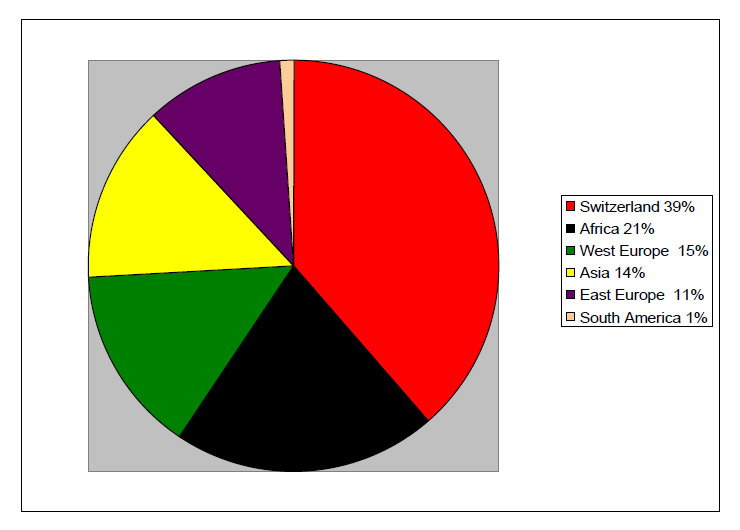
Figure 1
Origin of the patients.
DOI: https://doi.org/10.4414/smw.2011.13176
Hepatitis D virus (HDV) is a defective RNA virus that requires the helper function of hepatitis B virus (HBV) for its assembly and transmission [1]. HDV is the smallest animal virus and the only one to possess a circular RNA genome and a single structural protein, the hepatitis delta antigen (HDAg), surrounded by an envelope containing the hepatitis B surface antigen (HBsAg) [2]. Since it uses the surface antigen of the HBV to enter hepatocytes as well as for cell-to-cell spread, HDV can only infect those who are already chronic HBV carriers (super-infection) or co-infect people simultaneously with HBV (co-infection) [3].
From an epidemiological point of view, this means that HDV should be searched for in HBsAg- positive patients, especially those with abnormal transaminases levels despite low viral loads since HDV exerts a suppressive effect on HBV replication [4]. Worldwide, more than 350 million people are considered to have chronic HBV infection, and 15–20 million of them are thought to be infected with HDV [5]. Few countries know their HDV prevalence, since studies concerning this disease are scarce. Additionally, those prevalences are changing, according to intravenous drug-user policies (needle exchange for example) and immigration from highly prevalent areas like central Africa, Turkey or Brazil to Europe [6].
Since epidemiological data for Switzerland is missing, the aim of the current study was to evaluate the prevalence of HDV infection and describe the profile of patients infected by HDV in this country.
In Switzerland, most hepatitis-infected patients are followed by gastroenterologists, hepatologists and infectious diseases specialists. In 2008, a questionnaire regarding HDV and HBV infections was sent to all those physicians in Switzerland (total 349: 219 in the German speaking part, 111 in the French part and 19 in the Italian part). Physicians were asked to declare the number of HBV and HDV patients they followed and to complete an anonymous questionnaire for every HDV positive patient. All responses were recorded in a data base. The results were completely anonymous.
A patient was considered HDV positive if one of the following tests was positive: IgG and/or IgM anti-HDV, serum HDAg, serum HDV RNA, or immunohistochemistry for HDAg done on a liver biopsy. Only IgG anti-HDV (Anti Delta Murex, Abbott) and immunohistochemistry assays are currently available in Switzerland but some tests, like the viral load, could be done elsewhere in Europe. Most centres sent patients' samples either to MVZ Labor Prof Seeling, Karlsruhe, Germany (HDV assay used: RT-NAA), or to Laboratoire de Virologie- Hôpital Avicenne, Bobigny, France (details of HDV assay used are in reference [7]) in order to measure HDV viral load.
Physicians were asked to answer demographic questions (age, sex, origin of the patients), and to give epidemiological, clinical and laboratory information. Questions about hepatitis B included: estimated year of infection, mode of transmission, viral load, detailed serology and questions about hepatitis D: estimated year of infection, mode of transmission, vertical, co- or super-infection, viral load, how the diagnosis was obtained, treatment and evolution. Results of the liver tests and liver biopsy were requested. Physicians were also asked about their patients' co-infections of HIV and HCV (defined by an anti-HCV positive), but were permitted to conduct their necessary evaluations at their discretion.
All HDV positive patients could be included, whether they were cured, alive or dead. The questionnaire did not evaluate the rate of acute HDV infection.
A total of 78 physicians answered the questionnaire (22%). Most of the others did not usually follow patients suffering from infectious hepatitis. We were able to collect data from 101 patients, 70 of whom were followed at a university hospital. Mean follow-up was 6 years (3 months – 22 years). Median age was 44 years (range 20–79), and there were 76 males (75%) and 25 females (25%). Patients came from all over Switzerland: 24 Bern, 12 Basel, 12 Neuchâtel, 11 Geneva, 9 Ticino, 9 Vaud, 6 Argau, 6 Fribourg, 6 Solothurn, 6 St. Gallen, 4 Zürich, 3 Lucerne, 3 Valais, 2 Jura, 2 Thurgovia. Figure 1 shows the origin of the patients. While Swiss citizens were the majority, foreigners were over-represented, coming mostly from African or European countries (particularly from Turkey and Italy). At diagnosis, the median age of the patients was 36 years (range 19–79). The method of transmission was only known for 52 (52%) of them (fig. 2). The majority had been infected by intravenous drug use (62%). Out of 19 patients for whom this information could be obtained, 16 were likely to be co-infected and 3 super-infected, although these results have to be analysed with caution as it supposes that the physicians of these patients regularly followed HDV serologies in their HBV positive patients. Eight (8%) were probably infected by vertical transmission. For all others (73%), the time and modality of the infection (i.e. co-infection vs. super-infection) remained unknown.

Figure 1
Origin of the patients.
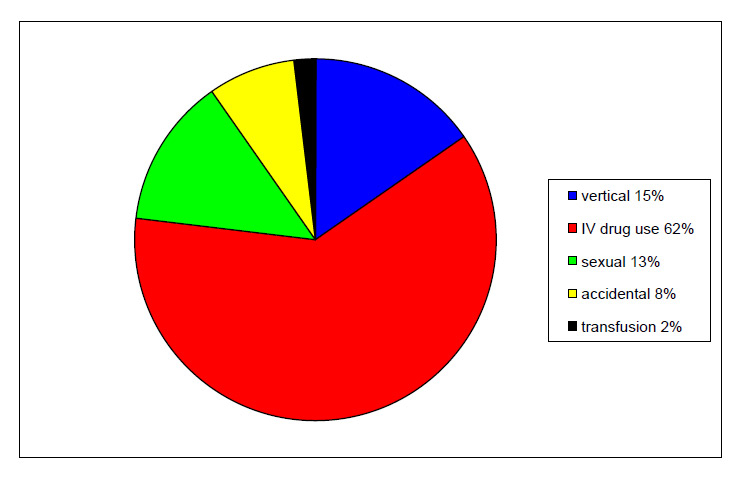
Figure 2
Method of transmission.
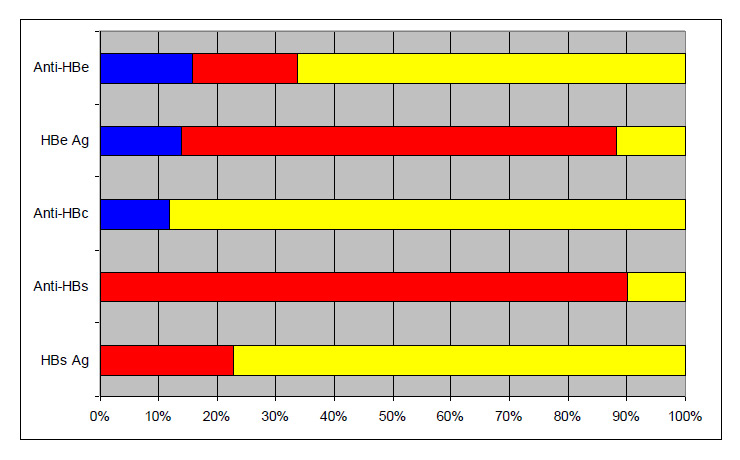
Figure 3
Detailed HBV co-infection serologies (n = 101).
All physicians also had to indicate the total number of chronic hepatitis B patients they were following. A total of 1699 hepatitis B patients could therefore be collected, of whom 101 were co-infected by HDV resulting in a prevalence of 5.9%.
Table 1 shows the co-morbidities, the viral load of both HBV and HDV, transaminases level and liver biopsy results. Most patients were co-infected with another virus (other than HBV), mostly HCV (45 anti-HCV positive from whom 20 had a detectable viral load, 22 patients not tested) and HIV (18 positive, 2 patients not tested). About a third were suffering from alcohol abuse or from a psychiatric disease (present before INF-treatment). Information on HBV viral load was available for 58 patients. A total of 43 (74%) had an undetectable or very low (<103 UI/ml) HBV viral load. Seven (12%) had a viral load between 103–105 UI/ml and 8 (14%) had a viral load greater than 105 UI/ml. HDV viral load could only be measured for 37 patients because it was not available in Switzerland, that is, until recently. Among those, none had a HBV viral load greater than 103. From those patients who had a liver biopsy (n = 68), a majority had advanced liver disease (33 [48.5%] Metavir score ≥F3), and 19 (28%) already had significant fibrosis (Metavir F2).
Figure 3 shows the detail of HBV serologies. Anti-HBs was reported in ten patients (10%) considered as cured from HDV-HBV. The majority were anti-HBe positive (65% positive, 18% negative and 17% unknown) and 4% had positive anti-HBc as a unique serological marker of HBV infection (anti-HBc alone). Interestingly, 13 patients (13%) were HBsAg-negative (and anti-HBs negative) which is usually the gold-standard marker of HBV infection (two of them were co-infected with HIV).
A total of 50 patients (50%) were treated against HDV, HBV or both, but only 21 (21%) by a validated drug, namely 15 peginterferon α-2a and 6 interferon-α. Of those, only half could be treated for at least one year. Seven patients had an undetectable viral load during treatment, but only 2 developed Anti-HBs and were considered cured. Treatment interruption was mainly due to significant side effects. The others were treated by a nucleoside analogue, mostly lamivudine or tenofovir + emtricitabine. Although follow-up was scantily reported, 8 patients had already undergone a liver transplant, one was lost for follow-up and one patient ultimately died from a disseminated hepatocellular carcinoma.
| Table 1: Clinical and laboratory characteristics of patients with HDV (n = 101). | |
| Co-morbidities | |
| HCV co-infection | 45 (45%) |
| HIV co-infection | 18 (18%) |
| Alcohol abuse | 19 (19%) |
| Psychiatric | 13 (13%) |
| Hepatocellular carcinoma | 5 (5%) |
| Liver transplantation | 8 (8%) |
| Biological characteristics | |
| Transaminases (ALAT) >2.5 x N | 44 (44%) |
| HDV viral load | |
| Qualitative | 33 (33%) |
| Quantitative: 4 x 103–4 x 107 | 7 (7%) |
| HBV viral load (n = 58) | |
| Undetectable | 23 (40%) |
| <103 UI/ml | 20 (34%) |
| 103–104 UI/ml | 4 (7%) |
| 104–105 UI/ml | 3 (5%) |
| 105–106 UI/ml | 4 (7%) |
| >106 UI/ml | 4 (7%) |
| Liver biopsy (n = 68) | |
| F0 | 5 (8%) |
| F1 | 11 (16%) |
| F2 | 19 (28%) |
| F3 | 9 (13%) |
| F4 | 24 (35%) |
HDV is an infection with a significant impact on global health and yet it is vastly underestimated. Its prevalence varies between different parts of the world going from very low (China) to prevalence rates of up to 30% in other regions like Moldavia, Romania, southern Italy and other Mediterranean countries [8]. According to the results of the current study, the prevalence of HDV in chronic hepatitis B patients in Switzerland is about 6%. This low prevalence compared to other European countries might be due to under-diagnosed infections, but is also secondary to diagnostic problems. The commercial assays available for the diagnosis of HDV infection detects total anti-HDV. In acute hepatitis D, anti-HDV appears very late and may be missed if repeated testing is not performed [5]. Thus, the true occurrence of acute hepatitis D may be underestimated.
There is a decreasing prevalence of both acute and chronic HDV infections in the Mediterranean area and in many other parts of the world, which has been attributed to a decline in the prevalence of chronic HBsAg carriers in the general population [6]. This prevalence has decreased in Italy to 8% (from about 20% in 1987) for HBsAg carriers [9], in Turkey from 38% before 1995 to 25% after 1995 [10], and in Germany from 12% to 8% between 1992 to 2006 [11]. Unfortunately, there is little data for Switzerland but in 1985 out of 21 Swiss patients with HBsAg-positive chronic hepatitis hospitalised between 1997 and 1982 at the Stadtspital Waid in Zurich, only one patient was anti-HDV positive (prevalence of 5%) [12].
In European countries, there is a high prevalence of HDV infection in intravenous drug addicts with HBV infection [9, 13] as is also most likely in Switzerland. In our study, 62% of the patients came from this risk factor group. Despite the decreasing prevalence of HDV in southern Europe, the disease still represents a major health burden in central Europe where its prevalence is mostly attributable to the immigration of individuals from highly endemic areas [14]. More than 20% of our patients came from an African country.
Due to interference mechanisms that are not well understood, HBV replication is suppressed in most HDV-infected individuals. An interesting finding of the current survey was that some HDV positive patients were co-infected with HBV without HBsAg even though they were not cured (no anti-HBs) (13%) and some showed anti-HBc alone (4%). These serological profiles make the diagnosis of hepatitis D very difficult, so clinicians should reconsider any positive HBV result.
Concerning co-morbidities, 18% of our patients were co-infected by HIV and 45% by HCV. Some authors found an increased risk of hepatitis flares, liver cirrhosis, hepatic decompensation and death in patients with HIV-HBV-HDV or HCV-HBV-HDV triple infection compared to HIV-HBV or HCV-HBV-infected patients without hepatitis D [15, 16]. Moreover, in a longitudinal study, the severe course of chronic liver disease was confirmed and hepatic decompensation, rather than hepatocellular carcinoma, was identified to be the primary clinical event in European HDV patients [17]. Five patients from our series suffered from hepatocarcinoma, but hepatic decompensations were unfortunately not recorded in our questionnaire.
Patients with HDV infection pose a major therapeutic challenge because most of them have advanced liver disease and belong to difficult to treat patient groups like IV drug users or those with psychiatric co-morbidities (nearly 70% of our patients). The current state-of-the-art and approved regimen against hepatitis D is treatment with peginterferon-α [8]. Only 21% of our patients could be treated by interferon-α or peginterferon-α. Swiss gastroenterologists, hepatologist and specialists in infectious diseases should try to enhance the rate of treated patients.
The current study had several limitations. First, not all hepatologists and infectious disease specialists follow hepatitis patients, therefore only a fraction of them even replied to the survey. Since most of those who did reply, however, followed HDV patients, there may be an inevitable bias in our results. Secondly, prevalence may be underestimated because HDV may not be systematically investigated in all HBV patients. On the other hand, as only those specialists who followed HDV patients answered the questionnaire, this could overestimate the true prevalence, since other specialists who followed HBV patients not co-infected with HDV were excluded and, therefore, their patients were not considered in our survey. Finally, the advanced liver disease reported in the patients (48.5% ≥ Metavir F3) could be explained by the fact that a majority was drawn from a tertiary hospital, which usually focuses on the worst cases.
In conclusion, with a prevalence of 5.9% of hepatitis D in HBsAg-positive patients, Switzerland seems less affected than most other European countries; it is, however, possible that this infection is under-diagnosed. Every individual who is HBsAg positive or anti-HBc alone should be tested for anti-HDV IgG antibodies, especially if belonging to a high risk group (intravenous drug users and immigrants from endemic regions). A prospective study supported by Swiss authorities should be implemented to define the actual prevalence of HDV infection in all HBV carriers, as well as its burden of disease in Switzerland.
We thank Goldi Riyahi, Hans H. Siegrist and Francesco Negro for critically reviewing the manuscript.
Partial financial support by Roche SA. No other potential conflict of interest relevant to this article was reported.
1 Rizzetto M, Canese MG, Arico S, Crivelli O, Bonino F, Trepo CG, et al. Immunofluorescence detection of a new antigen/antibody system (delta/anti-delta) associated with hepatitis B virus in liver and serum of HBsAg carriers. Gut. 1977;18:997–1003.
2 Wang KS, Choo QL, Weiner AJ, Ou JH, Najarian C, Thayer G, et al. Structure, sequence and expression of the hepatitis delta viral genome. Nature. 1986;323:508–14.
3 Rizzetto M, Ponzetto A, Forzani I. Epidemiology of hepatitis delta virus: Overview. Prog Clin Biol Res. 1991;364:1.
4 Hadziyannis SJ. Review: Hepatitis delta. J Gastroenterol Hepatol. 1997;12(4):289–98.
5 Colombo P, et al. Smouldering hepatitis B virus replication in patients with chronic liver disease and hepatitis delta virus superinfection. J Hepatol. 1991;12:64–9.
6 Aragona M, Macagno S, Caredda F, et al. Serological response to the hepatitis delta virus in hepatitis D. Lancet. 1987;1:478–82.
7 Le Gal F, Gordien E, Affolabi D, Hanslik T, Alloui C, Déni P, et al. Quantification of hepatitis delta virus RNA in serum by consensus real-time PCR indicates different patterns of virological response to interferon therapy in chronically infected patients. J Clin Microbiol. 2005;43:2363–9.
8 Grabowski J, Wedemeyer H. Hepatitis Delta: Immunopathogenesis and clinical challenges. Dig Dis. 2010;28:133–8.
9 Gaeta GB, Stroffolini T, Chiaramonte M, Ascione T, Stornaiuolo G, Lobello S, et al. Chronic hepatitis D: a vanishing disease? An Italian multicenter study. Hepatology. 2000;32:824–7.
10 Degertekin H, Yalçin K, Yakut M, Yurdaydin C. Seropositivity for delta hepatitis in patients with chronic hepatitis B and liver cirrhosis in Turkey: a meta-analysis. Liver Int. 2008;4:494–8.
11 Heidrich B, Deterding K, Tillmann HL, Raupach R, Manns MP, Wedemeyer H. Virological and clinical characteristics of delta hepatitis in central Europe. J Viral Hepat. 2009;16:883–94.
12 Frösner GG, Franco E, Altorfer J, Decker RH, Mushahwar IK. Prevalence of Antibody to delta hepatitis virus population groups in west Germany and Switzerland. Eur J Clin Microbiol. 1985;4;147–9.
13 Wedemeyer H, Heidrich B, Manns MP. Hepatitis D virus infection – not a vanishing disease in Europe. Hepatology. 2007;45:1331–2.
14 Wedemeyer H, Manns MP. Epidemiology, pathogenesis and management of hepatitis D: update and challenges ahead. Nat Rev Gastroenterol Hepatol. 2010;7:31–40.
15 Sheng W, Hung C, Kao J, Chang S, Chen M, Hsieh S, et al. Impact of hepatitis D virus infection on the long-term outcomes of patients with hepatitis B virus and HIV coinfection in the era of highly active anti-retroviral therapy: a matched cohort study. Clin Infect Dis. 2007;44:988–95.
16 Raimondo G, Brunetto MR, Pontisso P, Smedile A, Maina AM, Saitta C, et al. Longitudinal evaluation reveals a complex spectrum of virological profiles in hepatitis B virus/hepatitis C virus-coinfected patients. Hepatology. 2006;43:100–7.
17 Calle Serrano B, Heidrich B, Jaroszewicz J, Deterding K, Raupach R, Cornberg M, et al. Long-term outcome of hepatitis delta: A 14-year single center experience. Hepatology. 2009;50:737A.