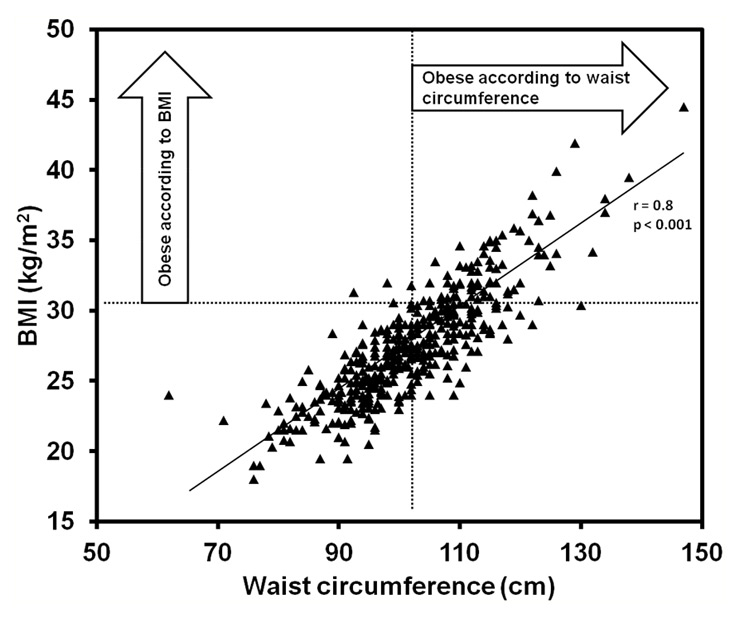
Figure 1
Correlation of waist circumference and body-mass index (BMI).
DOI: https://doi.org/10.4414/smw.2011.13153
Results from the Swiss CaRe study
1 These authors contributed equally to the work.
Obesity has emerged as one of the most serious public health concerns in the 21st century [1, 2]. It is associated with impaired quality of life [3, 4], considerable medical co-morbidities [5] and, in particular, shortened life expectancy [6]. The prevalence of obesity is rising continuously in industrialised nations [7, 8] as well as in developing countries [1, 9]. A detrimental lifestyle, including decreased physical activity and increased calorie intake, is the main factor generating this development [10].
Until recently, calculation of the body-mass index (BMI) has been the method of choice for the classification of obesity. However, BMI does not necessarily reflect the amount of body fat mass, and the waist circumference [11] and waist-to-hip ratio [11, 12] were introduced for cardiovascular risk assessment. Measuring waist circumference to assess abdominal obesity (AO) can be performed easily in everyday practice and, after adjustment for other risk factors, is linked closely to the risk of myocardial infarction [11].
Coronary artery disease (CAD) is the single most important factor contributing to increased mortality in obese patients [13]. Cardiac rehabilitation (CR) programmes are intended to decrease morbidity and mortality in CAD patients, in particular through lifestyle interventions and by reducing cardiovascular risk factors. One of these risk factors is AO, but the burden of AO and its association with other cardiovascular risk factors is unknown in CAD patients attending CR. The aim of this study was, therefore, to investigate the prevalence of AO in a large cohort of CAD patients attending CR and to evaluate differences in cardiovascular risk factors in AO and non-AO patients, thus sensitising physicians to this medical entity.
Patients included in this analysis are part of the Swiss CaRe Study, a prospective cohort study of consecutive patients attending the outpatient CR clinic at University Hospital Bern, Switzerland, starting from April 2004. All patients gave informed consent for prospective data acquisition, and the protocol was approved by the local Ethical Committee.
Waist circumference was measured by a non-stretchable standard tape measure on the unclothed abdomen at the midpoint between the costal margin and the iliac crest. This was performed by study physicians who were instructed by one of the authors. According to international standards, patients were considered abdominally obese when waist circumference was ≥102 cm in males [14]. Additionally, BMI was calculated by body weight in kilograms divided by the square of the height in metres. Height and weight were self-reported by the patients.
Cardiovascular risk factors, including diabetes mellitus, arterial hypertension, dyslipidaemia, current or past smoking (more than one pack year), history of current or past depression and family history of CAD (at least one first degree relative with a history of CAD before the age of 55 years for male and before the age of 65 years for female relatives), were assessed by taking the medical history. For 256 patients a fasting lipid profile, including total cholesterol, low-density lipoprotein (LDL-C), high-density lipoprotein (HDL-C) and triglyceride level, was analysed before the start of rehabilitation in the central laboratory of the University Hospital, Inselspital, Bern.
In the first week of rehabilitation, patients filled in the Hospital Anxiety and Depression Scale (HADS) [15], the Type D personality score (DS14) [16], the Short-Form 36 Health Survey (SF-36) [17] and a questionnaire concerning education and economic status. The HADS is a self-reported questionnaire measuring depression and anxiety. Scores of 11 or more on either subscale are considered to be a significant ‘case’ of psychological morbidity. Type D personality is a combination of social inhibition and negative affectivity predictive of worse prognosis in CAD [18]. Patients were considered to have Type D personalities when their scores for social inhibition and negative affectivity were each ≥10 points [16]. The SF-36 is a health survey designed to assess health-related quality of life that is not disease-, treatment- or age-specific. The scores are converted to a scale of 0 to 100, a higher score indicating a better quality of life on a mental and physical health subscale.
Symptom-limited exercise testing was performed using an upright computer-controlled, rotational speed independent bicycle (Ergo-metrics 800S, Ergoline® GmbH, Bitz, Germany). After a 3-minute warm-up phase, during which patients cycled at 20W, workload was increased by 10 to 20W/minute by a ramp protocol aimed at reaching a maximal exercise time of between 8 and 12 minutes. A 12-lead ECG was recorded throughout the test and blood pressure was measured every 2 minutes. After interruption of the exercise test, patients were advised to continue pedalling slowly and the ECG was recorded for an additional 3 to 5 min. Heart rate recovery was defined as the difference between the maximum heart rate and the heart rate one minute after test interruption.
All statistical analysis was performed using the SPSS® for Windows® software (version 15, SPSS® Inc., Chicago, Illinois, USA). Unless indicated otherwise, data are expressed as mean values ± standard deviation. Comparison of categorical variables was performed by the chi-square or Fisher’s exact test. For comparison of continuous variables, a linear multivariate analysis of co-variance was performed for the lipid profile, the psychosocial scores and the ergometry data. All analyses of the lipid profile were adjusted for statin use and of the ergometry data for age and beta-blocker use. Furthermore, all multivariate analyses were adjusted for obesity measured by BMI, permitting interpretation of the impact of AO on risk factors independently of BMI. Correlations between BMI and waist circumference were tested by linear regression and expressed by Pearson’s correlation coefficient. All statistical tests were two-tailed and a value of p <0.05 was considered statistically significant. All authors had full access to the data and take full responsibility for their integrity.
Mean waist circumference of 415 consecutive male CAD patients (mean age 58 ± 11 years) was 102 ± 11 cm. Mean BMI was 27 ± 4 kg/m2. Waist circumference and BMI were highly correlated (fig. 1, r = 0.80, p <0.001). However, while 50% of all patients were considered obese according to waist circumference, only 23% met this criterion according to the BMI cut-off (30 kg/m2). Table 1 shows the characteristics of patients classified as AO and non-AO. Concerning education, AO was significantly associated with lower educational level (p = 0.021) parallel to a higher BMI in this category (p = 0.022).

Figure 1
Correlation of waist circumference and body-mass index (BMI).
The association of AO with other cardiovascular risk factors is shown in table 2. AO was associated significantly with diabetes (p = 0.003) and hypertension (p <0.001), whereas BMI equal or higher than 30 kg/m2 was only associated with diabetes (p = 0.036, not shown in the table). Furthermore, there was a trend towards higher depression scores in AO patients (p = 0.056). Surprisingly, there was no interaction between educational level and other cardiovascular risk factors, despite a trend towards more diabetics in the lower educational class (p = 0.055).
Results of multivariate analyses on the lipid profile in AO versus non-AO patients are shown in table 3 (all analyses were adjusted for statin use and obesity measured by BMI). AO patients showed an unfavourable lipid profile compared to non-AO patients. AO patients exhibited lower HDL-C levels (p <0.001) and higher triglyceride levels (p = 0.006) than non-AO patients. The LDL-C and total cholesterol levels did not differ significantly. In contrast, obesity measured by BMI was not associated with an unfavourable lipid profile.
Results of exercise testing are shown in table 3 (all analyses were adjusted for age, beta-blocker use and obesity measured by BMI). The ergometry parameters differed depending on patients’ AO status. No significant difference in blood pressure, maximal exercise capacity and heart rate recovery in AO versus non-AO patients was noted, but AO patients had a higher resting heart rate (p = 0.021). In contrast, no differences were found when BMI was used as a measure of obesity.
Scores for HADS, DS14 and SF-36 were not significantly different between AO and non-AO patients, as shown in table 4 (all analyses were adjusted for obesity measured by BMI). Type D personality incidence was not different between groups (26.9% in AO versus 23.4% in non-AO patients, p = 0.559).
| Table 1: Sociodemographic and clinical characteristics of abdominally obese (AO) versus non-AO patients (BMI = body-mass index, PCI = percutaneous coronary intervention, CABG = coronary artery bypass graft). | |||
| non-AO 50% (n = 209) | AO 50% (n = 206) | p-value | |
| Age (years) | 57 ± 11 | 59 ± 11 | 0.056 |
| BMI (kg/m2) | 24.8 ± 2.4 | 30.1 ± 3.3 | <0.001 |
| Waist circumference (cm) | 93 ± 6 | 111 ± 8 | <0.001 |
| Grade of coronary artery disease: | 0.267 | ||
| 1-vessel disease (%) | 41 | 34 | |
| 2-vessel disease (%) | 28 | 32 | |
| 3-vessel disease (%) | 31 | 34 | |
| Left ventricular ejection fraction (%) | 53 ± 11 | 54 ± 10 | 0.315 |
| Intervention: | 0.217 | ||
| PCI (%) | 78 | 76 | |
| CABG (%) | 19 | 16 | |
| None (%) | 2 | 5 | |
| Education: | 0.021 | ||
| College or university (%) | 26 | 21 | |
| Apprenticeship training (%) | 59 | 53 | |
| Primary school (%) | 15 | 26 | |
| Table 2: Cardiovascular risk factors in abdominally obese (AO) versus non-AO patients (“Smoking” refers to current smokers or a history of smoking). | |||
| non-AO 50% (n = 209) | AO 50% (n = 206) | p-value | |
| Family history (%) | 26 | 29 | 0.868 |
| Smoking (%) | 74 | 84 | 0.099 |
| Dyslipidaemia (%) | 63 | 71 | 0.096 |
| Diabetes (%) | 11 | 21 | 0.003 |
| Hypertension (%) | 47 | 67 | <0.001 |
| Depression (%) | 8 | 14 | 0.056 |
| Table 3: Laboratory assessment and exercise testing in abdominally obese (AO) versus non-AO patients (p-values were corrected for age, beta-blocker use, statin use and obesity measured by BMI). | |||
| non-AO | AO | p-value | |
| Total cholesterol (mmol/L) | 5.0 ± 1.3 | 4.9 ± 1.3 | 0.668 |
| High density lipoprotein cholesterol (mmol/L) | 1.3 ± 0.4 | 1.2 ± 0.3 | <0.001 |
| Low density lipoprotein cholesterol (mmol/L) | 2.9 ± 1.1 | 2.6 ± 1.2 | 0.164 |
| Triglycerides (mmol/L) | 1.8 ± 1.3 | 2.4 ± 2.1 | 0.006 |
| Resting systolic blood pressure (mm Hg) | 120 ± 20 | 124 ± 17 | 0.185 |
| Resting heart rate (bpm) | 67 ± 11 | 69 ± 12 | 0.021 |
| Exercise capacity (Watt) | 140 ± 45 | 139 ± 45 | 0.644 |
| Maximal heart rate (bpm) | 133 ± 24 | 128 ± 23 | 0.630 |
| Heart rate recovery | 23 ± 10 | 21 ± 12 | 0.948 |
| Table 4: Hospital Anxiety and Depression Scale (HADS), Type D personality score (DS14) and Short-form 36 Health Survey (SF-36) in abdominally obese (AO) versus non-AO patients (all analyses were adjusted for obesity measured by BMI). | |||
| non-AO | AO | p-value | |
| Anxiety (HADS) | 4.2 ± 3.4 | 4.8 ± 3.4 | 0.199 |
| Depression (HADS) | 3.1 ± 3.4 | 3.5 ± 3.3 | 0.415 |
| Negative affectivity (DS14) | 8.8 ± 5.4 | 8.8 ± 4.5 | 0.908 |
| Social inhibition (DS14) | 9.0 ± 5.5 | 9.1 ± 5.1 | 0.871 |
| Physical health (SF-36) | 42.8 ± 10.0 | 43.7 ± 11.1 | 0.530 |
| Mental health (SF-36) | 51.5 ± 9.6 | 49.5 ± 10.4 | 0.160 |
We investigated the burden and the association of AO, corrected for BMI, with the cardiovascular risk profile, health related quality of life and psychosocial profile in a large cohort of consecutive CAD patients attending CR. Half of the patients were considered abdominally obese according to the waist circumference cut-off. In contrast, only a quarter of patients met the criterion for obesity according to BMI categories. Of all abdominally obese patients, 56% were not considered obese according to the established 30 kg/m2 BMI cut-off, which corresponds to an underestimation of the burden of obesity by BMI [19].
In a cross-sectional, population-based random sample of more than 6000 men and women aged 35–75 years (53.1 ± 10.8 years) in Switzerland, the prevalence of AO was 23.9% in men [19]. In contrast to this sample of the general population, the rate of AO in our cohort was, alarmingly, more than twice as high. Although the mean age, which is 5 years higher in our cohort, might account for some of the difference, the main reason is the association of AO with CAD. The epidemic of obesity has reached secondary prevention in CR.
Obesity is known to be associated with impaired quality of life [3, 4], depression and other mood disorders [20], as well as reduced physical and mental wellbeing [4, 21]. In our study population AO was by trend associated with depression, which may be explained by a lower motivation of depressed patients to engage in physical activity and by less favourable diet. The rate of AO was higher in patients with a lower educational level, which is in accordance with previous findings of an inverse correlation between education and obesity in the metabolic syndrome [22, 23]. Instantaneous scores at the beginning of CR for anxiety and depression (HADS), social inhibition and negative affectivity (DS14) and physical and mental wellbeing (SF-36) did not differ between AO and non-AO patients. The lack of difference in these scores may be due to the simultaneously increased physical and mental distress affecting both groups equally after a recent myocardial infarction.
Resting heart rate is one of the most powerful predictors of overall mortality [24, 25]. Blood pressure, maximal exercise capacity and heart rate recovery were not significantly different between AO and non-AO patients after adjustment for age, beta-blocker use and obesity measured by BMI. However, AO patients had significantly higher resting heart rates, suggesting an increased cardiovascular risk independently of obesity measured by BMI. The higher resting heart rate in AO patients is due to an increase in sympathetic tone coupled with a reduction in parasympathetic tone [26].
An atherogenic lipid profile is characterised by elevated triglycerides and low levels of HDL-C [27], and an inverse correlation of HDL-C level and CAD has been consistently shown in population studies [28, 29]. In our study cohort AO was, independently of BMI, associated with an elevated triglyceride level and a low HDL-C level. This is in accordance with the inverse correlation of visceral adipose tissue with HDL-C levels and a positive correlation of visceral adipose tissue with triglyceride levels [30].
Abdominal obesity worsens the prognosis in patients with CAD [31, 32]. The characteristics in AO patients we showed here are independent of obesity measured by BMI and may account for some of the prognostic difference.
Since only patients who attended our three-month ambulatory CR programme were included in this analysis, the results may not be extrapolated to unselected CAD patients. To avoid confounding by gender differences, female patients were omitted. This study is a cross-sectional not longitudinal survey, so future cardiovascular events cannot be anticipated from the results. Although measurements were standardised, anthropomorphic measurements may show substantial inter-observer variability [33, 34].
AO is highly prevalent in patients with CAD attending outpatient CR. AO is, independently of BMI, associated with metabolic lipid disorders and autonomic cardiovascular dysregulation, suggesting increased cardiovascular risk. AO patients, therefore, need particular attention during CR and follow-up, with regard to lifestyle change including substantial weight loss by changing eating behaviour and by increasing physical activity. Interventional studies will clarify the best approach to reducing the burden of AO.
The authors of this manuscript have no conflict of interest to declare. The study was supported by an unrestricted educational grant from Sanofi Aventis (Suisse) SA.
1 McLellan F. Obesity rising to alarming levels around the world. Lancet. 2002;359:1412.
2 Probst-Hensch NM. Chronic age-related diseases share risk factors: do they share pathophysiological mechanisms and why does that matter? Swiss Med Wkly. 2010; 140:w13072.
3 Duval K, Marceau P, Lescelleur O, et al. Health-related quality of life in morbid obesity. Obes Surg. 2006;16:574–9.
4 Jia H, Lubetkin EI. The impact of obesity on health-related quality-of-life in the general adult US population. J Public Health. (Oxf) 2005;27:156–64.
5 Overweight, obesity, and health risk. National Task Force on the Prevention and Treatment of Obesity. Arch Intern Med. 2000;160:898–904.
6 Fontaine KR, Redden DT, Wang C, Westfall AO, Allison DB. Years of life lost due to obesity. JAMA. 2003;289:187–93.
7 Hedley AA, Ogden CL, Johnson CL, et al. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA. 2004;291:2847–50.
8 Saely CH, Risch L, Frey F, et al. Body mass index, blood pressure, and serum cholesterol in young Swiss men: an analysis on 56784 army conscripts. Swiss Med Wkly. 2009;139:518–24.
9 Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the developing world – a growing challenge. N Engl J Med. 2007;356:213–5.
10 Hill JO, Peters JC. Environmental contributions to the obesity epidemic. Science. 1998;280:1371–4.
11 Yusuf S, Hawken S, Ounpuu S, et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet. 2005;366:1640–9.
12 See R, Abdullah SM, McGuire DK, et al. The association of differing measures of overweight and obesity with prevalent atherosclerosis: the Dallas Heart Study. J Am Coll Cardiol. 2007;50:752–9.
13 Seidell JC, Verschuren WM, van Leer EM, Kromhout D. Overweight, underweight, and mortality. A prospective study of 48,287 men and women. Arch Intern Med. 1996;156:958–63.
14 Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i-xii, 1-253.
15 Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52:69–77.
16 Denollet J. DS14: standard assessment of negative affectivity, social inhibition, and Type D personality. Psychosom Med. 2005;67:89–97.
17 Bullinger M. German translation and psychometric testing of the SF-36 Health Survey: preliminary results from the IQOLA Project. International Quality of Life Assessment. Soc Sci Med. 1995;41:1359–66.
18 Kupper N, Denollet J. Type D personality as a prognostic factor in heart disease: assessment and mediating mechanisms. J Pers Assess. 2007;89:265–76.
19 Marques-Vidal P, Bochud M, Mooser V, et al. Prevalence of obesity and abdominal obesity in the Lausanne population. BMC Public Health. 2008;8:330.
20 Scott KM, McGee MA, Wells JE, Oakley Browne MA. Obesity and mental disorders in the adult general population. J Psychosom Res. 2008;64:97–105.
21 Le Pen C, Levy E, Loos F, Banzet MN, Basdevant A. “Specific” scale compared with “generic” scale: a double measurement of the quality of life in a French community sample of obese subjects. J Epidemiol Community Health. 1998;52:445–50.
22 Silventoinen K, Pankow J, Jousilahti P, Hu G, Tuomilehto J. Educational inequalities in the metabolic syndrome and coronary heart disease among middle-aged men and women. Int J Epidemiol. 2005;34:327–34.
23 Wamala SP, Lynch J, Horsten M, et al. Education and the metabolic syndrome in women. Diabetes Care. 1999;22:1999–2003.
24 Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med. 1999;341:1351–7.
25 Jouven X, Empana JP, Schwartz PJ, et al. Heart-rate profile during exercise as a predictor of sudden death. N Engl J Med. 2005;352:1951–8.
26 Deniz F, Katircibasi MT, Pamukcu B, Binici S, Sanisoglu SY. Association of metabolic syndrome with impaired heart rate recovery and low exercise capacity in young male adults. Clin Endocrinol. (Oxf) 2007;66:218–23.
27 U.K. Prospective Diabetes Study 27. Plasma lipids and lipoproteins at diagnosis of NIDDM by age and sex. Diabetes Care. 1997;20:1683–7.
28 Castelli WP, Garrison RJ, Wilson PW, et al. Incidence of coronary heart disease and lipoprotein cholesterol levels. The Framingham Study. JAMA. 1986;256:2835–8.
29 Assmann G, Schulte H, von Eckardstein A, Huang Y. High-density lipoprotein cholesterol as a predictor of coronary heart disease risk. The PROCAM experience and pathophysiological implications for reverse cholesterol transport. Atherosclerosis. 1996;124(Suppl):S11–20.
30 Liu J, Fox CS, Hickson DA, et al. Impact of Abdominal Visceral and Subcutaneous Adipose Tissue on Cardiometabolic Risk Factors: The Jackson Heart Study. J Clin Endocrinol Metab.
31 Dagenais GR, Yi Q, Mann JF, et al. Prognostic impact of body weight and abdominal obesity in women and men with cardiovascular disease. Am Heart J. 2005;149:54–60.
32 Kragelund C, Hassager C, Hildebrandt P, Torp-Pedersen C, Kober L. Impact of obesity on long-term prognosis following acute myocardial infarction. Int J Cardiol. 2005;98:123–31.
33 Sebo P, Beer-Borst S, Haller DM, Bovier PA. Reliability of doctors’ anthropometric measurements to detect obesity. Prev Med. 2008;47:389–93.
34 Faeh D, Braun J, Bopp M. Underestimation of obesity prevalence in Switzerland: comparison of two methods for correction of self-report. Swiss Med Wkly. 2009;139:752–6.