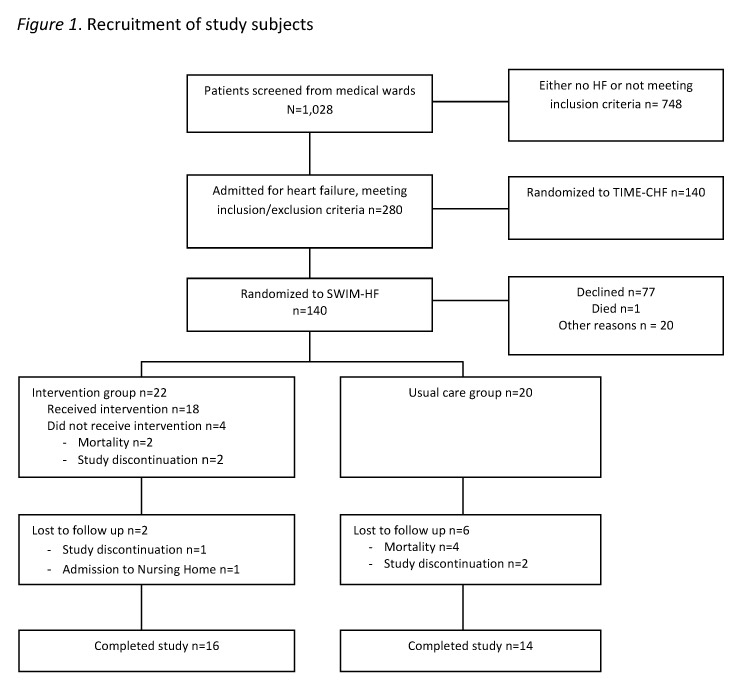
Figure 1
Recruitment of study subjects.
DOI: https://doi.org/10.4414/smw.2011.13171
A randomised controlled trial study of an outpatient inter-professional management programme for heart failure patients in Switzerland
As the incidence and prevalence of cardiovascular diseases are steadily increasing globally, new and more efficient care for these patients is needed. Heart failure (HF), the end stage condition for at least 10% of all patients with heart disease, presents a particular challenge for healthcare systems as patients with HF are primarily aged, have a high symptom burden and are susceptible to frequent, acute decompensation, subsequent re-admissions to hospital and have a high mortality [1]. About 2–3% of the population older than 20 years has HF [2, 3]. Median survival following a first hospitalisation for HF is 2.33 years in men and 1.79 years in women [4, 5]. Annual costs incurred by HF are estimated to represent approximately 2% of the total healthcare budget [6]. Hospital re-admission rates after an admission for HF are high, as 9% will be admitted within 1 week, 23% of the patients will be admitted within a month and 41% within a year following a HF hospitalisation. This challenges healthcare systems to institute improvements in care during hospitalisation, during the transition from hospital to home, and during post-hospitalisation follow-up [7, 8]. International and national guidelines recommend close observation and follow-up for early identification of changes in signs and symptoms to prevent clinical deterioration and subsequent emergency treatment and/or hospitalisation. Several systematic reviews and meta-analyses have shown disease management programmes for HF patients to have positive effects on re-hospitalisation, mortality, costs and quality of life [9–14]. These programmes, however, have been very heterogeneous and lack a shared definition of what elements are necessary for improved outcomes. In addition, the healthcare system has a significant influence not only on the outcome of such programmes but also on which elements of the programmes are most important and most effective. Very few programmes for chronic disease management exist at present in Switzerland, and only one involves patients with HF, although this programme has not been tested. In addition, a threatened shortage of primary care physicians, at least in the rural areas, will increase the need to develop programmes to help patients manage their conditions [15]. Guidelines recommend that nurses specialising in HF can provide the necessary education regarding medication, diet, exercise, weight monitoring and what to do in case of increasing symptoms, and supervision for patients and families to improve compliance with treatment recommendations [3, 16, 17]. As the success of disease management programmes depends heavily on the type and function of the system, there is a need to test such programmes in Switzerland before recommending their implementation. The Swiss Interdisciplinary Management Programme for Heart Failure (SWIM-HF) was designed as a randomised controlled trial (RCT) to test an outpatient educational and supportive programme for patients following a hospitalisation for HF in the Swiss healthcare setting. The SWIM-HF model includes an inter-professional team of HF nurses, cardiologists and primary care physicians. Primary hypotheses of the study were that study groups would differ in view of hospital re-admission rates and mortality. As secondary hypotheses, differences were expected in quality of life (QoL) and length of stay. Due to a prolonged recruiting time, however, the research team made the decision to stop recruitment after the first 42 patients and to evaluate the feasibility of the intervention and to make recommendations for future HF programmes within the Swiss healthcare system.
The SWIM-HF study reports on the first 42 patients recruited in a planned RCT, conducted at the University Hospital of Basel, Switzerland. According to the study proposal, 300 patients would have been needed to achieve 80% power for addressing the first study aim1. The convenience sample consisted of adult patients hospitalised with decompensated HF (NYHA II–IV), irrespective of left-ventricular ejection fraction, and a brain natriuretic peptide (BNP) ≥100 pg/ml. Additional inclusion criteria were: a history of dyspnea, increased fatigue or weakness, the ability to speak German and to comprehend a telephone conversation, and discharge to a home setting. Excluded were those who had had an acute myocardial infarction within 8 weeks prior to inclusion (Creatine Kinase (CK) >2x normal), severe myocardial or valvular obstructive disease or uncontrolled angina pectoris (Canadian Cardiovascular Society Functional Classification of Angina (CCS) >3), those who had co-morbid conditions compromising prognosis (life expectancy of less than 12 months), those who had planned (except heart transplantation) or had had previous cardiac surgery within 3 months, those who were on dialysis, had unstable psychiatric disorders or substance abuse, had cognitive impairment (Mini-Mental State Examination score <24) [18], or those who were enrolled in another study, or refused to sign an informed consent. The inclusion and exclusion criteria was assessed and adapted following recruitment of the first 10 patients due to the criteria being too restrictive (12 months post cardiac surgery was changed to 3 months), not adequately defined (e.g., CCS score was added to define angina), and the target population was broadened to include both patients with systolic dysfunction and patients with HF and a normal systolic function. The time of randomisation was changed from hospital discharge to discharge to home (either after hospitalisation or stationary rehabilitation). The study conforms to the Declaration of Helsinki and was approved by the Ethics Committee of Basel/Basel and, Switzerland.
1 The description of the calculation of the sample size can be obtained from the corresponding author.
Demographic and clinical background variables were collected from medical records. Demographic variables included age, gender, professional status, marital status and living situation (living alone/together). Clinical variables included risk factors for heart disease, co-morbidities as assessed by the Charlson Co-morbidity Index [19], laboratory values and left-ventricular ejection fraction as determined by echocardiography or ventricular radionuclide test. As additional background variables, we measured depressive symptomatology using the Geriatric Depression Scale and functional status with the Specific Activity Scale [20].
Primary study outcomes were mortality (all causes) and re-admission (HF related and all causes). Secondary outcomes were quality of life and length of stay. Hospitalisation due to HF was defined as any unplanned overnight admission to the hospital for treatment of HF or HF symptoms, or decompensation of HF during admission. All-cause hospitalisation included non-HF cardiac and non-cardiac reasons for admission. Information on hospitalisation was collected from the medical records. Death certificates were obtained from the Department of Birth and Death Records. Health-related quality of life (QoL) was measured using two instruments: 1) a general QoL instrument, the EuroQol-5D (EQ-5D) [21], and 2) a disease-specific instrument, the Minnesota Living with Heart Failure Questionnaire (MLHF) [22].
All patients received similar care during hospitalisation. This consisted of the normal medical and nursing care provided by hospital staff. In addition, all study patients were examined by the study HF-cardiologist who recommended lifestyle modifications to the patients and made suggestions for optimal medical management to the patient’s primary care physician. All patients were given a HF education booklet published by the Swiss Heart Foundation. These efforts were made to standardise usual care, to remove unnecessary variability in care provided to the control patients. Following hospitalisation, medical care was provided by the primary care physician (usual care group protocol).
Intervention group: In addition to the care described above, once patients were discharged to home, the intervention began as an ambulatory care programme. Intervention patients received one home visit by a specialised HF nurse approximately 1 week after returning home after discharge from either hospitalisation or rehabilitation, followed by 17 telephone calls in decreasing intervals over the next 12 months. The home visit consisted of a physical, psychosocial and environmental assessment, the provision of educational, behavioural, and supportive care to build self-care abilities, and individualised patient goal-setting to increase self-efficacy [23]. All intervention group patients were given a special kit published by the Swiss Heart Foundation that included in-depth explanations of HF and self-care procedures. The kit was explained to the patients and used to support subsequent education. Following the home visit, an individualised nursing care plan was developed that included the patient-identified goals and the goals that the nurse identified based on the results of the assessments. This plan was then discussed with the primary care physician to elicit his/her support and to coordinate and prioritise goals. Follow up telephone calls included discussions of questions or problems the patients had due to their HF, identification of signs and symptoms signifying possible decompensation of HF, review of current medications, reinforcement of self-care activities and setting new goals. In learning to self-manage signs and symptoms, patients were assisted in identifying situations where consultation with their physician was necessary. Primary care physicians were contacted by the HF nurse regarding questions or concerns that arose concerning their patient’s condition throughout the intervention period. The intervention included all domains of disease management recommended by the American Heart Association [24] thus fulfilling international guidelines (see table 1) .
| Table 1: Description of intervention programme according to the AHA Taxonomy. | |
| Patient population | Adults (>18 years) admitted to hospital with acute HF decompensation (systolic or diastolic) and discharged to home; excluded if acute MI had occurred within 8 weeks of index hospitalisation (CK >2x normal), severe myocardial or obstructive valvular disease, uncontrolled angina pectoris, co-morbid conditions compromising life expectancy (<12 months), planned or previous heart surgery within 3 months, dialysis, unstable psychiatric disorders, substance abuse, cognitive impairment (MMSE ≤23), refusal to sign informed consent |
| Intervention recipient | Patient |
| Intervention content | Patient education and support with self-care including recognition of warning signs of deterioration; advice on diet, fluids and sodium management; importance of daily weighing; identifying actions to take in case of increasing symptoms, individualised care plans, communication with primary care physician |
| Delivery personnel | HF-nurse specialists |
| Method of communication | HF-educational Booklet & Kit (Swiss Heart Foundation), face to face at home, personalised telephone follow-up calls |
| Intensity and complexity | Intervention duration 12 months, beginning with home visit, followed by 17 structured telephone calls (weekly x 4, bi-monthly x 4, monthly x 6) plus additional calls when needed; 1 call with primary care physician following home visit, additional calls when needed; Nurse consultation with study internist, study cardiologist or dietician when needed |
| Environment | Out-patient, in patients’ homes |
| Clinical Outcomes | All-cause hospitalisation, HF-hospitalisation, mortality, quality of life, length of stay |
During the study’s 20-month enrolment period (July 2003-February 2005), eligible patients were identified through bi-weekly screening of all patients admitted to the internal medicine departments of a university hospital due to dyspnea. A concurrent HF medical intervention study, TIME-CHF [25], was conducted during the same time period and at the same institution. The two studies shared screening responsibilities and patients eligible for both studies were randomly assigned 1:1 to be approached by one or the other study. The study nurse discussed participation of potential patients with their treating physicians. When the patient’s condition had stabilised, the study nurse visited the patient, explained the study and obtained written informed consent from the patient. Baseline questionnaires were completed either by the patient alone or by interview with the study nurse. Once the patient was discharged home, either directly from hospital or following an inpatient rehabilitation programme, the patient was randomised by an independent centre, according to a computer generated list (blocked, variable block size). The study nurse called the randomisation centre (Lausanne), stated the chronological study recruitment number and was given the group assignment. Patients were notified of their group assignment by telephone. The primary care physicians received written notification of their patient’s participation and group assignment, along with the cardiologist’s recommendations for pharmacological treatment. Follow up outcome data were collected at 3, 6, 9 and 12 months. Patients were sent the follow-up study questionnaires with a pre-addressed, stamped reply envelope and an appointment for a follow-up telephone interview with a special data collector blinded to group assignments.
Questionnaires were entered into the database by research assistants, blinded to group assignment, and checked by random sample by the data analyst. Mortality data were obtained from the Department of Birth and Death Records and re-admission data were obtained from hospital records, examined and adjudicated by a senior researcher blinded to group assignment, and entered into the database by the study coordinator. Any incongruence in data was double checked using the original clinical or research records. Patients, care-givers, primary care physicians and the intervention nurses were not blinded to group assignment.
Detailed descriptive analyses of all data were performed prior to testing the study hypotheses. Percentages, means, standard deviations, medians and inter quartile ranges were used as appropriate. Kaplan Meier survival analysis was planned to compare time to mortality and time to re-admission. However, since less patients were included than was initially planned, preventing calculation of reliable estimates, only a graphical representation of the survival curves are given, without formal testing. The lower sample also necessitated a different approach regarding the evaluation of quality of life. Instead of performing an endpoint evaluation, we used this outcome’s longitudinal assessments in a repeated-measure type analysis. A mixed regression testing 1) group assignment, 2) time and 3) their interaction, allowed comparison of whether trends in quality of life differed between both study groups. Analyses were intention to treat-based and performed in SAS 9.1.
Out of 1028 patients who were screened, 280 presented with decompensated HF and met the study inclusion criteria. Following 1:1 randomisation (using computer generated randomisation) for assignment to TIME-CHF or SWIM-HF, 140 patients were contacted to participate in the study (fig. 1). Agreement for participation was given by 42 patients. Some of the reasons given for refusing to participate included feeling too ill (8), feeling that a commitment of one year would be too long (5), not interested in being in a study (11), concern that their primary care physicians would be upset (2), and not meeting the inclusion criteria (3). Twenty patients were randomly allocated to the usual care group and twenty-two patients were randomised to the intervention group.

Figure 1
Recruitment of study subjects.
In the usual care group, four died and two were lost to follow up, resulting in a sample of 14 usual care group patients who completed the follow up. In the intervention group, 16 patients completed the full 12 months of the study intervention. Two patients withdrew participation prior to the home visit; both had been in extensive in-patient rehabilitation programmes and planned to continue in outpatient rehabilitation programmes. Two patients died prior to the home visit. One patient was censored after 3.5 months because of admission to long-term care, and one patient dropped out after 9 months due to increasing medical problems (hematologic) (see fig. 1). A total of 8 patients required extra phone calls, ranging from 1 to 8.
Baseline characteristics of the sample can be found in table 2. The average age was 77 years. The majority were male patients and were married. The prevalence of multi-morbidity was high with a mean number of co-morbidities of three. The mean Charlson Co-morbidity Index was eight [19]; 26% had diabetes and 64% had mild-moderate depressive symptoms.
| Table 2: Sample characteristics. | |||
| Total sample (n = 42) | Intervention group (n = 22) | Usual care group (n = 20) | |
| Demographic data | Mean ± std | Mean ± std | Mean ± std |
| Age in years | 77.0 ± 6.5 | 76.7 ± 7.1 | 77.6 ± 6.0 |
| % (n) | % (n) | % (n) | |
| Gender | |||
| Female | 38.1 (16) | 40.9 (9) | 35.0 (7) |
| Male | 61.9 (26) | 59.1 (13) | 65.0 (13) |
| Marital status | |||
| Single | 7.1 (3) | 9.1 (2) | 5.0 (1) |
| Married | 57.1 (24) | 54.5 (12) | 60.0 (12) |
| Divorced | 11.9 (5) | 9.1 (2) | 15.0 (3) |
| Widowed | 23.8 (10) | 27.3 (6) | 20.0 (4) |
| Risk factors and medical history | Mean ± std | Mean ± std | Mean ± std |
| Blood pressure | |||
| Systolic | 126.7 ± 21.1 | 124.1 ±19.9 | 129.7 ±22.5 |
| Diastolic | 71.9 ± 10.7 | 72.3 ±11.8 | 71.6 ±9.5 |
| BMI (n = 40) | 25.9 ± 4.9 | 26.3 ± 4.7 | 25.5 ± 5.1 |
| Charlson Co-morbidity Index – with age § | 7.9 ± 2.2 | 7.2 ± 1.7 | 8.5 ± 2.6 |
| Charlson Co-morbidity Index – without age | 3.7 ± 2.3 | 3.1 ± 1.8 | 4.3 ± 2.6 |
| Haemoglobin (mg/l) | 124.5 ± 14.01 | 123.3 ±12.8 | 125.8 ± 15.6 |
| Haematocrit (l/l) | 0.37 ± 0.04 | 0.36 ±0.04 | 0.38 ± 0.04 |
| Cholesterol LDL (mmol/l) | 2.28 ± 0.7 | 2.12 ±0.7 | 2.46 ± 0.7 |
| Cholesterol HDL (mmol/l) | 1.24 ± 0.5 | 1.15 ±0.4 | 1.33 ± 0.5 |
| Median (P25–P75) | Median (P25–P75) | Median (P25–P75) | |
| BNP pg/ml (n = 35) | 671 (306–1300) | 671 (270–1600) | 620 (314–1150) |
| Creatinine mg/dl | 108 (76–155) | 104 (75–155) | 112 (83–152) |
| Urea (mg/dl) | 10.2 (6.7–15) | 10.2 (6.7–13.2) | 10.2 (6.9–18.9) |
| Left ventricular ejection fraction % (n = 41) | 44 (30–60) | 45 (30–60) | 42 (28–57.5) |
| % (n) | % (n) | % (n) | |
| Left ventricular ejection fraction | |||
| <45% | 51.2 (21) | 52.4 (10) | 55.0 (11) |
| ≥45% | 48.8 (20) | 47.6 (11) | 45.0 (9) |
| Smoking | |||
| Currently not smoking | 74.1 (35) | 90.9 (20) | 75.0 (15) |
| Ever smoked | 64.3 (27) | 63.6 (14) | 65.0 (13) |
| Never smoked | 35.7 (15) | 36.4 (8) | 35.0 (7) |
| Diabetes mellitus | |||
| Yes | 26.2 (11) | 31.8 (7) | 20.0 (4) |
| No | 73.8 (31) | 68.2 (15) | 80.0 (16) |
| Specific Activity Scale (*) | |||
| Class I | 16.7 (6) | 15.0 (3) | 18.7 (3) |
| Class II | 44.4 (16) | 35.0 (7) | 56.3 (9) |
| Class III | 38.9 (14) | 50.0 (10) | 25.0 (4) |
| Geriatric Depression Scale | |||
| No depressive state (1–5) | 35.7 (15) | 40.9 (9) | 30.0 (6) |
| Mild depressive state (6–10) | 61.9 (26) | 54.5 (12) | 70.0 (14) |
| Severe depressive state (11–15) | 2.4 (1) | 4.5 (1) | 0.0 (0) |
| Missing data not reported, thus totals may not equal n = 42; § For each decade that a patient was over 40 years, one point was added to the index; (*) Physical Activity Scale defined by metabolic units: Class I = 10 Mets, Class II = 7 to 10 Mets, Class III = 4 to 7 Mets, and Class IV = 0 to 4 Mets. 1 MET = metabolic rate of energy consumption during quiet sitting. | |||
Mortality and re-admission: A total of 22 (52%) patients were either hospitalised or died over the 12 months. Figures 2, 3 and table 3 present mortality data. Four patients died in the usual care group (n = 4) and two in the intervention group (n = 2). In contrast, re-admissions were relatively frequent (n = 16; 38%), but more prevalent in the intervention group compared to the usual care group, both in terms of the number of patients hospitalised at least once (n = 10 vs n = 6), and the number of multiple hospitalisations in total (table 3). Our impression that patients with multiple admissions (n = 7) tended to be re-admitted soon after a discharge was substantiated by a strong negative correlation calculated between time to first re-admission and the number of re-hospitalisations (Spearman’s rho = –0.66). Very few patients were hospitalised due to decompensated HF: two were hospitalised from the usual care group (whose combined total length of stay was 61 days) and one from the intervention group (length of stay 79 days). The patient from the intervention group was hospitalised five times due to spontaneous acute decompensation, the reason for which could not be determined.
| Table 3: Prevalence of adverse events (mortality, hospital re-admissions). | ||
| Intervention group (n = 22) | Usual care group (n = 20) | |
| % (n) | % (n) | |
| Number of deaths | 9 (2) | 20 (4) |
| Number of re-hospitalisations | ||
| At least once | 45 (10) | 30 (6) |
| 1 re-admission | 27 (6) | 20 (4) |
| 2 re-admissions | 9 (2) | 5 (1) |
| 4 re-admissions | 0 (0) | 5 (1) |
| 5 re-admissions | 5 (1) | 0 (0) |
| 6 re-admissions | 5 (1) | 0 (0) |
| Number of HF-related re-hospitalisations | ||
| At least once | 5 (1) | 10 (2) |
| 1 re-admission | 0 (0) | 10 (2) |
| 5 re-admissions | 5 (1) | 0 (0) |
| Number of Cardiac non-HF re-hospitalisations | ||
| At least once | 14 (3) | 10 (2) |
| 1 re-admission | 9 (2) | 10 (2) |
| 2 re-admissions | 5 (1) | 0 (0) |
Quality of Life: figures 4 and 5 show how quality of life scores evolved from start to end of the study with each of the QoL instruments used. For both the EQ-5D and the MLHF, a switch in scores after the intervention could be observed, with the intervention group changing from lower to higher QoL. The VAS did not show this trend. Imposing linear time lines and testing for interactions between group and time showed no significant interaction for the MLHF (p = 0.11), although significant for the EQ-5D (p = 0.006; table 4).
| Table 4: Results of the random intercept regression analysis (n = 42). | ||||||
| Instrument | Parameter | Estimate | 95% CI | DF | t-value | p-value |
| Minnesota | Intercept | 38.6 | 19.3–57.9 | 40 | 4.04 | 0.0002 |
| living with HF | Time | –4.80 | –9.67–0.07 | 111 | –1.95 | 0.05 |
| Group | –4.45 | –16.6–7.65 | 111 | –0.73 | 0.47 | |
| Time*group | 2.50 | –0.61–5.61 | 111 | 1.60 | 0.11 | |
| EuroQol | Intercept | 1.70 | 1.32–2.08 | 40 | 9.11 | <.0001 |
| EQ-5D | Time | –0.09 | –0.18–0.01 | 110 | –2.26 | 0.03 |
| Group | –0.21 | –0.45–0.02 | 110 | –1.78 | 0.08 | |
| Time*group | 0.07 | 0.02–0.13 | 110 | 32.8 | 0.006 | |
| EuroQol | Intercept | 65.3 | 45.7–84.9 | 40 | 6.74 | <.0001 |
| VAS | Time | –0.91 | –5.50–3.70 | 109 | –0.39 | 0.70 |
| Group | 1.66 | –10.6–14.0 | 109 | 0.27 | 0.79 | |
| Time*group | 0.07 | –2.87–3.01 | 109 | 0.05 | 0.96 | |
The SWIM-HF study showed that an inter-professional behavioural and educational programme for patients following a hospital admission for acute HF is feasible and may be applied to HF patients in Switzerland, which may result in positive effects on QoL.
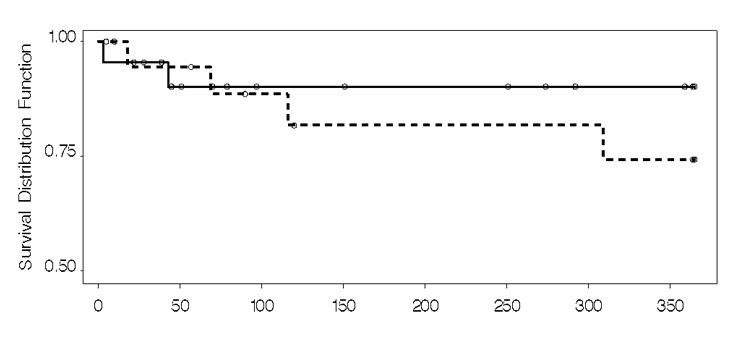
Figure 2
Kaplan Meier analysis: mortality.
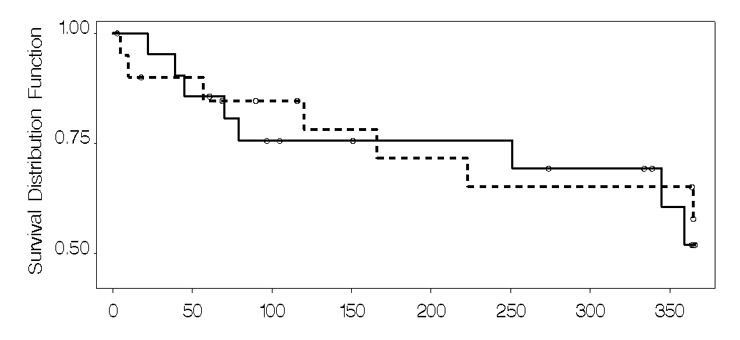
Figure 3
Kaplan Meier analysis: readmission.
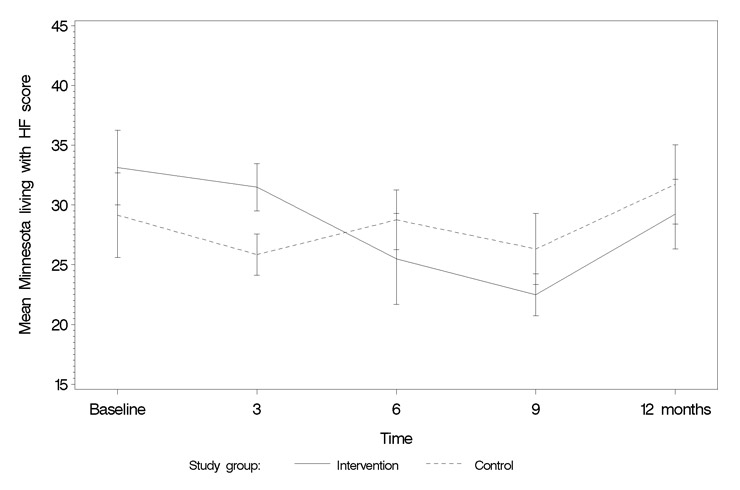
Figure 4
Minnesota Living with Heart Failure Questionnaire.
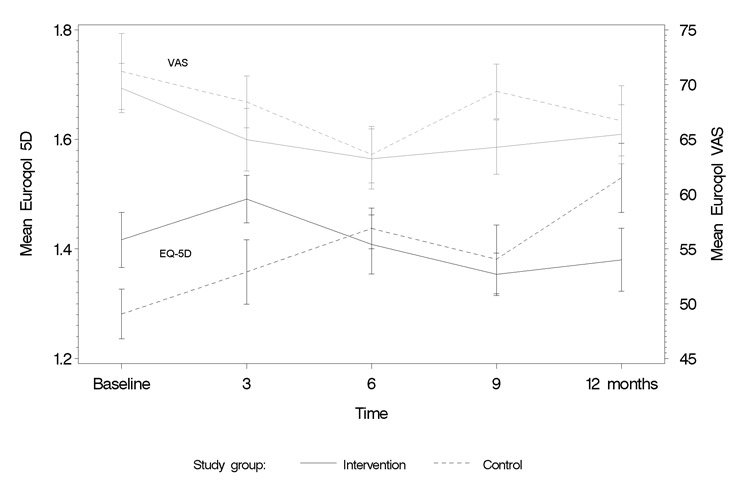
Figure 5
Euroqol (EQ-5D score and VAS score).
Several HF disease management programmes have shown benefits for patients in terms of re-admission, quality of life, and in some cases mortality [9–11], but this type of programme had not previously been tested in Switzerland. In addition to the efficacy of an inter-professional HF disease management programme in Switzerland, questions have been previously raised as to whether such a programme would be accepted by primary care physicians, whether patients would agree to participate, and whether the intervention, or self-care procedures, would be too burdensome for these very ill patients. This analysis of 42 patients of the SWIM-HF trial attempted to address these issues.
As most programmes published to date include both an optimisation of medical management in addition to education and support of patients’ self-care skills, the value of the intervention itself in contributing to a positive outcome is difficult to determine. The SWIM-HF intervention was similar to the study by Krumholz et al. [26] and the COACH study by Jaarsma et al. [27] where patients received an educational and supportive intervention while allowing medical care to be the same for all patients [26, 27]. In SWIM-HF, all patients were examined during hospitalisation by a cardiologist specialised in HF, patients were discharged on optimal medication treatment, and further recommendations for the medical treatment were sent to the primary care physicians of all recruited patients. The study cardiologist was available to the intervention nurse and the primary care physicians for consultation, if needed, but did not have any further follow-up appointments with the patients (as opposed to the COACH study where the study cardiologist continued to see all patients on a frequent basis [27]).
Compared to other European studies of HF disease management, SWIM-HF initially had a high refusal to participate rate (55%) (see fig. 1) [27]. However, once patients agreed to participate in SWIM-HF, the majority remained in the study the full 12 months (or until death). Only five patients (12%) elected to withdraw participation following randomisation highlighting a high acceptance of the SWIM-HF programme. In addition, primary care physicians were cooperative and made themselves available to the intervention nurses to discuss pertinent issues and strategies regarding their patient’s care. This was an important finding in a country where interdisciplinary collaboration between nurses and primary care physicians as part of disease management programmes is only emerging and where advanced nursing practice is a relatively new entity [28].
The study population in the SWIM-HF study was similar to those in other reported HF disease management trials in terms of age, gender and co-morbidities, but differed in that it included patients with both reduced and preserved left ventricular function [8, 26, 27, 29]. Over 12 months, 38% of the patients in SWIM-HF had at least one all-cause admission, and 52% experienced an all-cause admission or death. This number is lower than patients in Krumholz’s study where 57% (Intervention) and 82% (control) were hospitalised or died [26]. Patients in the COACH and TIME-CHF studies were followed over 18 months, and experienced 55% and 60% all-cause re-admissions, respectively [25, 27]. Cardiovascular admissions or death for SWIM-HF patients (33%) were also lower than expected as compared to a recently published, large (n = 1528), multinational population study without a disease management programme in which 43.2% of the patients either died or had cardiovascular hospitalisations over 12 months. Similar to the SWIM-HF results, this study found that 4.5% of the patients died in the first week following hospitalisation [8]. Case fatality data from Switzerland show that twice the number of patients die from HF outside of the hospital compared to within hospital, emphasising the need to improve ambulatory care and support for this vulnerable group [30].
The overall 12 month survival of the SWIM-HF patients was high; 91% in the intervention group and 80% in the usual care group survived compared to 80% and 72% in the Krumholz study [26]. The concurrent TIME-CHF study showed equally high 18 month survival rates of 84% and 78% when compared to those in the COACH study (75% and 71%) [27, 29]. Our lower mortality and re-hospitalisation rate suggests that patients were well treated, irrespective of group allocation. Furthermore, the study design might have added to this effect by calling patients every three months and asking about symptoms, doctor’s visits, hospitalisations and adherence to medications, salt and fluid restrictions, daily weighing and exercise.
Recent meta-analyses of disease management programmes found that most of the HF disease management programmes decrease hospitalisation, both HF and all-cause, and decrease the days in hospital per hospitalisation [9, 10]. One SWIM-HF intervention patient experienced multiple admissions for left ventricular decompensation. Apart from this patient, very few patients in the SWIM-HF study were hospitalised due to HF specifically, but did experience other cardiac hospitalisations such as for arrhythmias or cardiac surgery, and hospitalisations for non-cardiac reasons. Multiple hospitalisations seemed to be clustered within one time period and for the same diagnosis (e.g., GI-bleeding). Thus, the effect of a specific disease management programme might be diluted due to the high burden of co-morbidities as seen in our study population. It remains to be determined if a disease management programme that addresses all major diseases of an individual patient would be more efficient. Indeed, in such complex cases, personalised case management may help tailor interventions that are more appropriate for a given individual.
Disease management programmes for HF have shown that quality of life, in general, tends to improve over time following a HF-hospitalisation, but studies adding education in self-care show significantly greater improvements in QoL [9, 12]. As shown in the DIAL study, adding a telephone follow-up intervention to optimal medical treatment for ambulatory patients with stable heart failure results in a significant improvement in QoL of 4.4 points on MLHF (p = 0.001) [31, 32]. SWIM-HF showed a similar improvement in QoL (improvement of MLHF score = 4.2 points) and a significant improvement in the EuroQol score.
Due to the small numbers recruited for the study and the fact that it was conducted in only one section of the German-speaking region of Switzerland, it is difficult to generalise the findings for all HF patients in Switzerland. However, the data indicates that the intervention is feasible in the setting in which the study was conducted. Some patients did attend in-patient rehabilitation programmes following discharge, which may have influenced the course of their illness once they returned home. Additionally, special attention was given to all patients by the HF cardiologist which included specific individualised suggestions for treatment sent to the primary care physicians. This is not usually done.
To improve generalisability, future studies would optimally be positioned in a multicenter design comprising of several different regions of Switzerland. Moreover, it would be advisable to include “at home” heart failure patients in addition to hospitalised patients. Admittedly, recruitment was challenging in the current study and even greater efforts will need to be invested to involve all stakeholders (i.e. patient organisations, family physicians, home health nurses, cardiologists and internists, as well as organisations on the policy level) and to motivate them to participate and support this kind of study.As Switzerland increasingly realises the need for new care models in treating patients with chronic conditions, it is only a broad, well-embedded, and collaborative approach that will allow the testing and implementation of heart failure management interventions.
As has been shown in many European countries, this study design of an inter-professional programme with HF specialist nursing support for patients, also including primary care physicians, is feasible in Switzerland and shows promise in terms of improving quality of life. Due to the small number of patients included, no conclusions on efficacy can be made. However, evidence from similar trials in other countries supports the use of disease management for HF patients in Switzerland. Given the difficulties with recruitment, awareness for the importance of interdisciplinary management of HF patients must be improved for both patients and physicians. Such a model of interdisciplinary care requires open communication between specialised HF nurses, general practitioners and cardiologists, and all partners of such a programme must be included in developing and monitoring the programme. A further multicenter study from all the language regions of Switzerland would be advisable to test if such an intervention may improve outcomes in patients with HF.
We would like to thank the following people: Joan McDowell (deceased Sept 2009) for her advice, help and support, beginning with writing the proposal for the study and her guidance throughout the study; Germaine Eze for her support in conducting the study in Basel; Lyn Lindpainter for the medical support for the intervention; Stefano Muzarelli for help with screening; Brigitte Jenni, Nicole Zigan, Evelyne Haegi-Rieder, Laura Bogert and Cornelia Blauer for their support with the data; Heidi Petry and Kim Moody for their help in finalising the proposal and starting the study and Sandra Engberg, Peter Buser, Pedram Sendi, Jean-Blaise Wasserfallen, Jean Christophe Luthi, Diane Morin and Hélène Brioschi Levi for their help in developing the proposal.
Funding for this study was provided by the Swiss National Foundation # 3200-068000 ( http://www.snf.ch ) and the Swiss Heart Foundation. There are no potential competing interests.
1 Cardiovascular diseases: Fact sheet N°317. 2010 [cited March 7, 2010] Available from: www.who.int/mediacentre/factsheets/fs317/en/print.html
2 Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, et al. Heart disease and stroke statistics – 2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215.
3 Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole-Wilson PA, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur J Heart Fail. 2008;10:933–89.
4 Jhund PS, Macintyre K, Simpson CR, Lewsey JD, Stewart S, Redpath A, et al. Long-term trends in first hospitalization for heart failure and subsequent survival between 1986 and 2003: a population study of 5.1 million people. Circulation. 2009;119:515–23.
5 Ritter M, Laule-Kilian K, Klima T, Christ A, Christ M, Perruchoud A, et al. Gender differences in acute congestive heart failure. Swiss Med Wkly. 2006;136:311–7.
6 Stewart S, Jenkins A, Buchan S, McGuire A, Capewell S, McMurray JJ. The current cost of heart failure to the National Health Service in the UK. Eur J Heart Fail. 2002;4:361–71.
7 Ross JS, Chen J, Lin Z, Bueno H, Curtis JP, Keenan PS, et al. Recent national trends in readmission rates after heart failure hospitalization. Circ Heart Fail. 2010;3:97–103.
8 Dunlay SM, Gheorghiade M, Reid KJ, Allen LA, Chan PS, Hauptman PJ, et al. Critical elements of clinical follow-up after hospital discharge for heart failure: insights from the EVEREST trial. Eur J Heart Fail. 2010;12:367–74.
9 Whellan DJ, Hasselblad V, Peterson E, O'Connor CM, Schulman KA. Metaanalysis and review of heart failure disease management randomized controlled clinical trials. Am Heart J. 2005;149:722–9.
10 McAlister FA, Stewart S, Ferrua S, McMurray JJ. Multidisciplinary strategies for the management of heart failure patients at high risk for admission: a systematic review of randomized trials. J Am Coll Cardiol. 2004;44:810–9.
11 Phillips CO, Singa RM, Rubin HR, Jaarsma T. Complexity of program and clinical outcomes of heart failure disease management incorporating specialist nurse-led heart failure clinics. A meta-regression analysis. Eur J Heart Fail. 2005;7:333–41.
12 Phillips CO, Wright SM, Kern DE, Singa RM, Shepperd S, Rubin HR. Comprehensive discharge planning with postdischarge support for older patients with congestive heart failure: a meta-analysis. Jama: the Journal of the American Medical Association 2004;291:1358–67.
13 Brunner-La Rocca HP, F. W, Trigo-Trindade P, Leventhal M, Seydoux C, Schmid JP, et al. Empfehlungen zur Diagnose und Behandlung der Herzinsuffizienz Teil 1: ambulante Betreuung. Schweiz Med Forum. 2007;7:3S–14S.
14 Buser P, H.P. B-LR, Leventhal M, Mahrer R, Mohacsi P, Nuesch K, et al. Empfehlungen zur vernetzten Betreuung von Herzinsuffizienzpatienten in der Schweiz. Schweiz Ärztezeitung. 2006;87:1943–52.
15 Peytremann-Bridevaux I, Burnand B. Inventory and perspectives of chronic disease management programs in Switzerland: an exploratory survey. Int J Integr Care. 2009;9:e93.
16 Jessup M, Abraham WT, Casey DE, Feldman AM, Francis GS, Ganiats TG, et al. 2009 focused update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:1977–2016.
17 Heart Failure Society of America. HFSA 2006 Comprehensive Heart Failure Practice Guideline. J Card Fail. 2006;12:e1–2.
18 Folstein M, Folstein S, McHugh P. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98.
19 Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–51.
20 Goldman L, Hashimoto B, Cook EF, Loscalzo A. Comparative reproducibility and validity of systems for assessing cardiovascular functional class: advantages of a new specific activity scale. Circulation. 1981;64:1227–34.
21 Cleemput I, Kesteloot K, Moons P, Vanrenterghem Y, Van Hooff JP, Squifflet JP, et al. The construct and concurrent validity of the EQ-5D in a renal transplant population. Value Health. 2004;7:499–509.
22 Rector TS, Kubo SH, Cohn JN. Patients’ Self-Assessment of Their Congestive Heart Failure. Part 2: Content, Reliability and Validity of a New Measure, The Minnesota Living with Heart Failure Questionnaire. Heart Failure 1987; October/November: 198–200, 205–209.
23 Bandura A. Self-efficacy: The exercise of control. New York: W.H. Freeman; 1997.
24 Krumholz HM, Currie PM, Riegel B, Phillips CO, Peterson ED, Smith R, et al. A taxonomy for disease management: a scientific statement from the American Heart Association Disease Management Taxonomy Writing Group. Circulation. 2006;114:1432–45.
25 Brunner-La Rocca HP, Buser PT, Schindler R, Bernheim A, Rickenbacher P, Pfisterer M. Management of elderly patients with congestive heart failure – design of the Trial of Intensified versus standard Medical therapy in Elderly patients with Congestive Heart Failure (TIME-CHF). Am Heart J. 2006;151:949–55.
26 Krumholz HM, Amatruda J, Smith GL, Mattera JA, Roumanis SA, Radford MJ, et al. Randomized trial of an education and support intervention to prevent readmission of patients with heart failure. J Am Coll Cardiol. 2002;39:83–9.
27 Jaarsma T, van der Wal MH, Lesman-Leegte I, Luttik ML, Hogenhuis J, Veeger NJ, et al. Effect of moderate or intensive disease management program on outcome in patients with heart failure: Coordinating Study Evaluating Outcomes of Advising and Counseling in Heart Failure (COACH). Arch Intern Med. 2008;168:316–24.
28 De Geest S, Moons P, Callens B, Gut C, Lindpaintner L, Spirig R. Introducing Advanced Practice Nurses / Nurse Practitioners in health care systems: a framework for reflection and analysis. Swiss Med Wkly. 2008;138:621–8.
29 Pfisterer M, Buser P, Rickli H, Gutmann M, Erne P, Rickenbacher P, et al. BNP-guided vs symptom-guided heart failure therapy: the Trial of Intensified vs Standard Medical Therapy in Elderly Patients With Congestive Heart Failure (TIME-CHF) randomized trial. JAMA. 2009;301:383–92.
30 Meyer K, Murner N, Laederach-Hofmann K, Simmet A, Hess OM. Heart failure events, and case fatalities in Switzerland based on hospital statistics and cause of death statistics. Swiss Med Wkly. 2008;138:506–11.
31 Randomised trial of telephone intervention in chronic heart failure: DIAL trial. BMJ. 2005;331:425.
32 Ferrante D, Varini S, Macchia A, Soifer S, Badra R, Nul D, et al. Long-term results after a telephone intervention in chronic heart failure: DIAL (Randomized Trial of Phone Intervention in Chronic Heart Failure) follow-up. J Am Coll Cardiol. 2010;56:372–8.