th percentile), the median (50th percentile), the upper quartile (75th percentile), and the largest observation (sample maximum). Central tendency is reported as the median and degree of dispersion as lower to upper 95% confidence interval (95% CI). All analyses were performed using the Statistical Package for Social Sciences (SPSS) version 18.0.
| Table 1: Characteristics of the Study Cohort. | |
| Characteristic | Experimental group (N= 22) |
| Age (years) | 37.8 ± 7.9 |
| Body height (cm) | 168.2 ± 7.9 |
| Body mass (kg) | 62.3 ± 10.0 |
| Body mass index (kg/m2) | 22.0 ± 2.8 |
| Notes: Values are mean ± SD. | |
Figure 2 illustrates a significantly larger sway area during bipedal stance when wearing the Biodyn sandals compared to the barefoot condition (Z = –1.95; p = .026). The median (95% CI) for sway area was 1.74 (1.63–2.85) cm² for the Biodyn sandal and 1.57 (1.20–1.85) cm² for the barefoot condition. Comparison of sway area between the Biodyn sandal and the control sandal, 1.81 (1.32–1.93) cm², revealed no statistically significant difference (Z = –1.58; p = .114) (table 2). In terms of monopedal stance, no significant differences in sway area were found between the test conditions.
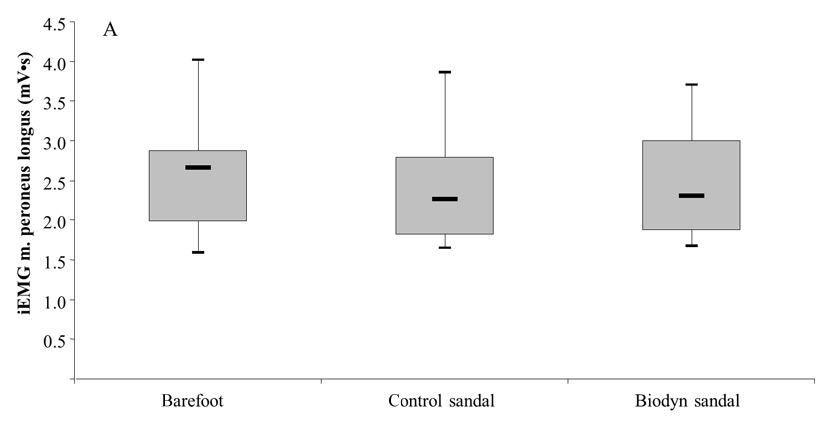
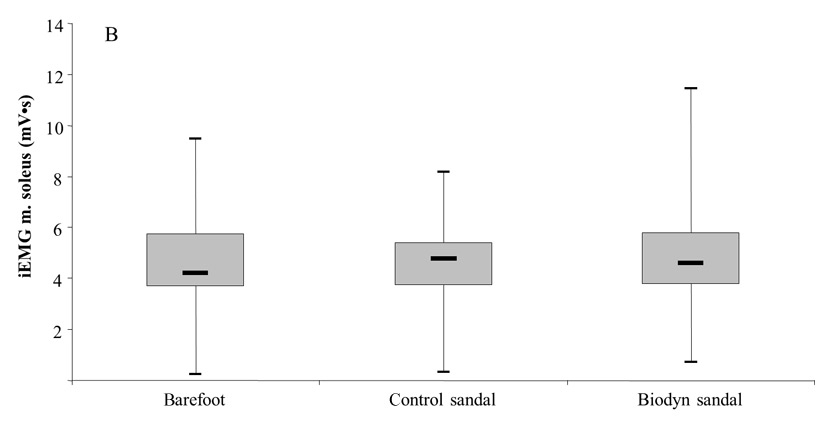
Figures 5A and 5B
Box plots comparing muscle activity (iEMG) between testing conditions, of the m. peroneus longus during the swing phase (5A) and the m. soleus during the stance phase (5B) of a regular walk at preferred gait speed.
Figure 3 demonstrates a significantly higher muscle activity during bipedal stance in the PL (Z = –1.93; p = .027) when wearing the Biodyn sandal, 1.18 (0.96–1.25) mV•s, compared to the control sandal, 1.11 (0.88–1.15) mV•s. Comparison of PL activity between the Biodyn sandal and the barefoot condition, 1.19 (0.83–1.19) cm², revealed no statistically significant difference (Z = –1.16; p = .244). Furthermore, a tendency towards higher muscle activity in the GM was observed for the Biodyn sandal compared to the control sandal (Z = –1.51; p = .065). The respective median values (95% CI) amounted to 1.11 (1.01–1.21) mV•s for the Biodyn sandal and 1.01 (0.93–1.16) mV•s for the control sandal. Comparison of GM activity between the Biodyn sandal and the barefoot condition, 1.06 (0.87–1.18) cm², revealed no statistically significant difference (Z = -0.86; p = .390) (table 2).
Figure 4 indicates that step width coefficient of variation (CV) was significantly greater for the Biodyn sandal compared to the barefoot condition (Z = –2.58; p = .005). The respective median values (95% CI) amounted to 20.00 (18.55–24.10) % for the Biodyn sandal and 17.52 (16.99–22.91) % for the barefoot condition. Comparison of step width CV between the Biodyn sandal and the control sandal, 19.46 (17.63–23.70) %, revealed no statistically significant difference (Z = –1.55; p = .121) (table 2). No significant differences between test conditions were observed for the parameters of stride time/length CV.
Figures 5A and 5B show that muscle activities in PL and SO were significantly higher during the swing phase (PL: Z = –1.64; p = .050) and the stance phase (SO: Z = -1.70; p = .044) when walking with the Biodyn sandal compared to the control sandal. The respective median value for the PL amounted to 2.30 (2.13–2.78) mV•s for the Biodyn sandal and 2.26 (2.06–2.70) mV•s for the control sandal. For the SO, median values of 4.56 (4.03–6.08) mV•s (Biodyn sandal) and 4.73 (3.91–5.40) mV•s (control sandal) were detected. Comparison of the Biodyn sandal with the barefoot condition revealed no statistically significant difference for PL activity during the swing phase (Z = –1.81; p = .070) and for SO activity during the stance phase (Z = -1.19; p = .236) (table 2).
| Table 2: Median (lower to upper 95% CI), Wilcoxon statistics (Z-value), and Bonferroni corrected p-values for the three testing conditions (Biodyn sandal, control sandal and barefoot) separated into standing and walking performance. | |||||
| Median (lower to upper 95% CI) | Comparisons (Z-value, p-value) | ||||
| Biodyn sandal | Control sandal | Barefoot condition | Biodyn vs. control sandal | Biodyn vs. barefoot | |
| Standing | |||||
| CoP_C90_area | 1.74 (1.63–2.85) | 1.81 (1.32–1.93) | 1.57 (1.20–1.85) | Z = –1.58, p = .114 | Z = –1.95, p = .026 |
| PL | 1.18 (0.96–1.25) | 1.11 (0.88–1.15) | 1.19 (0.83–1.19) | Z = –1.93, p = .027 | Z = –1.16, p = .244 |
| GM | 1.11 (1.01–1.21) | 1.01 (0.93–1.16) | 1.06 (0.87–1.18) | Z = –1.51, p = .065 | Z = –0.86, p =.390 |
| Walking | |||||
| CV_step width | 20.00 (18.55–24.10) | 19.46 (17.63–23.70) | 17.52 (16.99–22.91) | Z = –1.55, p = .121 | Z = –2.58, p = .005 |
| PL | 2.30 (2.13–2.78) | 2.26 (2.06–2.70) | 2.65 (2.24–2.98) | Z = –1.64, p = .050 | Z = –1.81 p = .070 |
| SO | 4.56 (4.03–6.08) | 4.73 (3.91–5.40) | 4.20 (3.94–5.59) | Z = –1.70, p = .044 | Z = –1.19 p = .236 |
| Notes: 95% CI: confidence interval; CoP_C90_area: surface area covered by the trajectory of the centre of pressure with a 90% confidence interval in cm2; CV: coefficient of variation in %; GM: m. gastrocnemius medialis in mV•s; PL: m. peroneus longus in mV•s; SO: m. soleus in mV•s. | |||||
To the authors’ knowledge, this is the first study that has investigated the effects of a functional sandal, with a regularly shaped sole and an unstable element integrated into the sole of the sandal, on measures of postural control and muscle activity in healthy women. From a functional point of view, the most interesting finding of the present study was that the unstable sandal construction produced higher activities of muscles encompassing the ankle joint during standing and walking compared to regular sandals. In addition, greater postural sway during bipedal standing and greater step width variability during walking were found compared to the barefoot condition.
These findings are in accordance with the literature regarding the acute effects of unstable shoe constructions on variables of static and dynamic postural control and lower extremity muscle activity. In fact, Romkes et al. [25] found significantly larger COP excursions during bipedal stance with the MBT shoe compared to standing barefoot, for both the anterior-posterior and the medio-lateral direction. Notably, no differences were observed between the unstable and stable condition during monopedal stance. This was also found in the present study and may indicate that the restricted functionality of the respective unstable shoe constructions (i.e., MBT, Biodyn) during monopedal stance could be caused by the increased impact of body mass during one-legged compared to two-legged stance.
Our finding of a larger sway area during unstable bipedal stance was accompanied by increased activity of the m. peroneus longus compared to the stable condition. This provides support that the Biodyn sandal was effective in inducing unstable conditions that helped to increase activity of muscles encompassing the ankle joint, which could represent a sufficient training stimuli. Landry et al. [15] reported similar results for an unstable shoe when using an EMG circumferential linear array. These authors were able to show that standing in the unstable shoe increased activity of the flexor digitorum longus, peroneal and anterior compartment muscles of the lower leg.
In the present study, the Biodyn sandal did not only produce postural instability during standing but also during walking. In fact, we were able to detect larger step width CV while walking in the Biodyn sandal compared to the control sandal. Of note, step width variability in particular has been identified as a marker of gait stability/instability [26, 27]. The greater instability during walking in the unstable sandal was associated with increased activity of the m. soleus and m. peroneus longus during the stance and swing phase of the gait cycle. This is in accordance with a recent study conducted by Stoggl et al. [28] who observed a 35% higher variability in kinetic (i.e., peak foot force) and kinematic (i.e., joint angles) measures when walking with an unstable compared to a stable shoe condition. Furthermore, Romkes et al. [16] and Nigg et al. [14] found higher activities in muscles encompassing the ankle joint when walking in unstable compared to conventional shoe constructions. In the present study, standing/walking in the Biodyn sandal compared to standing barefoot did not result in different levels of muscle activity. This finding is in line with the study of Landry et al. [15] and could be explained by the concept of unstable shoe constructions where the biomechanics of barefoot movement are mimicked. In fact, Nigg [29] stated in his review that depending on the shoe concept and the shape of the foot, the kinematics and/or the feeling of barefoot walking/running is copied.
Given the similarity in findings regarding the acute effects of the Biodyn sandal and other unstable shoe constructions on measures of postural control and muscle activity, it appears plausible to argue that both shoe types can be used for strengthening and conditioning lower extremity muscles. In fact, various longitudinal studies were able to demonstrate both improved balance performance and reduced extremity muscle activity after a prolonged use of unstable shoe constructions in different age and patient groups [15, 17, 30, 31]. Recently, Ramstrand et al. [30] investigated the effects of wearing the MBT shoe as often as possible over a period of eight weeks on measures of postural control in women aged over 50 years. The authors found significant improvements in the training group but not in the control group when balancing on an unstable surface with eyes closed, in stabilising their COP following a rapid, downward rotating perturbation, and in directional control when performing a limits of stability test. Our preliminary results are promising and may indicate that the prolonged use of the Biodyn sandal could produce an activating and strengthening effect on lower extremity muscles that might be similar to that seen after wearing other unstable shoe constructions or even in balance training. However, caution is needed when transferring the results from other unstable shoe constructions to the Biodyn sandal, which is why future studies have to be conducted to prove the long-term effects of the Biodyn sandal.
In summary, this study was able to show that a newly developed Biodyn sandal with a regularly shaped sole and an unstable element integrated into the sole of the sandal induced postural instability during standing and walking which was associated with higher lower extremity muscle activity. This finding may have implications for challenging the postural control system during activities of daily living as well as strengthening and conditioning lower extremity muscles.
While Biodyn Human Footwear Technology, Germany provided the unstable sandals and financial support, they had no role in (a) the study design, (b) the collection, analysis, and interpretation of the data, and (c) the writing of the manuscript, or (d) the decision to submit the manuscript for publication.
1 Granacher U, Muehlbauer T, Gollhofer A, Kressig RW, Zahner L. An Intergenerational Approach in the Promotion of Balance and Strength for Fall Prevention - A Mini-Review. Gerontology. 2010; Epub ahead of print.
2 Era P, Sainio P, Koskinen S, Haavisto P, Vaara M, Aromaa A. Postural balance in a random sample of 7,979 subjects aged 30 years and over. Gerontology. 2006;52(4):204–13.
3 Wang HK, Chen CH, Shiang TY, Jan MH, Lin KH. Risk-factor analysis of high school basketball-player ankle injuries: a prospective controlled cohort study evaluating postural sway, ankle strength, and flexibility. Arch Phys Med Rehabil. 2006;87(6):821–5.
4 Tropp H, Ekstrand J, Gillquist J. Stabilometry in functional instability of the ankle and its value in predicting injury. Med Sci Sports Exerc. 1984;16(1):64–6.
5 Verghese J, Buschke H, Viola L, Katz M, Hall C, Kuslansky G, Lipton R. Validity of divided attention tasks in predicting falls in older individuals: a preliminary study. J Am Geriatr Soc. 2002;50(9):1572–6.
6 Maki BE, Holliday PJ, Topper AK. A prospective study of postural balance and risk of falling in an ambulatory and independent elderly population. J Gerontol A Biol Sci Med Sci. 1994;49(2):72–84.
7 Talbot LA, Musiol RJ, Witham EK, Metter EJ. Falls in young, middle-aged and older community dwelling adults: perceived cause, environmental factors and injury. BMC Public Health. 2005;5:86.
8 Kahl H, Dortschy R, Ellsasser G. Injuries among children and adolescents (1–17 years) and implementation of safety measures. Results of the nationwide German Health Interview and Examination Survey for Children and Adolescents (KiGGS). Bundesgesundheitsblatt-Gesundheitsforschung-Gesundheitsschutz. 2007;50(5-6):718–27.
9 Schneider S, Weidmann C, Seither B. Epidemiology and risk factors of sports injuries--multivariate analyses using German national data. Int J Sports Med. 2007;28(3):247–52.
10 Barnett A, Smith B, Lord SR, Williams M, Baumand A. Community-based group exercise improves balance and reduces falls in at-risk older people: a randomised controlled trial. Age Ageing. 2003;32(4):407–14.
11 Clemson L, Singh MF, Bundy A, Cumming RG, Weissel E, Munro J, Manollaras K, Black D. LiFE Pilot Study: A randomised trial of balance and strength training embedded in daily life activity to reduce falls in older adults. Aust Occup Ther J. 2010;57(1):42–50.
12 Granacher U, Muehlbauer T, Taube W, Gollhofer A, Gruber M. Sensorimotor training. In Cardinale M, ed. Strength and conditioning: Biological principles and practical applications. San Francisco: Wiley; 2011, pp 399–409.
13 Wahl MJ, Behm DG. Not all instability training devices enhance muscle activation in highly resistance-trained individuals. J Strength Cond Res. 2008;22(4):1360–70.
14 Nigg B, Hintzen S, Ferber R. Effect of an unstable shoe construction on lower extremity gait characteristics. Clin Biomech. 2006;21(1):82–8.
15 Landry SC, Nigg BM, Tecante KE. Standing in an unstable shoe increases postural sway and muscle activity of selected smaller extrinsic foot muscles. Gait Posture. 2010;32(2):215–9.
16 Romkes J, Rudmann C, Brunner R. Changes in gait and EMG when walking with the Masai Barefoot Technique. Clin Biomech. 2006;21(1):75–81.
17 Korsten K, Mornieux G, Walter N, Gollhofer A. Gibt es Alternativen zum sensomotorischen Training? Schweiz Z Sportmed. 2008;56(4):150–5.
18 Kapteyn TS, Bles W, Njiokiktjien CJ, Kodde L, Massen CH, Mol JM. Standardization in platform stabilometry being a part of posturography. Agressologie 1983;24(7):321–6.
19 Coren S. The lateral preference inventory for measurement of handedness, footedness, eyedness, and earedness: Norms for young adults. Bull Psych Soc. 1993;31(1):1–3.
20 Owings TM, Grabiner MD. Measuring step kinematic variability on an instrumented treadmill: how many steps are enough? J Biomech. 2003;36(8):1215–8.
21 Huxham F, Gong J, Baker R, Morris M, Iansek R. Defining spatial parameters for non-linear walking. Gait Posture. 2006;23(2):159–63.
22 Kressig RW, Beauchet O. Guidelines for clinical applications of spatio-temporal gait analysis in older adults. Aging Clin Exp Res. 2006;18(2):174–6.
23 Hermens HJ, Freriks B, Merletti R, Stegeman DF, Blok J, Rau G, et al. European recommendations for surface electromyography: results of the SENIAM project. Roessingh Research and Development bv; 1999.
24 Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–91.
25 Romkes J. Statische Gleichgewichtskontrolle mit dem MBT-Schuh. Schweiz Z Sportmed. 2008;56(2):61–5.
26 Owings TM, Grabiner MD. Variability of step kinematics in young and older adults. Gait Posture. 2004;20(1):26–9.
27 Owings TM, Grabiner MD. Step width variability, but not step length variability or step time variability, discriminates gait of healthy young and older adults during treadmill locomotion. J Biomech. 2004;37(6):935–8.
28 Stoggl T, Haudum A, Birklbauer J, Murrer M, Muller E. Short and long term adaptation of variability during walking using unstable (Mbt) shoes. Clin Biomech. 2010;25(8):816–22.
29 Nigg B. Biomechanical considerations on barefoot movements and barefoot shoe concepts. Footwear Sci. 2009;1(2):73–9.
30 Ramstrand N, Thuesen AH, Nielsen DB, Rusaw D. Effects of an unstable shoe construction on balance in women aged over 50 years. Clin Biomech. 2010;25(5):455–60.
31 Ramstrand N, Andersson CB, Rusaw D. Effects of an unstable shoe construction on standing balance in children with developmental disabilities: a pilot study. Prosthet Orthot Int. 2008;32(4):422–33.