Figure 1
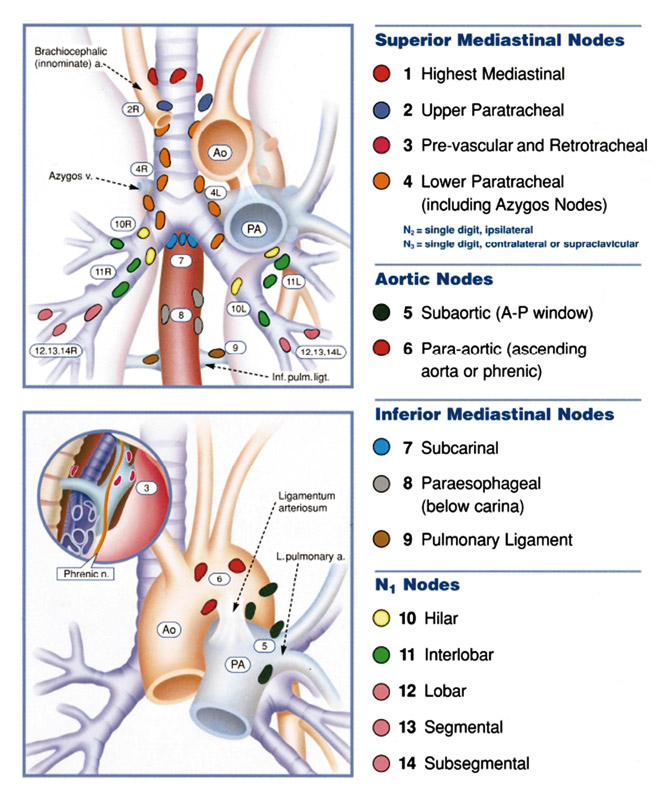
Regional lymph node stations for lung cancer staging (from Mountain CF, Dresler CM. Chest 1997;11:1718–23 [12]; reprint with permission).
DOI: https://doi.org/10.4414/smw.2011.13168
Accurate staging is required to provide precise information on the extent of the disease and to determine the most appropriate therapy in patients with non-small-cell lung cancer (NSCLC). It is also important for estimating prognosis and for comparison of studies. It has been shown that patients with clinical stage III are a heterogeneous group in which the outcome is very poor when treated with surgery or radiotherapy alone. In order to improve the outcome in this subgroup of patients, the concept of multimodality treatment has been introduced. Several studies have shown a significant benefit on survival with potential curability after an induction therapy followed by surgery in the case of disappearance of the tumour from mediastinal nodes (downstaging, observed in about 50–60%) and complete resection in the patients with stage IIIA-N2 disease (tumour infiltration of the mediastinal nodes) [1–5]. Therefore, an accurate pre-induction (primary staging) and post-induction (restaging) lymph-node staging is mandatory. It is evident that both in primary staging and restaging, not every staging technique is available in every centre. Therefore, the choice of the technique used is dependent on the availability and expertise of the centre.

Figure 1
Regional lymph node stations for lung cancer staging (from Mountain CF, Dresler CM. Chest 1997;11:1718–23 [12]; reprint with permission).
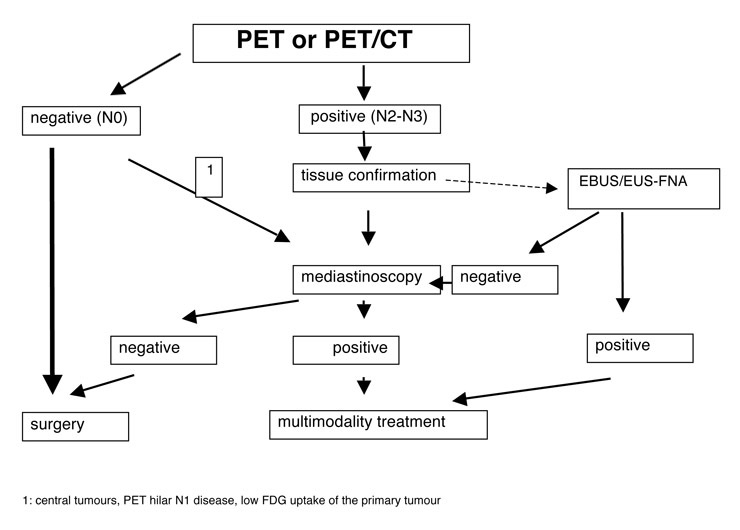
Figure 2
Algorithm (De Leyn P, Lardinois D, Van Schil P, Rami-Porta R, Passlick B, Zielinski M, Waller D, Lerut T, Weder W. ESTS guidelines for preoperative lymph node staging for NSCLC. Eur J Cardiothorac Surg 2007).
In order to obtain the most precise (re)staging, an integration of these procedures is highly recommended, especially in the context of clinical trials and to maintain the morbidity as low as possible.
Computed tomography (CT) of the chest is part of the traditional work-up used for staging purposes. CT provides morphologic information on the extent of the disease but has limited usefulness in the assessment of mediastinal lymph-node involvement. A diameter larger than 1 cm in the short axis is generally considered as the standard criterion for a suspicious lymph node. However, metastases have been found in up to 20% of small nodes in patients with clinical stage cT1N0 and cT2N0, and only about 50% of the nodes with a diameter of 1.5 cm to 2 cm are metastatic [6]. Therefore, lymph-node size does not predict malignancy. Several meta-analyses have reported low sensitivities and specificities of CT in the assessment of mediastinal lymph-node involvement, ranging from 50% to 65% and from 65% to 85%, respectively [6].
This performance is insufficient for clinical decisions, but CT can be of help in selecting the most appropriate procedure for tissue sample of the suspect lymph nodes.
Mediastinoscopy remains the gold standard for invasive complete staging of the upper mediastinum in patients with potentially operable lung cancer. Cervical mediastinoscopy is the most commonly used and was introduced by Carlen in 1959. It is a surgical biopsy technique under general anaesthesia [7].
According to the lymph-node map proposed by Montain and Dresler [8; fig. 1], the following lymph- node stations can be evaluated by cervical mediastinoscopy: the high mediastinal station (level 1), the right and left superior paratracheal station (level 2 right, level 2 left), the right and left lower paratracheal station (level 4 right, level 4 left), and the subcarinal station (level 7).
There is no internationally accepted recommendation regarding how many lymph-node stations should be examined at cervical mediastinoscopy but ideally, the following nodal stations should be biopsied [9]:
– right and left superior paratracheal nodes (stations 2R and 2L)
– right and left inferior paratracheal nodes (stations 4R and 4L)
– subcarinal (station 7)
An advantage of mediastinoscopy over fine needle aspiration is that a full mediastinal staging can be performed. This might be important in the differentiation and treatment planning of patients with single and multi-level N2-disease. The sensitivity of cervical mediastinoscopy is reported to be between 72% and 89%, on average 81% with a negative predictive value of 91% [10]. The results of the suboptimal sensitivity can partly be explained by the fact that some lymph node (LN) stations (stations 5, 6, 7 posteriorly and stations 8 and 9) are not accessible by cervical mediastinoscopy.
More recently, mediastinoscopy is performed by the use of video-mediastinoscope [7, 11]. This definitely improves the visualisation of the operative field and may lead to a higher accuracy in staging and to a better systematisation of the technique [11, 12]. The technique of video-mediastinoscopy seems relatively simple to perform but should be reserved for experienced centres due to the proximity of some large vessels (superior vena cava, right pulmonary artery, vena innominate and brachio-cephalic trunk) and of the left recurrent nerve on the left side of the trachea. Some large published series have shown that the morbidity and mortality of this staging technique can be very low in experienced hands [13].
Non-invasive lung cancer staging was substantially improved by the use of positron emission tomography with 18F-fluoro-2-deoxy-D-glucose (FDG-PET), which gives information about the metabolism of the cells. A large number of accuracy studies and already six meta-analyses [6, 14–18] have demonstrated that PET is superior to CT scans for mediastinal staging in potential operable non-small-cell lung cancer.
Comparable high sensitivities and negative predictive values have been observed for PET and mediastinoscopy (table 1). However, the positive predictive value and the specificity of FDG PET-scan are lower than mediastinoscopy due to the fact that FDG is also taken up by inflammatory processes.
Due to the high negative predictive value of PET-scans, invasive staging procedures like mediastinoscopy can generally be omitted in patients with negative mediastinal PET images. However, in case of patients with central tumours, central hilar N1-disease on CT scan, bronchio-alveolar cell carcinoma or in all situations with weak FDG-uptake in the primary [19, 20], more invasive mediastinal staging by use of mediastinoscopy or endoscopic techniques is recommended. It is estimated that the introduction of PET has reduced the number of mediastinoscopies by 65% [19]. In case of positive mediastinal PET, invasive mediastinal staging is still needed to confirm lymph-node metastasis or not.
The actual resolution of PET scans is about 5 mm. The main drawback of PET is the poor quality of its anatomical information. The exact localisation of a single focal abnormality can be difficult or even impossible with use of PET alone. Ten years ago, integrated PET-CT scanners were introduced. The great advantages of this technique consist of the precise anatomical correlation of the radionuclide uptake, an identical positioning of the patient, no time interval for data acquisition and no additional work for collecting data. The first available studies showed an increased diagnostic accuracy of integrated PET-CT which respects the nodal staging in comparison to all other standard imaging methods [21]. Integrated PET/CT was also demonstrated to be superior to all other imaging techniques in the evaluation of chest wall infiltration, of mediastinum invasion, and in the exact localisation of occult distant metastases, which are found in up to 15% of the patients with potentially operable NSCLC [21].
Endoscopic techniques are minimally invasive approaches that provide histological or cytological confirmation of nodal tumour involvement. A transbronchial needle aspiration (TBNA) of mediastinal lymph nodes can be performed. TBNA has been shown to be safe and useful in patients with enlarged mediastinal lymph nodes. However, this technique has a moderate yield (ATS 4, 7), is a “blind” technique, operator dependent, and the results depend on the size of the lymph node [22]. In an overview, a sensitivity of 76% and a false negative rate of 29% were reported for conventional transbronchial needle aspiration in clinical N2 disease [10, 23] (table 1). This high false negative rate compromises the use of conventional transbronchial needle aspiration for routine complete mediastinal lymph-node staging.
The accuracy can be improved by guidance of endoscopic ultrasonography (the endobronchial or esophageal ultrasound-guided fine needle aspiration, EBUS-TBNA and EUS-FNA respectively). A prospective study demonstrated an improvement of the diagnostic accuracy of EBUS-TBNA in comparison with TBNA, especially for stations 2 and 4 [24]. The yield of EBUS-TBNA is comparable with mediastinoscopy (upper mediastinum) but the hilar (ATS 10) and intrapulmonary nodal stations can be biopsied additionally.
The EUS-guided FNA are mainly suitable for the assessment of LNs in the posterior part of levels 4L, 5 and 7, and in the inferior mediastinal at levels 8 and 9, as described on the Mountain-Dresler map (fig. 1).
A review of the literature reported a pooled sensitivity of 88%, a specificity of 91%, a positive predictive value of 98% and a negative predictive value of 77%.
However, these studies were performed in patients with enlarged lymph nodes and a high suspicion of N2-N3 disease [25].
When the prevalence of involved mediastinal lymph nodes is high, an improved sensitivity is to be expected which does not reflect the accuracy in patients with normal sized lymph nodes. It is generally accepted that endoscopic techniques are suitable to prove suspicious mediastinal lymph nodes (PET-positive finding), especially when the lymph node is not reachable by mediastinoscopy, but cannot be used to exclude mediastinal lymph-node disease because of the low negative predictive value. Transbronchial-/oesophageal US-guided FNA is therefore not a substitute for mediastinoscopy. These techniques should be considered as complementary staging methods.
– The accuracy of CT scans in the evaluation of mediastinal lymph nodes is insufficient to guide clinical decisions.
– Invasive staging can be omitted in patients with negative mediastinal PET images. However, in case of central tumours, PET hilar N1 disease, low FDG uptake of the primary tumour, invasive staging with mediastinoscopy remains indicated.
– PET positive mediastinal findings should be histologically or cytologically confirmed.
– Transbronchial needle aspiration, ultrasound-guided bronchoscopy (EBUS-TBNA) and esophagoscopy (EUS-FNA) are techniques that provide cytological/histological diagnosis and are minimally invasive. They can be complementary to surgical invasive staging technique. Their specificity is high, but their negative predictive value is low. Due to this, if they yield negative results, an invasive surgical technique is indicated.
– Cervical mediastinoscopy provides the advantage that a full mapping of mediastinal lymph nodes can be performed. At least one ipsilateral, one contralateral and the subcarinal LNs should be biopsied.
| Table 1:Performance of different locoregional staging techniques (adapted from Toloza 2003). | |||||
| Sensitivity (%) | Specificity (%) | NPV (%) | PPV (%) | Prevalence (%) | |
| CT | 57 | 82 | 83 | 56 | 28 |
| PET | 84 | 89 | 93 | 79 | 32 |
| Blind TBNA | 76 | 96 | 71 | 100 | 70 |
| EUS-FNA | 88 | 91 | 77 | 98 | 69 |
| Mediastinoscopy | 81 | 100 | 91 | 100 | 37 |
| NPV: negative predictive value; PPV: positive predictive value; TBNA: transbronchial needle aspiration; EUS-FNA: esophageal ultrasound guided fine needle aspiration: Prevalence: proportion of patients with metastatic mediastinal nodes in the study cohorts. | |||||
Mediastinal restaging after induction therapy is required to aid proper selection of patients likely to benefit from surgical resection. This is important because resection following induction therapy may increase post-operative morbidity and mortality compared with resection without induction treatment [26].
The mediastinum can be restaged by CT-scan, mediastinoscopy or remediastinoscopy, PET scan, PET-CT-scan and fine needle aspiration.
In primary staging, CT-scanning has proved to have a low accuracy. It is not surprising that the accuracy of CT-scans in restaging the mediastinum is also low, with a sensitivity of 50%, a specificity of 65%, and an accuracy of about 60% [27, 28].
Remediastinoscopy offers the advantage of providing histological evidence of response after induction therapy. The technique is often challenging, due to fibrosis and severe adhesions.
Only a few centres have reported their experience with repeat mediastinoscopy. All this series showed a lower sensitivity and a lower negative predictive value in comparison with primary mediastinoscopy [28–30]. The sensitivity to detect residual mediastinal disease was 70%, the specificity was 100% and accuracy was 80%. The problem is that remediastinoscopy was often inadequate or incomplete due to technical difficulties. A prospective study evaluated the accuracy of remediastinoscopy and PET-CT in restaging the mediastinum after mediastinoscopy proven N2 disease in 30 patients [27]. In this experience, remediastinoscopy was technically feasible but inaccurate due to severe adhesions and fibrosis. In all this series involving a limited number of patients, no mortality and only minimal morbidity was reported. However, technical difficulties of remediastinoscopy may prevent the generalisation of its use for restaging purposes.
In the restaging of mediastinal lymph nodes after induction therapy, PET-scan is less sensitive than before induction treatment with a higher rate of false negative interpretations. In most studies [31–35], the sensitivity was reported to be 50–60% with a good specificity of 85–90%. The reason for this poor sensitivity is not clear. A very small mass of tumour, such as post-treatment microscopic foci surrounded by fibrosis, may be more difficult to detect. Changes in the microenvironment of the tumour such as altered perfusion due to post-chemotherapy changes may also impair presentation of FDG to the metastatic lymph nodes. The time interval between induction therapy and post-induction PET also seems to play an important role in the accuracy of this technique for mediastinal restaging.
Use of PET-CT fusion images significantly increases the specificity through better localisation of focal FDG uptake in mediastinum [27].
It seems that SUV max values from two PET/CT scans, before and after induction therapy allow prediction of the histopathological response in the primary tumour and mediastinal lymph nodes and have prognostic value [36]. Several studies have shown that high residual activity (≥4) in primary tumour and in mediastinal nodes is associated with poor prognosis and a high rate of incomplete resections (up to 46%).
There is still little experience with transbronchial or transesophageal ultrasound guided biopsy for restaging of the mediastinum. First results indicate a diagnostic accuracy of about 80%. The problem remains a low negative predictive value as shown in a recent prospective study [37, 38]. Although the use of the endoscopic techniques may avoid the difficulties of remediastinoscopy, their role for a complete mediastinal restaging has not yet been defined.
– Restaging of the mediastinum after induction treatment is necessary to select the patients who can benefit from surgery.
– At the present time, there are no imaging techniques which can accurately determine the biological response of the tumour to the induction treatment. Neither CT, PET or PET-CT seem good enough to make further therapeutic decisions, based on their results.
– The accuracy of PET in mediastinal restaging is not optimal, mainly due to its low sensitivity. Fusion images with PET-CT seem to improve the results with a very favourable sensitivity, specificity and accuracy.
– An invasive technique providing cytohistological information is necessary. For restaging techniques, endoscopic techniques or surgical invasive techniques can be used. If they yield a positive result, definitive non-surgical treatment seems to be indicated in most patients.
– Remediastinoscopy has proven to be feasible but due to adhesions and fibrosis, the intervention is technically challenging.
– Less invasive techniques, such as EBUS fine needle aspiration, seem to obtain similar results as remediastinoscopy.
There are internationally accepted definitions for intra-operative lymph-node staging in NSCLC, however there are some unanswered questions regarding the extent, nomenclature definition, and surgical procedure of intra-operative lymph node evaluation.
Although it is clear that nodal staging of non-small-cell lung cancer should be as accurate as possible, the extent of mediastinal lymph node assessment during surgery is controversial and there is no consensus [39].
Different techniques are used, ranging from simple visual inspection of the unopened mediastinum to an extended bilateral lymph node dissection. Furthermore, different terms are used to define these techniques.
– Sampling: Sampling is the removal of one or more lymph nodes guided by pre- or intra-operative findings which are thought to be representative. Systematic sampling means a predetermined selection of the lymph node stations specified by the surgeon.
– Systematic nodal dissection: All the mediastinal tissue containing the lymph nodes is dissected and removed systematically within anatomical landmarks. It is recommended that at least 3 mediastinal nodal stations (but always subcarinal) should be excised as a minimum requirement. The nodes are separately labelled and examined histologically. Beside the mediastinal nodes, the hilar and the intrapulmonary lymph nodes are dissected as well [40].
– Lobe-specific systematic node dissection: In this procedure, the mediastinal tissue containing specific lymph node stations are excised, depending on the lobar location of the primary tumour. The subcarinal front should always be removed.
– Extended lymph node dissection: In this procedure, bilateral mediastinal and cervical lymph node dissection is performed through median sternotomy and cervicotomy.
For complete resection of non-small-cell lung cancer, a systematic nodal dissection is recommended in all cases (also after induction therapy) [41–43]. Ideally, this should be done as an en-bloc resection, when possible, of the upper mediastinal nodes on the right side (stations 2R and 4R), and of the lower mediastinum, including the fatty tissue from the diaphragm to the subcarinal space (stations 7, 8, and 9). On the left side, removal of the lymph node stations 4–9 should be performed, including the sub-aortic (aorto-pulmonary window, number 5) and para-aortic (number 6) stations. For a complete nodal dissection of the left upper mediastinum, division of the ligamentum arteriosus allowing mobilisation of the aortic arch is recommended, with special care not to injure the left recurrent laryngeal nerve [43].
All the nodal stations excised should be put in different vials with separate labelling. It is important to note that although systematic mediastinal nodal dissection is recommended, only 1/3 of the surgeons in North America perform this type of lymphadenectomy.
– For peripheral squamous cell carcinoma T1, a more selective nodal dissection depending on the lobar location of the primary tumour (lobe-specific systematic nodal dissection) is acceptable, based on the detailed analysis of lobe-specific lymphatic drainage [44, 45]. It has been shown that the probability of unforeseen N2 disease is very low (less than 5%) in such patients [46, 47].
With this technique, a minimal dissection of at least 3 mediastinal nodal stations depending on the lobar location of the primary tumour has been recommended [48]. Dissection and histological examination of hilar and interlobar nodes have to be tumour-free on frozen section analysis.
Right upper and middle lobe: 2R, 4R and 7
Right lower lobe: 4R, 7, 8 and 9
Left upper lobe: 5, 6 and 7
Left lower lobe: 7, 8 and 9.
In total, the lymphadenectomy specimen should include at least 6 nodes.
– High risk patients: Intra-operative lymph node assessment can be minimised in high-risk patients undergoing minimal invasive video assisted wedge resections, but if the patient can tolerate a lobectomy, standard recommendation of lymph node assessment should be followed [49].
Whether extending the lymph-node dissection influences survival or recurrence rate of the disease remains to be determined [50–53]. There are data which clearly show that systematic sampling or nodal dissection improves intra-operative staging, especially in the detection of multi-level N2 disease which is associated with a poorer prognosis [50, 51, 54].
Three retrospective studies have shown a survival benefit (5-, and 10-y survival) from complete mediastinal dissection in stage I NSCLC. One prospective study indicates improved survival in stage II and IIIA after systematic dissection [51]. A prospective non randomised trial comparing 50 patients undergoing systematic lymph node dissection and 50 patients undergoing systematic sampling showed a significant longer disease-free survival and a significant lower local recurrence rate (13% vs. 45%) after systematic nodal dissection in patients with stage I NSCLC. There was no higher morbidity (intra-operative blood loss, need for transfusion, bronchopleural fistula) after systematic nodal dissection, and the duration of chest tube drainage and of hospitalisation was comparable in the two groups [53].
Recently, a meta-analysis of 3 randomised controlled trials indicated a significant reduction in the risk of death at 4 years after systematic nodal dissection compared to sampling in patients with stage I-IIIA NSCLC undergoing resection [55].
– The technique of lymph-node assessment during surgery for NSCLC is not standardised to date.
– An accurate intra-operative staging is necessary to compare the results from different institutions and to conduct multi-institutional trials.
– Systematic mediastinal lymph-node dissection is recommended in all cases for complete resection of NSCLC, and improves pathologic staging and the prospect for adjuvant therapy.
– The role of mediastinal lymphadenectomy regarding overall survival and local control remains controversial but systematic lymph-node dissection might be associated with a better outcome in early-stage NSCLC.
– Lobe-specific systematic nodal dissection is acceptable for peripheral squamous T1 tumours, if hilar and interlobar nodes are negative on frozen section studies.
1 Betticher DC, Hsu Schmitz SF, Totsch M, et al. Mediastinal lymph node clearance after docetaxel-cisplatin neoadjuvant chemotherapy is prognostic of survival in patients with stage IIIA pN2 non-small cell lung cancer: a multicenter phase II trial. J Clin Oncol. 2003;21:1752–9.
2 Bueno R, Richards WG, Swanson SJ, et al. Nodal stage after induction therapy for stage IIIA lung cancer determines patient survival. Ann Thorac Surg. 2001;70:1826–31.
3 Lorent N, De Leyn P, Lievens Y, et al. Long-term survival of surgically staged IIIA-N2 non-small cell lung cancer treated with surgical combined modality approach: analysis of a 7-year experience. Ann Oncol. 2004;15:1645–53.
4 Albain KS, Swann RS, Rusch VR, Turrisi AT, Shepherd FA, Smith CJ, et al., North American Lung Cancer Intergroup. Phase III study of concurrent chemotherapy and radiotherapy (CT/RT) versus CT/RT followed by surgical resection for stage IIIA (pN2) nonsmall cell lung cancer (NSCLC): Outcomes update of North American Intergroup 0139 (RTOG 9309). The Oncologist. 2006;11:43.
5 Van Meerbeeck JP, Kramer G, Van Schil PE, Legrand C, Smit EF, Schramel FM, et al., EORTC-Lung Cancer Group. A randomized trial of radical surgery (S) versus thoracic radiotherapy (TRT) in patients (pts) with stage IIIA-N2 nonsmall cell lung cancer (NSCLC) after response to induction chemotherapy (ICT) (EORTC 08941). The Oncologist. 2006;11:42–3.
6 Toloza EM, Harpole L, McCrory DC. Noninvasive staging of non-small cell lung cancer: A review of the current evidence. Chest. 2003;123:137S–146S.
7 Martin-Ucar AE, Chetty GK, Vaughan R, Waller DA. A prospective audit evaluating the role of video-assisted cervical mediastinoscopy (VAM) as a training tool. Eur J Cardiothorac Surg. 2004; 26:393–5.
8 Mountain CF, Dresler CM. Regional lymph node classification for lung cancer staging. Chest. 1997;111:1718–23.
9 Lardinois D, De Leyn P, Van Schil PE, Rami Porta R, Waller, Passlick B, et al. ESTS guidelines for intraoperative lymph node staging in non-small-cell lung cancer. Eur J Cardiothorac Surg. 2006;30:787–92.
10 Toloza EM, Harpole L, Detterbeck F. Invasive staging of non-small cell lung cancer: A review of the current evidence. Chest. 2003;123:157S–166S.
11 Lardinois D, Schallberger A, Betticher D, Ris HB. Postinduction video-mediastinoscopy is as accurate and safe as video-mediastinoscopy in patients without pretreatment for potentially operable non-small cell lung cancer. Ann Thorac Surg. 2003;75:1102–6.
12 Mouroux J, Venissac N, Alifano M. Combined video-assisted mediastinoscopy and video-assisted thoracoscopy in the management of lung cancer. Ann Thorac Surg. 2001;72:1698–704.
13 De Leyn P, Lerut T. Videomediastinoscopy, in Yim AP, Hazelrigg SR, Izzat MB, Landreneau RJ, Mack MJ, Naunheim KS (ed). Minimal access in cardiothoracic surgery. WB Saunders. 2000;169–74.
14 Dwamena BA, Sonnad SS, Angobaldo JO, et al. Metastases from non-small cell lung cancer: mediastinal staging in the 1990s. Meta-analytic comparison of PET and CT. Radiology. 1999;213:530–6.
15 Fischer BM, Mortensen J, Hojgaard L. Positron emission tomography in the diagnosis and staging of lung cancer: a systematic, quantitative review. Lancet Oncol. 2001;2:659–66.
16 Hellwig D, Ukena D, Paulsen F, et al. Meta-analysis of the efficacy of positron emission tomography with F-18-fluorodeoxyglucose in lung tumors. Basis for discussion of the German Consensus Conference on PET in Oncology 2000 (in German). Pneumologie. 2001;55:367–77.
17 Gould MK, Kuschner WG, Rydzak CE, et al. Test performance of positron emission tomography and computed tomography for mediastinal staging in patients with non-small cell lung cancer: a meta-analysis. Ann Intern Med. 2003;139:879–92.
18 Birim O, Kappetein AP, Stijnen T, et al. Meta-analysis of positron emission tomographic and computed tomographic imaging in detecting mediastinal lymph node metastases in non-small cell lung cancer. Ann Thorac Surg. 2005;79:375–82.
19 Vansteenkiste JF, Stroobants SG, De Leyn PR, et al. Mediastinal lymph node staging with FDG-PET scan in patients with potentially operable non-small cell lung cancer: A prospective analysis of 50 cases. Chest. 1997;112:1480–6.
20 Verhaghen AT, Bootsma GP, Tjan-Heijnen VCG, van der Wilt GJ, Cox AL, Brouwer MHJ, et al. FDG-PET in staging lung cancer. How does it change the algorithm? Lung cancer. 2004;44:175–81.
21 Lardinois D, Weder W, Hany TF, et al. Staging of non-small cell lung cancer with integrated positron-emission tomography and computed tomography. N Engl J Med. 2003;348:2500–7.
22 Holty JEC, Kuschner WG, Gould MK. Accuracy of transbronchial needle aspiration for mediastinal staging of non-small cell lung cancer: a meta-anlysis. Thorax. 2005;60:949–55.
23 Detterbeck FC, Jones DR, Parker Jr LA. Intrathoracic staging. In: Detterbeck FC, Rivera MP, Socinski MA, Roseman JG (Editors). Diagnosis and treatment of lung cancer. An evidence-based guide for the practicing clinician. WB Saunders Company, Philadelphia, 2001;73–93.
24 Annema JT, Verteegh MI, Veselic M, Voigt P, Rabe KF. Endoscopic ultrasound fine-needle aspiration in the diagnosis and staging of lung cancer and its impact on surgical staging. JCO. 2005;23:8357–61.
25 Kramer H, Groen HJM. Current concepts in the mediastinal lymph node staging of nonsmall cell lung cancer. Ann Thorac Surg. 2003;238:180–8.
26 Martin J, Ginsberg RJ, Venkatraman ES. Long-term results of combined-modality therapy in resectable non-small cell lung cancer. J Clin Oncol. 2002;15:1989–95.
27 De Leyn P, Stoobants S, Dewever W, Lerut T, Coosemans W, Decker G, et al. Prospective comparative study of integrated PET-CT versus re-mediastinoscopy in the assessment of residual mediastinal disease after induction chemotherapy for mediastinoscopy proven stage IIIa-N2 non-small cell lung cancer. JCO, 2006
28 Mateu-Navarro M, Rami-Porta R, Bastus-Piulats R, et al. Remediastinoscopy after induction chemotherapy in non-small cell lung cancer. Ann Thorac Surg. 2000;70:391–5.
29 Pitz CCM, Maas KW, Van Swieten HA, et al. Surgery as part of combined modality treatment in stage IIIB non-small cell lung cancer. Ann Thorac Surg. 2002;74:164–9.
30 Van Schil P, Van Der Schoot J, Poniewierski J, et al. Remediastinoscopy after neoadjuvant therapy for non-small cell lung cancer. Lung Cancer. 20022;37:281–5.
31 Akhurst T, Downey RJ, Ginsberg MS, et al. An initial experience with FDG-PET in the imaging of residual disease after induction therapy for lung cancer. Ann Thorac Surg. 2002;73:259–66.
32 Ryu JS, Choi NC, Fischman AJ, et al. FDG-PET in staging and restaging non-small cell lung cancer after neoadjuvant chemoradiotherapy: correlation with histopathology. Lung Cancer. 2002;35:179–87.
33 Cerfolio RJ, Ojha B, Mukherjee S, et al. Positron emission tomography scanning with 2-fluoro-2-deoxy-d-glucose as a predictor of response of neoadjuvant treatment for non-small cell carcinoma. J Thorac Cardiovasc Surg. 2003;15:938–44.
34 Port JL, Kent MS, Korst RJ, et al. Positron emission tomography scanning poorly predicts response to preoperative chemotherapy in non-small cell lung cancer. Ann Thorac Surg. 2004;77:254–9.
35 Hellwig D, Graeter TP, Ukena D, et al. Value of F-18-fluorodeoxyglucose positron emission tomography after induction therapy of locally advanced bronchogenic carcinoma. J Thorac Cardiovasc Surg. 2004;128:892–9.
36 Pöttgen C, Levegrün S, Theegarten D, Marnitz S, Grehl S, Pink R, et al. Value of 18F-Fluoro-2-Deoxy-D-Glucose-Positron emission tomography/computed tomography in non-smal-cell lung cancer for prediction of pathologic response and times to relapse after neoadjuvant chemoradiotherapy. Clin Cancver Res. 2006;12:97–106.
37 Annema JT, Veselic M, Versteegh MIM, Willems LNA, Rabe KF. Mediastinal restaging: EUS-FNA offers a new perspective. Lung Cancer. 2003;42:311–8.
38 Herth FJ, Annema JT, Eberhardt R, Yasufuku K, Ernst A, Krasnik M, Rintoul RC. Endobronchial ultrasound with transbronchial needle aspiration for restaging the mediastinum in lung cancer. J Clin Oncol. 2008;26:3346–50.
39 Silverberg SG, Connolly JL, Dabbs D, Muro-Cacho CA, Page DL, Ray MB, Wick MR. Association of directors of anatomic and surgical pathology. Recommendations for processing and reporting of lymph node specimens submitted for evaluation of metastatic disease. Am J Clin Pathol. 2001;115:799–801.
40 Goldstraw P. Report on the international workshop on intrathoracic staging. London. October 1996. Lung Cancer. 1997;18:107–11.
41 Graham A, Chan K, Pastorino U, Goldstraw P. Systematic nodal dissection in the intrathoracic staging of patients with non-small-cell lung cancer. J Thorac Cardiovasc Surg. 1999;117:246–51.
42 Rami-Porta R, Wittekind C, Goldstraw P. Complete resection in lung cancer surgery: proposed definition. Lung Cancer. 2005;49:25–33.
43 Lardinois D, De Leyn P, Van Schil P, Porta RR, Waller D, Passlick B, et al. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur J Cardiothorac Surg. 2006;30:787–92.
44 Naruke T, Tsuchiya R, Kondo H, Nakayama H, Asamura H. Lymph node sampling in lung cancer: how should it be done? Eur J Cardiothorac Surg. 1999;16:S17–S24.
45 Ichinose Y, Kato H, Koike T, Tsuchiya R, Fujisawa T, Shimizu N, et al., Japanese Clinical Oncology Group. J Thorac Cardiovasc Surg. 2001;122:803–8.
46 De Leyn P, Vansteenkiste J, Cuypers P, Deneffe G, Van Raemdonck D, Coosemans W, et al. Role of cervical mediastinoscopy in staging of non-small cell lung cancer without enlarged mediastinal lymph nodes on CT-scan. Eur J Cardiothorac Surg. 1997;12:706–12.
47 Verhagen A, Bootsma G, Tjan-Heijnen V, van der Wilt G, Cox A, Brouwer M, et al. FDG-PET in staging lung cancer. How does it change the algorithm? Lung Cancer. 2004;44:175–81.
48 GCCB-S (Grupo Cooperativo de Carcinoma Broncogencio de la Sociedad Espanola de Neumologia y Cirugia Toracica). Intraoperative lymph node staging in bronchogenic carcinoma surgery. Consensus report. Arch Bronconeumol. 2001;37:495–503.
49 Fry WA. Assessment of operability and respectability in lung cancer. Malignant tumors of the lung. Springer. 2004;179–82.
50 Izbicki JR, Passlick B, Karg O, Bloechle C, Pantel K, Knoefel WT, Thetter O. Impact of radical systematic mediastinal lymphadenectomy on tumor staging in lung cancer. Ann Thorac Surg. 1995;59:209–14.
51 Keller SM, Adak S, Wagner H, Johnson DH. Mediastinal lymph node dissection improves survival in patients with stages II and IIIA NSCLC. Ann Thorac Surg. 2000;70:358–66.
52 Wu Y, Huang Z, Wang S, Yang X, Ou W. A randomized trial of systematic nodal dissection in resectable NSCLC. Lung Cancer. 2002;36:1–6.
53 Lardinois D, Suter H, Hakki H, Rousson V, Betticher D, Ris HB. Morbidity, survival, and site of recurrence after mediastinal lymph-node dissection versus systematic sampling after complete resection for non-small cell lung cancer. Ann Thorac Surg. 2005;80:268–74.
54 Keller SM, Vangel MG, Wagner H, Schiller JH, Herskovic A, Komaki R, et al.; Eastern Cooperative Oncology Group. Prolonged survival in patients with resected non-small cell lung cancer and single-level N2 disease. J Thorac Cardiovasc Surg. 2004;128:130–7.
55 Wright G, Manser RL, Byrnes G, Hart D, Campbell DA. Surgery for non-small cell lung cancer: systematic review and meta-analysis of randomised controlled trials. Thorax. 2006;61: 597–603.
No financial support and no other potential conflict of interest relevant to this article was reported.