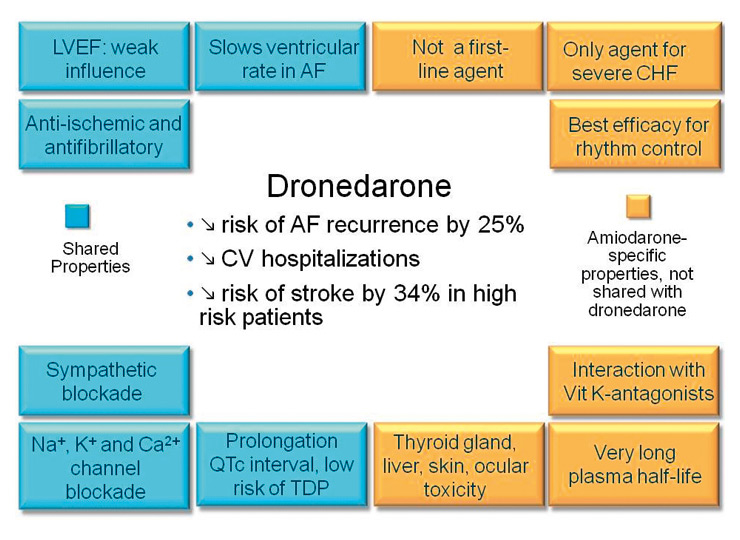
Figure 1
Dronedarone: differences to amiodarone. AF: atrial fibrillation. CHF: congestive heart failure. CV: cardiovascular. LVEF: left ventricular ejection fraction. TDP: torsade de pointes tachycardia.
DOI: https://doi.org/10.4414/smw.2011.13158
Abbreviations
AF atrial fibrillation
AFL atrial flutter
b.i.d. (bis in die) twice daily
CHADS2 congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke/transient ischaemic attack
CHF congestive heart failure
CI confidence interval
CV cardiovascular
FU follow-up period
HR hazard ratio
HT hypertension
INR international normalised ratio
LVEF left ventricular ejection fraction
LVH left ventricular hypertrophy
n.s. not significant
NYHA New York Heart Association
RAAS renin angiotensin aldosterone system
RCT randomised controlled trial
TDP torsade de pointes tachycardia
Atrial fibrillation (AF), the most common arrhythmia, affects approximately 1–1.5% of the population. AF prevalence increases exponentially with age, reaching more than 8% in patients over 80, and is constantly rising due to aging of the population [1–3]. AF is a major cause of morbidity and mortality, increasing the risks of death, congestive heart failure and embolic complications including stroke [1, 4, 5]. Therapy of AF has two major goals: symptom relief and prevention of complications including stroke and heart failure. During the past decade, catheter ablation of AF has evolved rapidly from an experimental to a commonly performed procedure. Catheter ablation is currently recommended in patients with symptomatic AF refractory to antiarrhythmic drugs, whereas it is performed as primary treatment in a minority of patients. Thus, pharmacological therapy remains the mainstay of treatment in many patients. Current antiarrhythmic drug agents for the prevention of AF recurrence carry a substantial risk of adverse effects or have limited efficacy, especially with long-term use. Several molecules are being developed for the management of AF. However, only a few novel agents show promising results with respect to safety issues and efficacy. Dronedarone (Multaq®) is one of these new compounds developed for the treatment of AF. Large clinical studies have demonstrated both the rhythm- and rate-controlling efficacy of dronedarone compared to placebo. In Switzerland, market release and health insurance reimbursement was obtained in February 2010.
Dronedarone, a benzofuran derivative, is structurally similar to amiodarone. Dronedarone was originally developed with the aim of leveraging amiodarone’s antiarrhythmic efficacy and cardiac safety, but with less organ toxicity. The most significant structural changes are the removal of iodine and the addition of a methane sulfonyl group. The removal of iodine results in freedom from amiodarone’s iodine-related organ toxicity (skin, lung, liver and thyroid gland), and the second molecular change decreases lipophilicity, thus shortening the half-life and reducing tissue accumulation (which in turn also lessens the risk of organ toxicity; fig. 1).

Figure 1
Dronedarone: differences to amiodarone. AF: atrial fibrillation. CHF: congestive heart failure. CV: cardiovascular. LVEF: left ventricular ejection fraction. TDP: torsade de pointes tachycardia.
Dronedarone has an absolute bioavailability under fed conditions of only 15–20%, with an extensive first-pass metabolism. The drug undergoes extensive hepatic metabolism, mainly (>84%) by CYP3A4, with a steady-state elimination half-life of approximately 30 hours. The metabolites are excreted primarily in faeces (>80%). One of the metabolites displays similar but less potent pharmacologic activity compared with the parent compound. With twice daily administration dronedarone reaches steady state in 5–7 days without the need for a loading dose.
Like amiodarone it is primarily a class III anti-arrhythmic drug but exhibits properties from all four Vaughan Williams classes: dronedarone blocks transmembrane potassium currents, L-type calcium and sodium currents, and also has alpha- and beta-blocking properties. In the rabbit and guinea pig hearts both amiodarone and dronedarone prolong the ventricular and atrial refractory period, suppress the automaticity of the sino-atrial node due to prolongation of the action potential duration, suppress phase 4 depolarisation as a result of their antiadrenergic effect, and decrease the maximum slope of action potential upstroke, which reflects blocking of fast sodium channel activity of the myocardium [6, 7]. Dronedarone slows the sinus rate and causes a dose-dependent lengthening of the corrected QT interval, and, with escalating doses, causes PR interval prolongation.
All clinical studies with dronedarone were multinational, multicentre, double-blind, placebo-controlled, of parallel design and comparable demographics, except the DIONYSOS trial which was actively-controlled (see table 1 for details).
The DAFNE study (dronedarone atrial fibrillation study after electrical cardioversion) [8] was a prospective, randomised study including patients with persistent AF with a duration of 72 hours to 12 months, designed to establish the optimal therapeutic dose of dronedarone for rhythm control of AF during a period of 6 months. Patients were randomised to dronedarone 400 mg b.i.d., 600 mg b.i.d., 800 mg b.i.d. or placebo (199 out of 270 patients were allocated in the dronedarone arms). If they failed to convert to sinus rhythm within 5–7 days of dronedarone therapy, patients were electrically cardioverted. The primary endpoint was the time to first recurrence of AF during the 6 months of follow-up. Dronedarone 400 mg b.i.d. was found to prolong the period to first recurrence of the arrhythmia significantly (median time: 60 days vs 5 days for placebo, p = 0.001). After 6 months the percentage of patients back in atrial fibrillation was 65% (dronedarone 400 mg b.i.d.) vs 90% (placebo) and is considered clinically relevant. The higher doses (600 and 800 mg b.i.d.) were found to lead to higher discontinuation rates due to (principally gastrointestinal) side effects. Pro-arrhythmia did not occur, and significant corrected QT interval prolongation was only observed in the 800 mg b.i.d. arm. In conclusion, the DAFNE study demonstrated the safety and moderate efficacy of dronedarone 400 mg b.i.d. for the management of persistent AF.
The European trial in atrial fibrillation or flutter patients receiving dronedarone for the maintenance of sinus rhythm (EURIDIS) and the American-Australian-African trial with dronedarone in atrial fibrillation or flutter patients for the maintenance of sinus rhythm (ADONIS) [9] were sister trials which evaluated dronedarone for the maintenance of sinus rhythm in 828 patients who received dronedarone and 409 patients who received placebo, with a follow-up duration of 12 months. At the time of randomisation all patients had been in sinus rhythm for at least one hour and had had at least one ECG-documented episode of AF in the previous three months. In the combined analysis, dronedarone lowered the risk of AF recurrence or atrial flutter recurrence by 25% (64.1% vs 75.2%, hazard ratio [HR] 0.75) within the 12-month study period compared with placebo. Time to AF recurrence – the primary endpoint of the trial – was significantly longer (by a factor of 2.19) in dronedarone-treated patients. Furthermore, heart rate at the first recurrence of AF was lowered significantly with dronedarone. Importantly, dronedarone reduced the incidence of hospitalisation or death at 12 months by 26% compared with placebo (22.8% vs 30.9%, respectively, p = 0.01). In conclusion, both studies showed that dronedarone is of moderate efficacy in decreasing AF recurrence; this effect translates into a significant decrease in hospitalisations.
Dronedarone has also been considered a rate controlling agent in AF. The ERATO trial (efficacy and safety of dronedarone for the control of ventricular rate during atrial fibrillation) [10] enrolled patients with permanent AF of more than 6 months’ duration and had a 6-month follow-up period. Patients had to have resting heart rates of ≥80 beats/min and had already been on conventional rate controlling therapy including beta blockers, digitalis or calcium channel blockers. Out of 200 patients screened 174 were randomised to dronedarone 400 mg b.i.d. (n = 85) or placebo (n = 89) on top of standard therapy. At day 14 the mean 24-hour heart rate of patients in the dronedarone arm was 11.7 beats/min lower than that in the placebo arm (p <0.0001). Dronedarone therapy was also found to reduce mean heart rate during maximal exercise by 24.5 beats/min compared to placebo (p <0.0001), without any negative effect on exercise tolerance. After 4 months the mean 24-hour heart rate of patients in the dronedarone arm was still significantly lower compared to that in the placebo arm, indicating a sustained rate controlling effect of dronedarone. The efficacy of dronedarone therapy also appeared to be additive to the effects of background conventional rate controlling therapy. The rate controlling effect of dronedarone was confirmed in all other trials with dronedarone, with mean heart rate during AF significantly lower in the dronedarone group than in the placebo group. In conclusion, dronedarone is an effective rate controlling agent, both at rest and during exercise, without negative effects on exercise tolerance. In patients who suffer AF recurrence, dronedarone may decrease symptoms and consequently lower hospital admissions and emergency visits.
Time to AF recurrence or prevention of AF recurrence are common endpoints in the studies addressing maintenance of sinus rhythm, but are of somewhat limited clinical interest. Arrhythmia burden and changes in quality of life are more important endpoints but are difficult to assess in patients with AF. AF-related hospitalisations are one of the major factors in significant impairment of patients’ quality of life, represent an increasing socioeconomic burden and are an interesting endpoint. However, it is of first importance to assess the effect of a new antiarrhythmic drug on mortality. The AFFIRM trial (atrial fibrillation follow-up investigation of rhythm management) has shown that rhythm control strategies do not translate into survival benefit [11]. However, there were some limitations in AFFIRM, including the use of diverse strategies to maintain sinus rhythm. A subgroup analysis of AFFIRM actually showed that patients in sinus rhythm had a better prognosis [12] than those in AF, and there is no final agreement as to whether rate-controlled AF is better than sinus rhythm. A trial assessing AF-related hospitalisations and mortality is therefore of first importance. The ATHENA study (a placebo-controlled, double-blind, parallel arm trial to assess the efficacy of dronedarone 400 mg b.i.d. for the prevention of cardiovascular hospitalisation or death from any cause in patients with atrial fibrillation/atrial flutter [AFL]) [13] evaluated 4628 high-risk patients with paroxysmal or persistent AF/AFL. Patients were required to be aged over 75 or, in the presence of at least one other risk factor, over 70. These risk factors included diabetes, hypertension, history of stroke, reduced left ventricular ejection fraction ≤40% or left atrial enlargement. Patients with heart failure NYHA IV were excluded. The follow-up period lasted 21 ± 5 months. Patients’ characteristics and drug treatment were similar in both arms. Dronedarone reduced by 24% the risk of meeting the primary endpoint, the first hospitalisation for cardiovascular events or death (31.9% of patients in the dronedarone group compared with 39.4% in the placebo group, HR 0.76, p <0.0001). This finding was mainly driven by a reduction in the number of hospitalisations for AF, as all-cause mortality was similar in both study arms. The reduction in the need for repeated hospitalisations for cardiovascular events was due to fewer hospital admissions for AF treatment and for therapy of acute coronary syndromes. The latter may be related to some dronedarone-induced effects, such as rate slowing in the case of atrial fibrillation recurrence, a drug-associated reduction in blood pressure, and some vasodilating effect (similar to amiodarone). Among patients in sinus rhythm at baseline, the median time to first recurrence of AF or AFL was significantly prolonged in the active-therapy group: 737 days vs 498 days on placebo (HR 0.75, p <0.001) [14]. Discontinuation of the study drug was similar in both groups, indicating good tolerability of dronedarone.
The effect of dronedarone on stroke prevention was evaluated in a post hoc analysis of the ATHENA trial data [15]. The baseline risk factors for stroke were well balanced between the two groups, and the percentage of patients receiving oral anticoagulants and with therapeutic INR was similar. Dronedarone reduced the risk of stroke by 34%, from 1.8% per year to 1.2% per year (HR 0.66, p = 0.027). The effect of dronedarone was similar whether or not patients were receiving oral anticoagulant therapy, and there was a significantly greater effect of dronedarone in patients with higher CHADS2 scores (calculated by assigning points for the presence of congestive heart failure, hypertension, age 75 years or over, diabetes mellitus and history of stroke or transient ischaemic attack).
In conclusion, dronedarone proved to be effective and safe in reducing cardiovascular outcomes (cardiovascular hospitalisation or death) in patients with AF or AFL. Post hoc analysis of ATHENA showing a reduction of stroke with dronedarone in high-risk patients needs to be confirmed by further studies.
The presence of NYHA III-IV heart failure was one of the exclusion criteria of most of the trials with dronedarone, with the exception of the ATHENA study which included stable NYHA III patients. The ANDROMEDA trial (anti-arrhythmic trial with dronedarone in moderate-to-severe congestive heart failure evaluating morbidity decrease) [16] was designed to evaluate the effect of dronedarone on morbidity in patients with unstable NYHA class II-IV heart failure. 627 patients with symptomatic heart failure and a left ventricular ejection fraction below 35% were enrolled. The study was stopped prematurely after 7 months due to increased mortality in the dronedarone group (25 of 310 patients vs 12 of 317 patients died, HR 2.13, p = 0.03) primarily due to worsening heart failure. A post hoc analysis suggested that increased mortality was associated with the absence or discontinuation of drugs blocking the renin angiotensin aldosterone system (RAAS). Indeed, due to the increase in creatinine levels under dronedarone (see section “safety”, below) RAAS blocking agents were discontinued in some of the patients and absence of adequate RAAS blockade in heart failure patients may account for the increased mortality.
The ATHENA trial [13, 17] included 209 patients with NYHA class II/III heart failure and a left ventricular ejection fraction below 40% at baseline (114 placebo and 95 dronedarone patients). A primary outcome event occurred in 59/114 placebo patients compared with 42/95 dronedarone patients (HR 0.78, 95% CI = 0.52–1.16). 20 of 114 placebo patients and 12/95 dronedarone patients died during the study (HR 0.71, 95% CI = 0.34–1.44). 54 placebo and 42 dronedarone patients were hospitalised for an intermittent episode of NYHA class IV heart failure (HR 0.78, 95% CI = 0.52–1.17). In conclusion, in patients with stable congestive heart failure dronedarone did not increase mortality and showed a reduction of cardiovascular hospitalisation or death similar to the overall population. However, in the light of the ANDROMEDA trial, dronedarone is contraindicated in patients with NYHA class III and IV or in patients with recent exacerbation of heart failure [18].
A comparison of dronedarone and amiodarone was made in the trial titled efficacy and safety of dronedarone vs amiodarone for the maintenance of sinus rhythm in patients with AF (DIONYSOS) [19]. The 504 patients were randomised to amiodarone (loading dose of 600 mg daily for 28 days followed by 200 mg once daily) or dronedarone (400 mg twice daily) and an electrical cardioversion was performed if necessary. The median treatment duration was only 7 months. The incidence of AF recurrence in the dronedarone arm was higher than in the amiodarone arm (36.5% versus 24.3% respectively), whereas adverse events leading to premature drug discontinuation tended to be less frequent in the dronedarone arm. The superiority of amiodarone in preventing AF recurrence is consistent with an indirect metaanalysis based on 4 studies of amiodarone and 4 studies of dronedarone [20]. In conclusion, DIONYSOS had a too short study duration for adequate comparison of dronedarone with amiodarone, bearing in mind that amiodarone side effects usually occur after several months or years of treatment, but it clearly confirms that dronedarone is less effective than amiodarone in maintaining sinus rhythm in patients with a history of AF.
| Table 1: Summary of clinical trials with dronedarone. | ||||
| Trial acronym | Subjects enrolled | Design and endpoints | Treatment and follow-up period | Main outcome |
| DAFNE [8] | 270Persistent AF | RCTDose-ranging study – Time to first AF recurrence | 199 patients on dronedarone 400 mg b.i.d., 600 mg b.i.d., or 800 mg b.i.d. 71 patients on placebo. Electrical cardioversion in patients without conversion to sinus rhythm within first 7 daysFU: 6 months | Median time to first AF recurrence prolonged with dronedarone 400 mg b.i.d. (60 days vs 5.3 days for placebo, p = 0.001). Dose-dependent effect. Frequent gastrointestinal effect with higher dosage |
| EURIDISand ADONIS [9] | 612 in EURIDISand 625 in ADONISAt least 1 episode of AF/AFL within the last 3 months | RCTTime to first AF recurrence | 828 patients who received dronedarone 400 mg b.i.d. and 409 patients who received placeboFU: 12 months | Combined results: symptomatic recurrences in 37.7% and 46% of the patients in the dronedarone and placebo arms, respectively (p <0.001). Median time to first recurrence increased by a factor 2.19 |
| ERATO [10] | 174Persistent AF of >6 months of duration, resting heart rates of ≥80 beats/min on conventional rate controlling agents | RCTHeart rate reduction during 24h ECG, submaximal and maximal exercise testing | Dronedarone 400 mg b.i.d. vs placeboFU: 6 months | Reduction of 11.7 beats/min in mean ventricular rate (24h ECG) and reduction of 25.6 and 27.4 beats/min during submaximal and maximal exercise at day 14 (p <0.0001 for both); this effect was sustained for the duration of trial |
| ANDROMEDA [16] | 627 of 1000 plannedModerate to severe heart failure and reduced left ventricular ejection fraction | RCTComposite endpointof death from any cause or hospitalisationfor heart failure | Dronedarone 400 mg b.i.d. vs placebo 13 months (including additional 6 months after premature discontinuation of study) | Premature termination of trial after 7 months due to excess mortality related to the worsening of heart failure in dronedarone group (HR 2.13; 95% CI 1.07–4.25; p <0.05) |
| ATHENA [13] | 4628paroxysmal/persistent AF and:• age ≥75 years with/ without additional risk factors• age ≥70 years and ≥1 risk factor* | RCTTime to first cardiovascularhospitalisation or death from any cause | Dronedarone 400 mg b.i.d. vs placeboFU: 21 months | First hospitalisation due to cardiovascular events or death reduced by 24% (31.9% in dronedarone group vs 39.4% in placebo group, HR 0.76; 95% CI 0.69–0.84; p <0.001, mainly driven by difference in hospitalisation).All-cause mortality was similar in both study arms.Reduction of cardiovascular death in the dronedarone group (2.7% vs 3.9% in the placebo group, HR 0.71, p = 0.03)Post hoc analysis: reduction of stroke by 34% (from 1.8% per year to 1.2% per year, HR 0.66, p = 0.027). |
| DIONYSOS [19] | 504AF or AFL >72 hoursCardioversion as needed after initiation of study drug | RCTCombined endpoint of recurrence of AF or premature drug discontinuation | Dronedarone 400 mg b.i.d. vs amiodarone 200 mg daily (after a loading dose of 16.8 g over 4 weeks)FU: 7 months | Incidence of AF recurrence or premature drug discontinuation for intolerance or lack of efficacy (composite primary endpoint) was 75.1% and 58.8% in the dronedarone and amiodarone groups respectively (HR 1.59, p <0.0001). Mainly driven by the AF recurrence component (in 36.5% of dronedarone patients and 24.3% of amiodarone patients). Premature drug discontinuation tended to be less frequent with dronedarone (10.4% vs 13.3%, p = n.s.) |
| AF: atrial fibrillation; AFL: atrial flutter; b.i.d.: twice daily; CI: confidence interval; FU: follow-up period; HR: hazard ratio; n.s.: non significant; RCT: prospective randomised double-blind placebo-controlled trial; * risk factors included: hypertension, diabetes, prior stroke / transient ischaemic attack, left atrial diameter ≥50 mm, left ventricular ejection fraction ≤40%. | ||||
The safety evaluation in the targeted AF patient population pooled across all randomised trials (DAFNE, EURIDIS/ADONIS, ERATO and ATHENA excluding DIONYSOS) included 6285 patients of whom 3410 were treated with dronedarone and 2875 with placebo. The extra-cardiac safety problems reported with amiodarone, in particular thyroid disorders, eye and skin disorders, nervous system disorders, liver disorders, respiratory, thoracic and mediastinal disorders, were reported in comparable frequencies with dronedarone and placebo. This argues in favour of a better extra-cardiac safety profile for dronedarone than amiodarone (fig. 1). Accordingly, the main safety endpoint (adverse event or premature study drug discontinuation following an adverse event) in the DIONYSOS study tended to be reached less frequently in the dronedarone group compared to the amiodarone group, and although the study duration was too short, the study suggested a better safety profile for dronedarone, chiefly due to fewer thyroid, neurological, skin and ocular events. The most common side effects of dronedarone are gastrointestinal adverse effects including diarrhoea and nausea, which are more frequent than with amiodarone or with placebo. In ATHENA, nausea was mentioned by 5.3% versus 3.1% of the patients on dronedarone versus placebo respectively, and diarrhoea occurred in 9.7% versus 6.2% of the patients under dronedarone and placebo respectively [13]. In our experience, however, gastrointestinal side effects appear more pronounced than is suggested by the small difference in their incidence under placebo versus dronedarone in randomised trials. Adverse events in heart failure patients were discussed in a separate section above. In patients and healthy individuals dronedarone increased serum creatinine by 10–15% without adverse effects on glomerular filtration rate or renal plasma flow. This is due to partial inhibition of tubular organic cation transporters, which is also seen during amiodarone treatment [21, 22].
With respect to vitamin K antagonists, drug interactions were less frequent with dronedarone than with amiodarone with fewer haemorrhagic events [21, 23]. Notwithstanding, some drug interactions may be relevant [24]. The plasma level of dronedarone may be increased severalfold in patients taking a strong inhibitor of CYP3A4, e.g., ketokonazole, itraconazole, voriconazole, telithromycin, clarithromycin, ciclosporine and ritornavir, and is contraindicated in such situations. In addition, grapefruit juice is contraindicated in patients under dronedarone. On the other hand, during treatment with dronedarone potent CYP 3A4 inducers such as phenobarbital, carbamazepine, phenytoin or St John’s wort are not recommended as they decrease dronedarone exposure up to fivefold [24]. Dronedarone (400 mg b.i.d.) increased simvastatin and simvastatin acid exposure 4- and 2-fold respectively, and increased exposures of lovastatin, atorvastatin, and pravastatin within the same range as simvastatin [24]. However, although interaction with some statins (CYP3A4 substrates) could hypothetically lead to increased risk of statin dose-related adverse events, especially myopathy, the data from the ATHENA trial and the integrated analyses did not confirm this assumption. Dronedarone may increase the plasma levels of some beta-blockers [25] and calcium channel blockers, and dose adjustments of beta-blocker treatment may be needed after introduction of dronedarone. In the ERATO trial the mean increase in digoxin levels in the dronedarone arm compared to placebo was 41.4%, but the number of patients with digoxin levels outside the normal range in the two groups did not differ significantly. Although no signal for hepatic toxicity was detected during phase III trials of dronedarone, post-marketing surveillance has recently identified several cases of liver injury including two cases of severe hepatic failure leading to transplantation amongst approximately 180 000 patients exposed worldwide (Sanofi letter from January 21st, 2011). No definite causal relationship between dronedarone and liver injury has been established, nonetheless, close monitoring of liver function test is recommended (see practical aspects below).
The initial therapy after onset of AF should always include adequate control of the ventricular rate. For long-term treatment, rate control with beta-blocker, calcium-antagonist, and/or digoxin (a combination of 2 agents is often needed) should be considered first, and on the basis of current data the use of dronedarone as a first-line agent against AF is not recommended. Treatment with antiarrhythmic drugs (“rhythm control”) should generally be considered only when symptoms persist despite adequate rate control [26]. In addition, rhythm strategy is reasonable way to improve symptoms, especially in young patients, patients who are highly symptomatic and those with a higher activity level [26]. The recently released European Society of Cardiology 2010 Guidelines for the Management of Atrial Fibrillation [26] emphasise the importance of choosing safer although possibly less efficacious antiarrhythmic agents before selecting more effective but less safe medication (fig. 2). Due to possible proarrhythmic effects, class Ic agents should only be used by physicians with previous experience of these drugs. Despite its efficacy, the use of amiodarone is often limited by its extensive side effect profile and is not currently recommended as a first choice antiarrhythmic agent, except in patients with heart failure or congenital heart disease. Compared with amiodarone, dronedarone has lower efficacy in preventing recurrent AF, but has an attractive safety profile in all patients except those with advanced heart failure (fig. 1). The ATHENA study was the first to demonstrate a reduced rate of death and cardiovascular hospitalisations for antiarrhythmic management of AF. The trial also showed that dronedarone has additional effects beyond simple rhythm control, i.e. beneficial effects of cardiovascular outcomes including a reduction of stroke risk. Dronedarone has not been tested against flecainide, sotalol or propafenone in randomised trials, and thus a direct comparison in terms of antiarrhythmic efficacy between these drugs is not possible. Sotalol and class Ic drugs are prescribed mainly by cardiologists, due to their possible proarrhythmic effects, and class Ic agents are contraindicated in patients with left ventricular hypertrophy, systolic dysfunction of the left ventricle, and other structural heart diseases. These are important limitations on the management of AF patients who are primarily followed by general practitioners and specialists in internal medicine. In contrast, safety data suggest that dronedarone can be prescribed by non-specialists in almost all patient groups, except in patients with NYHA III-IV heart failure or in patients with recent severe heart failure exacerbation (fig. 2).
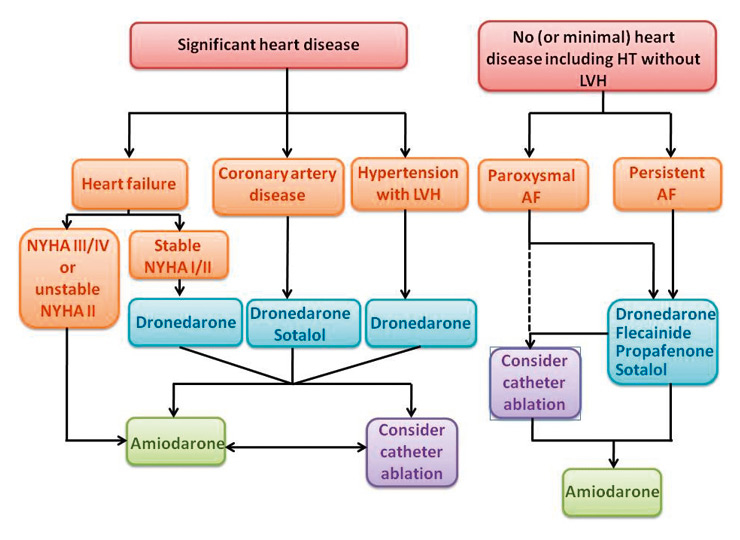
Figure 2
Rhythm control strategy (whenever rate control unsatisfactory). Choice of antiarrhythmic drug and ablation according to underlying pathology. Adapted from [18]. AF: atrial fibrillation; HT: hypertension; LVH: left ventricular hypertrophy; NYHA: New York Heart Association.
When considering treatment with dronedarone, contraindications and possible interactions should be assessed (table 2), and patients should be informed of possible side effects. An ECG should be done before and after one week of treatment. If the Bazett QTc is >500 ms (Bazett QTc = QT/ √ RR), dronedarone should be stopped. Dronedarone causes a slight increase in serum creatinine that does not reflect a change in underlying renal function. Dronedarone reduces creatinine clearance, without affecting glomerular filtration rate, as a result of aspecific partial inhibition of tubular organic-cation transporters. In patients not known to have renal insufficiency or heart failure there is no need to measure serum creatinine. In heart failure patients, inhibitors of the RAAS should be continued without a dose change even if the creatinine increases. Patients should be informed that gastrointestinal side effects are possible and sometimes transient, and that the treatment should be continued for at least one week if possible. Serum liver enzymes and bilirubin should be checked before treatment, monthly during 6 months, and at month 9 and 12 under dronedarone treatment or if the patients experience signs and symptoms consistent with hepatic injury or toxicity. If hepatic injury is found (ALT >3 times the normal value), dronedarone should be promptly discontinued and appropriate treatment should be initiated. Dronedarone should not be restarted in patients without another explanation for the observed liver injury.
| Table 2: Practical aspects in prescribing dronedarone. | |
| Consider possiblecontraindications: | Heart failure NYHA IV or recent heart failure exacerbation |
| Severe renal insufficiency (creatinine >250 µmol/l) | |
| Concomitant use of a strong CYP3A inhibitor (ketoconazole and derivates, cyclosporine, clarithromycin, and ritonavir) | |
| QTc interval ≥500 ms or concomitant use of drugs or herbal products that prolong the QT interval and may induce torsade de pointes | |
| Second- or third-degree atrioventicular block or sick sinus syndrome | |
| Severe hepatic impairment | |
| Consider possibleinteractions: | In patients under amiodarone, a 2-week wash-out is recommended |
| In patients under beta-blocker, consider reducing beta-blocker dosage | |
| In patients under digoxin, check digoxin levels after 1 week | |
| Class Ic anti-arrhythmic drugs are contraindicated | |
| Patients must avoid taking grapefruit | |
| Consider statin dose reduction in patients under high dose statin | |
| Others | Inform patients about possible gastrointestinal side effects, which may be transient |
| In elderly patients, consider starting treatment at a lower dosage | |
| Consider oral anticoagulation according to guidelines (no dose adaptation needed) | |
In the available data there is a discrepancy between the negative impact of AF on prognosis and the outcome of “rate versus rhythm” trials that favour rate control [4, 11, 27, 28]. Even in AFFIRM, patients in sinus rhythm had a better prognosis than those in AF [12]. In addition, many patients perceive a benefit in remaining in sinus rhythm. Consequently, there is a need for rhythm control strategies that are safe, and dronedarone may have an interesting role.
Despite considerable data available from large studies, further clinical trials (including head to head comparison with other conventional antiarrhythmics) are still required to determine the place of dronedarone in the management of AF. In the meantime, clinical experience with dronedarone compared to class Ic antiarrhythmic drugs will be one of the factors influencing drug prescription.
Dronedarone is a newer benzofuran derivative structurally similar to amiodarone except that it lacks the iodine moiety and there is addition of a methane sulfonyl group. The safety of dronedarone was shown in over 3500 patients in randomised trials, and no significant, ocular, or neurological toxic effects have been observed. Nor was there any significant thyroid or skin toxicity. In addition to its benefits for rate and rhythm control, dronedarone reduced hospitalisations in patients with AF and was shown to reduce the risk of stroke in high-risk patients with AF. However, dronedarone only has a moderate effect in preventing AF recurrence, and clearly appears less effective than amiodarone. Discontinue dronedarone if hepatic injury is found (ALT 73). Check serum liver enzymes and bilirubine before treatment and monthly during 6 months under treatment, as well as after 9 and 12 months. Dronedarone should not replace rate control as initial treatment of AF, but can be used by non-specialists whenever a rhythm control strategy is considered. It can be safely used in patients with heart disease, with the exception of patients with heart failure NYHA III and IV or recent heart failure exacerbation. Rare cases of severe liver injury were reported in post-marketing surveillance, thus close monitoring of liver function tests is recommended.
1 Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22(8):983–8.
2 Flegel KM, Shipley MJ, Rose G. Risk of stroke in non-rheumatic atrial fibrillation. Lancet. 1987;1(8532):526–9.
3 Conen D, Osswald S, Albert CM. Epidemiology of atrial fibrillation. Swiss Med Wkly. 2009;139(25–26):346–52.
4 Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98(10):946–52.
5 Stewart S, Hart CL, Hole DJ, McMurray JJ. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am J Med. 2002;113(5):359–64.
6 Sun W, Sarma JS, Singh BN. Electrophysiological effects of dronedarone (SR33589), a noniodinated benzofuran derivative, in the rabbit heart: comparison with amiodarone. Circulation. 1999;100(22):2276–81.
7 Gautier P, Guillemare E, Marion A, Bertrand JP, Tourneur Y, Nisato D. Electrophysiologic characterization of dronedarone in guinea pig ventricular cells. J Cardiovasc Pharmacol. 2003;41(2):191–202.
8 Touboul P, Brugada J, Capucci A, Crijns HJ, Edvardsson N, Hohnloser SH. Dronedarone for prevention of atrial fibrillation: a dose-ranging study. Eur Heart J. 2003;24(16):1481–7.
9 Singh BN, Connolly SJ, Crijns HJ, Roy D, Kowey PR, Capucci A, et al. Dronedarone for maintenance of sinus rhythm in atrial fibrillation or flutter. N Engl J Med. 2007;357(10):987–99.
10 Davy JM, Herold M, Hoglund C, Timmermans A, Alings A, Radzik D, et al. Dronedarone for the control of ventricular rate in permanent atrial fibrillation: the Efficacy and safety of dRonedArone for the cOntrol of ventricular rate during atrial fibrillation (ERATO) study. Am Heart J. 2008;156(3):527 e1–9.
11 Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EB, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347(23):1825–33.
12 Corley SD, Epstein AE, DiMarco JP, Domanski MJ, Geller N, Greene HL, et al. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study. Circulation. 2004;109(12):1509–13.
13 Hohnloser SH, Crijns HJ, van Eickels M, Gaudin C, Page RL, Torp-Pedersen C, et al. Effect of dronedarone on cardiovascular events in atrial fibrillation. N Engl J Med. 2009;360(7):668–78.
14 Page R, Connolly S, Crijns H, Eickels M, Gaudin C, Torp-Petersen C, et al. Abstract 4097: Rhythm- and Rate-Controlling Effects of Dronedarone in Patients with Atrial Fibrillation: Insights From the ATHENA Trial. Circulation. 2008;118: p. S_827.
15 Connolly SJ, Crijns HJ, Torp-Pedersen C, van Eickels M, Gaudin C, Page RL, et al. Analysis of stroke in ATHENA: a placebo-controlled, double-blind, parallel-arm trial to assess the efficacy of dronedarone 400 mg BID for the prevention of cardiovascular hospitalization or death from any cause in patients with atrial fibrillation/atrial flutter. Circulation. 2009;120(13):1174–80.
16 Kober L, Torp-Pedersen C, McMurray JJ, Gotzsche O, Levy S, Crijns H, et al. Increased mortality after dronedarone therapy for severe heart failure. N Engl J Med. 2008;358(25):678–87.
17 Hohnloser SH, Crijns HJ, van Eickels M, Gaudin C, Page RL, Torp-Pedersen C, et al. Dronedarone in patients with congestive heart failure: insights from ATHENA. Eur Heart J. 2010;31(14):1717–21.
18 Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, et al. Guidelines for the management of atrial fibrillation: The Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J.
19 Le Heuzey JY, De Ferrari GM, Radzik D, Santini M, Zhu J, Davy JM. A short-term, randomized, double-blind, parallel-group study to evaluate the efficacy and safety of dronedarone versus amiodarone in patients with persistent atrial fibrillation: the DIONYSOS study. J Cardiovasc Electrophysiol. 21(6):597–605.
20 Piccini JP, Hasselblad V, Peterson ED, Washam JB, Califf RM, Kong DF. Comparative efficacy of dronedarone and amiodarone for the maintenance of sinus rhythm in patients with atrial fibrillation. J Am Coll Cardiol. 2009;54(12):1089–95.
21 Le Heuzey JY, De Ferrari GM, Radzik D, Santini M, Zhu J, Davy JM. A short-term, randomized, double-blind, parallel-group study to evaluate the efficacy and safety of dronedarone versus amiodarone in patients with persistent atrial fibrillation: the DIONYSOS study. J Cardiovasc Electrophysiol. 2010;21(6):597–605.
22 Tschuppert Y, Buclin T, Rothuizen LE, Decosterd LA, Galleyrand J, Gaud C, et al. Effect of dronedarone on renal function in healthy subjects. Br J Clin Pharmacol. 2007;64(6):785–91.
23 Shirolkar SC, Fiuzat M, Becker RC. Dronedarone and vitamin K antagonists: a review of drug-drug interactions. Am Heart J. 160(4):577–82.
24 http://www.fda.gov/ohrms/dockets/ac/09/briefing/2009-4417b1-02-Sanofi_Aventis.pdf. last access dec 14 2010.
25 Damy T, Pousset F, Caplain H, Hulot JS, Lechat, P. Pharmacokinetic and pharmacodynamic interactions between metoprolol and dronedarone in extensive and poor CYP2D6 metabolizer healthy subjects. Fundam Clin Pharmacol. 2004;18(1):113–23.
26 Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J. 2010;31(19):2369–429.
27 Jouven X, Desnos M, Guerot C, Ducimetiere P. Idiopathic atrial fibrillation as a risk factor for mortality. The Paris Prospective Study I. Eur Heart J. 1999;20(12):896–9.
28 Dries DL, Exner DV, Gersh BJ, Domanski MJ, Waclawiw MA, Stevenson LW. Atrial fibrillation is associated with an increased risk for mortality and heart failure progression in patients with asymptomatic and symptomatic left ventricular systolic dysfunction: a retrospective analysis of the SOLVD trials. Studies of Left Ventricular Dysfunction. J Am Coll Cardiol. 1998;32(3):695–703.
ED received speaker fees from Sanofi-Aventis and is a principal investigator and the Swiss coordinator for a Sanofi-Aventis-sponsored study.