Figure 1
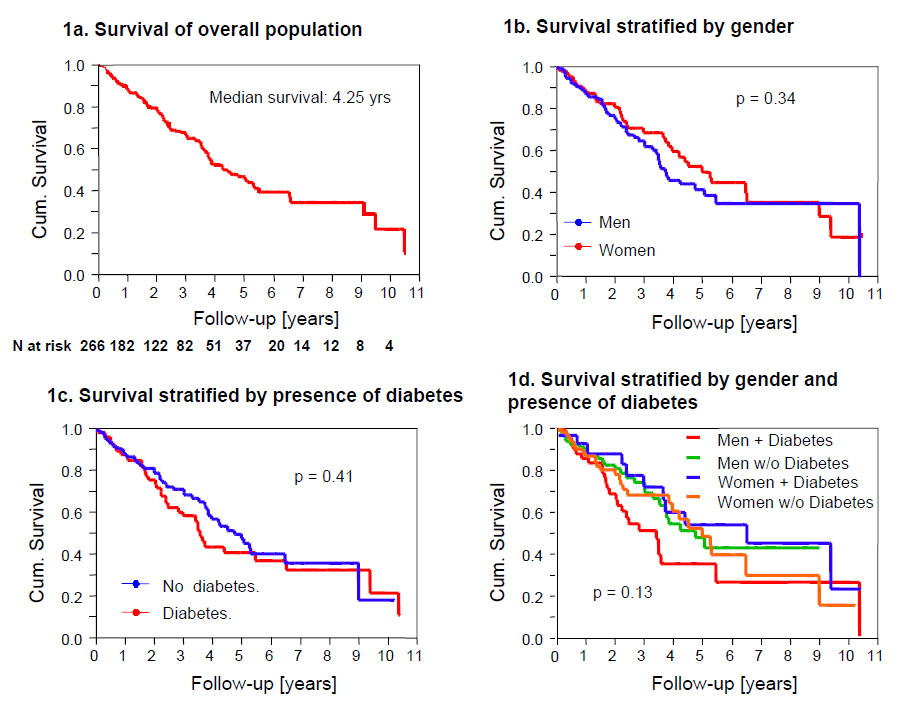
Overall survival on chronic haemodialysis (1a); survival stratified by gender (1b); survival stratified by presence of diabetes (1c); survival stratified by gender and presence of diabetes (1d).
DOI: https://doi.org/10.4414/smw.2011.13150
A 10-year Swiss single centre analysis
Since the first successful acute dialysis performed by Willem J. Kolff in 1946 and the opening of the first chronic outpatient dialysis centre by Belding Scribner in Seattle in 1962, chronic haemodialysis has become one of the mainstays of treating end stage renal disease (ESRD) [1]. Today, some 40 yearslater, nearly 300,000 patients in Europe and 1.5million worldwide are undergoing chronic dialysis [1, 2].
Despite the opportunities of chronic dialysis treatment, patients with end stage renal disease (ESRD) undergoing maintenance haemodialysis remain at a substantially increased risk of death. According to the latest European Renal Association Annual Report [2] the expected remaining lifetime of a 50 year old dialysis patient is less than 8 years, which is over 20 years less than the expected remaining lifetime of the age matched general population. Even the remaining lifetime of an 80 year old dialysis patient is shortened by over five years compared to the age-matched general population [2]. Additionally, it is becoming increasingly clear that outcomes among dialysis patients differ considerably between regions and countries. Held and colleagues were the first to report significant mortality differences between the renal replacement population in the United States, Europe and Japan [3]. In their study, the mortality risk for the renal replacement population in the United States was 15% higher than mortality risk in Europe and 33% above the Japanese risk. Later results from the “Dialysis Outcomes and Practice Patterns Study” (DOPPS) confirmed [4] and extended these findings by showing survival differences between European countries [5]. According to DOPPS analysis mortality risk was significantly higher in the United Kingdom compared to the mortality risk of dialysis patients in Italy, France or Germany. No significant mortality differences existed between the Italian, French or German dialysis population [5]. Unfortunately, data concerning the survival of the Swiss haemodialysis population are limited, since Swiss dialysis centres participated in neither the European Renal Association Annual Report nor the DOPPS study.
We therefore aimed to assess morbidity and mortality of haemodialysis patients at a Swiss dialysis centre.
Included were all patients who entered the chronic haemodialysis program of the University Hospital Basel and its affiliated centre between January 1995 and end of June 2006. There were no exclusion factors to the enrolment into this analysis. Due to dialysis-free intervals following kidney transplantation 12 patients entered the chronic haemodialysis program repeatedly. These patients were entered into the analysis only once. Three patients had to be excluded due to missing datasets. Overall, 266 patients were eligible for the analysis. The last follow-up was on 30 June 2006, median follow-up time was 5.18 years (range 0.003 to 11.48 years).
During the observation period the estimated incidence and prevalence rates for patients on haemodialysis were 0.01% and 0.034% per 100,000 inhabitants covered by our dialysis unit. Standard dialysis prescription consisted of four hour dialysis sessions three times a week with target blood flow >200 ml/min (>300 ml/min from 2002), and anticoagulation with low molecular heparins (dalteparin, enoxaparin). Haemodiafiltration was prescribed in >90% of patients from the beginning and since 2002 all dialysis machines had online haemodiafiltration equipment. High flux filters with 1.7 to 2.1 m2surface from different companies were used.
In case of withdrawal from dialysis, the process of decision was usually initiated by the patient`s wish to stop dialysis. Then, withdrawal from dialysis was evaluated and discussed by the dialysis care team (nurses, physicians and psychologists) and subsequently re-discussed with the patient and his relatives. Dialysis was only stopped if all of the involved persons consented, whereby the predominant factor of decision was the patient’s wish. In cases of incapability of the patient to participate in this process (dementia, cognitive impairment, cerebrovascular disease) the presumed wish of the patient was explored as far as possible by studying the living will and extensively discussing and evaluating the situation with the closest relatives. Importantly, whenever possible first “end of life” discussion were initiated soon after the entrance of the patient into the haemodialysis program. After withdrawal of dialysis, patients who remained in the hospital were treated with the best of palliative care to avoid pain, shortness of breath and anxiety. Patients who wished to die at home were usually attended by their relatives with close support by the family doctor and the home nursing care team.
From the beginning of the dialysis program at the University Hospital Basel all clinical data were prospectively and continuously collected in standardized flow sheets and in medical records. From 01.09.2002 flow sheets were replaced by an electronic database. A single trained researcher (C.M.-B.) abstracted data from the medical records. To verify the accuracy of the chart abstraction, a board-certified nephrologist (M.M.) re-evaluated all data points. Discrepancies between the original extractions and the reassessments were all corrected.
The diagnosis of a glomerulonephritis or interstitial nephropathy as the cause of end stage renal disease (ESRD) was histology based (kidney biopsy or nephrectomy). In cases of clinically suspected glomerulonephritis or interstitial nephropathy but missing biopsy proof, the underlying kidney pathology was grouped as “unknown”. Co-morbid conditions were confirmed by the abstractors based on medical history, current medication and clinical testing. In detail, diabetes mellitus was defined by use of antidiabetic medication or a history of diabetes mellitus. Coronary heart disease was defined by a positive stress test, a positive cardiac angiogram, a history of percutaneous coronary angioplasty or coronary bypass surgery, or a clear history of a coronary event. Peripheral vascular disease was defined by duplex ultrasound, angiography, a history of percutaneous angioplasty or bypass surgery, or a proven clinical event. Cerebrovascular disease was recorded if there was a history of a corresponding clinical event. Malignancies were based on a histological diagnosis. The diagnosis of autoimmune disease was based on the decision of the "Interdisciplinary Vasculitis Board" of the University Hospital Basel.
The cause of death was adjudicated by two board certified nephrologists (D.G. and M.M.) after reviewing all medical records pertaining to the patient.
The study was carried out according to the principles of the Declaration of Helsinki.
Survival on chronic haemodialysis was the primary endpoint of this study. Pre-specified sub-group analyses were performed for female and diabetic patients. Patient exposure was censored for renal transplant, discontinuation of dialysis because of regaining renal function, switch to peritoneal dialysis and transfer of the patient to a non-participating dialysis unit.
The statistical analyses were performed using SPSS/PC (version 15.0, SPSS Inc., USA). A statistical significance level of <0.05 was used. Discrete variables are expressed as counts (percentage) and continuous variables as means ± standard deviation or median and range, unless stated otherwise. The comparison between the two groups was done with chi-square test and Fisher exact test for categorical variables and t-test for continuous variables if normally distributed or Mann-Whitney test if not normally distributed. Cumulative survival was calculated using Kaplan-Meier analysis and differences between the curves were evaluated by means of log-rank statistics. Median survival was defined as the time at which 50% of all patients were still alive.
Detailed baseline characteristics of the study population are summarized in table 1.

Figure 1
Overall survival on chronic haemodialysis (1a); survival stratified by gender (1b); survival stratified by presence of diabetes (1c); survival stratified by gender and presence of diabetes (1d).
The patients’ age ranged from 15 to 90 years (median 64.5) and the rate of cardiovascular co-morbidities was high. 71% of patients suffered either from coronary artery, peripheral artery or cerebrovascular disease. Importantly, obesity, diabetes mellitus type 2 and coronary artery disease were significantly more common in men than in women.
Patients suffering from diabetes mellitus were significantly older, more obese and were diagnosed with more cardiovascular co-morbidities. Table 2 displays the characteristics of diabetic versus non-diabetic patients.
Diabetic (17%) and vascular nephropathies (15%) were the most common causes of ESRD. Biopsy proven glomerulonephropathies and interstitial nephropathies were present in 14% and 2% of patients, respectively. The underlying kidney disease was based on histology results in 116 (43%) patients. The vast majority of patients enrolled into this study were new dialysis patients (n = 217 (82%)), including 28 patients suffering from kidney transplant failure. The remainder of the patients were switched from peritoneal dialysis to haemodialysis (n = 39 (14.5%)) or transferred from an external dialysis centre (n = 10 (3.5%)). Median time on external dialysis was 805 days (2.2 years, range 9 to 4959 days) and on peritoneal dialysis 274 days (0.7 years, range 2 to 4012 days) before entering the haemodialysis program of our unit.
Remarkably, the frequency of malignant diseases was strikingly high (table 3). During the observational period 96 malignomas were detected in 70 (26%) out of 266 patients, with dermal, gastrointestinal and urinary tract malignomas being most frequent. Twelve of the 96 cases occurred in patients who had previously undergone kidney transplantation.
During the study period 69 patients underwent kidney transplantation. Median time on haemodialysis to transplantation was 1.68 years (range 0–9.45 years). 47 patients (68%) received a kidney graft from a deceased and 22 patients (32%) from a living donor. Transplanted patients were significantly younger than non-transplant patients, had a lower incidence of cardiovascular co-morbidities and were less likely to suffer from diabetic nephropathy (table 4).
| Table 1:Baseline characteristics of 266 patients undergoing chronic haemodialysis. | ||||
| All | Male | Female | p-value° | |
| Gender | 266 (100) | 148 (56) | 118 (44) | |
| Age | 65 [15–90] | 63 [15–88] | 65 [21–90] | 0.93* |
| Body mass index | 23 [12–51] | 24 [17–51] | 22 [12–40] | <0.01* |
| Underlying Kidney Disease | ||||
| Diabetic nephropathy | 45 (17) | |||
| – DM Type 1 10 (22) | 4 (3) | 6 (5) | 0.24** | |
| – DM Type 2 35 (78) | 24 (16) | 11 (9) | 0.07** | |
| Glomerulonephritis | 36 (14) | 20 (14) | 16 (14) | 0.57** |
| Interstitial nephropathy | 4 (2) | 3 (2) | 1 (1) | 0.40** |
| Vascular nephropathy | 40 (15) | 25 (17) | 15 (13) | 0.22** |
| Analgesic nephropathy | 16 (6) | 3 (2) | 13 (11) | <0.01** |
| ADPKD | 24 (9) | 11 (7) | 13 (11) | 0.21** |
| Other nephropathies§ | 68 (25) | 44 (30) | 24 (20) | 0.05** |
| Unknown | 33 (12) | 14 (10) | 19 (16) | 0.08** |
| Co-Morbidities | ||||
| Diabetes mellitus | 90 (34) | |||
| – DM Type 1 10 (11) | 4 (3) | 6 (5) | 0.25** | |
| – DM Type 2 80 (89) | 53 (36) | 27 (23) | 0.01** | |
| Coronary artery disease | 74 (28) | 49 (33) | 25 (21) | 0.02** |
| Peripheral artery disease | 74 (28) | 42 (28) | 32 (27) | 0.45** |
| Cerebrovascular disease | 43 (16) | 25 (17) | 18 (15) | 0.42** |
| Obstructive pulmonary disease | 32 (12) | 22 (15) | 10 (9) | 0.08** |
| Autoimmune disease | 19 (7) | 8 (5) | 11 (9) | 0.20** |
| Malignancies | 70 (26) | 38 (26) | 32 (27) | 0.50** |
| Data are displayed as counts and percentages (%) or median plus range [r], *Mann-Whitney-U-test; **Fisher-exact-test; °p-values: comparing male and female; §includes among others nephrectomy due to renal cell carcinoma (n = 12), primary focal segmental glomerulosclerosis (n = 11), reflux nephropathy (n = 9). | ||||
| Table 2:Baseline characteristics stratified according to the presence of diabetes mellitus. | |||
| Diabetes (n = 90) | No-Diabetes (n = 176) | p-value° | |
| Female | 33 (37) | 85 (49) | 0.04** |
| Age | 66 [22–81] | 62 [15–90] | 0.05* |
| BMI | 24 [17–41] | 23 [12–51] | <0.01* |
| Underlying Kidney Disease | |||
| Diabetic nephropathy Type 1 | 10 (11) | 0 (0) | <0.01** |
| Diabetic nephropathy Type 2 | 35 (39) | 0 (0) | <0.01** |
| Glomerulonephritis | 6 (7) | 30 (17) | 0.01** |
| Interstitial nephropathy | 1 (1) | 3 (2) | 0.58** |
| Vascular nephropathy | 17 (19) | 23 (13) | 0.15** |
| Analgesic nephropathy | 2 (2) | 14 (8) | 0.05** |
| ADPKD | 3 (3) | 21 (12) | 0.01** |
| Other Nephropathies§ | 9 (10) | 59 (33) | <0.01** |
| Unknown | 7 (8) | 26 (15) | 0.07** |
| Co-Morbidities | |||
| Coronary artery disease | 38 (42) | 36 (21) | <0.01** |
| Peripheral artery disease | 42 (47) | 32 (18) | <0.01** |
| Cerebrovascular disease | 19 (21) | 24 (14) | 0.09** |
| Obstructive pulmonary disease | 9 (10) | 23 (13) | 0.30** |
| Autoimmune disease | 2 (2) | 17 (10) | 0.02** |
| Malignancies | 19 (21) | 51 (29) | 0.10** |
| Data are displayed as counts and percentages (%) or median plus range [r], *Mann-Whitney-U-test; **Fisher-exact-test; °p-values: comparing patients with diabetes and no-diabetes; §includes among others nephrectomy due to renal cell carcinoma (n = 12), primary focal segmental glomerulosclerosis (n = 11), reflux nephropathy (n = 9). | |||
| Table 3:Frequency of malignancies in 266 patients undergoing chronic haemodialysis. | ||
| Type of malignancies | N (%) | Post-transplant (n) |
| Dermal malignoma | 24 (9) | 7 |
| Thyroid carcinoma | 5 (2) | |
| Gastrointestinal carcinoma | 16 (6) | 2 |
| Lung cancer | 4 (2) | |
| Urinary-tract carcinoma | 21 (8) | 1 |
| Carcinoma of the female genital organs | 4 (2) | |
| Breast cancer | 8 (3) | 1 |
| Prostate cancer | 5 (2) | |
| Haematologic malignoma | 7 (3) | 1 |
| Others | 2 (1) | |
| Total | 96 (36) | 12 |
| Data are displayed as counts and percentages (%) of 266 patients. | ||
| Table 4:Baseline characteristics stratified according to transplantation status during the observational period. | |||
| Transplant (n = 69) | No-Transplant (n = 197) | p-value° | |
| Female | 34 (49) | 84 (43) | 0.21** |
| Age | 51 [15–70] | 67 [17–90] | <0.01* |
| BMI | 22 [17–40] | 24 [12–51] | 0.08* |
| Underlying Kidney Disease | |||
| Diabetic nephropathy Type 1 | 5 (7) | 5 (3) | 0.08** |
| Diabetic nephropathy Type 2 | 3 (4) | 32 (16) | <0.01** |
| Glomerulonephritis | 10 (15) | 26 (13) | 0.46** |
| Interstitial nephropathy | 2 (3) | 2 (1) | 0.27** |
| Vascular nephropathy | 4 (6) | 36 (18) | <0.01** |
| Analgesic nephropathy | 2 (3) | 14 (7) | 0.16** |
| ADPKD | 12 (17) | 12 (6) | <0.01** |
| Other nephropathies§ | 20 (29) | 48 (24) | 0.27** |
| Unknown | 11 (16) | 22 (11) | 0.20** |
| Co-Morbidities | |||
| Diabetes mellitus Type 1 | 5 (7) | 5 (3) | 0.08** |
| Diabetes mellitus Type 2 | 9 (13) | 71 (36) | <0.01** |
| Coronary artery disease | 10 (15) | 64 (33) | <0.01** |
| Peripheral artery disease | 8 (12) | 66 (34) | <0.01** |
| Cerebrovascular disease | 5 (7) | 38 (19) | 0.01** |
| Obstructive pulmonary disease | 6 (9) | 26 (13) | 0.22** |
| Autoimmune disease | 5 (7) | 14 (7) | 0.60** |
| Malignancies | 13 (19) | 57 (29) | 0.06** |
| Data are displayed as counts and percentages (%) or median plus range [r], *Mann-Whitney-U-test; **Fisher-exact-test; °p-values: comparing patients who received a kidney transplantation and patients who did not during the observation period; §includes among others nephrectomy due to renal cell carcinoma (n = 12), primary focal segmental glomerulosclerosis (n = 11), reflux nephropathy (n = 9). | |||
Overall 91 patients died on chronic dialysis during the observational period (table 5). These patients were significantly older at initiation of chronic dialysis (69 vs. 60yrs, p <0.01) and more likely to be diabetic (39% vs. 26%, p <0.01) or suffer from coronary artery (42% vs. 21%, p <0.01), peripheral artery (46% vs. 18%, p <0.01) or cerebrovascular (22% vs. 13%, p = 0.05) disease. Furthermore, analgesic nephropathy was more (11% vs. 3%, p = 0.02), cystic kidney disease less (3% vs. 12%, p = 0.01) common in patient deceasing during the observational period.
The median survival on chronic haemodialysis was 4.25 years (95%CI 3.66-5.50), with one, three and five year survival rates reaching 88%, 68% and 46% respectively (fig. 1a). There were no survival differences between male and female patients (p = 0.34), diabetic and non-diabetic patients (p = 0.41) and between male and female patients stratified according to the presence of diabetes (0.13) (fig. 1b–d). There was no difference in survival when patients were stratified by two different time periods in which they entered the haemodialysis program (01.01.1995 – 31.12.2000 (n = 118) compared with 1.1.2001 to 30.6.2006 (n = 148)) (p = 0.855). Further, survival analysis in patients stratified by their history of dialysis (patients new on haemodialysis (n = 217) compared to patients transferred from an external dialysis unit (n = 10) or switched from peritoneal dialysis (n = 39) showed no difference (p = 0.412) during the observation period.
The majority of patients dying during the observational period deceased in hospital (64%), autopsies corroborating the cause of death were available in 30% (27/91) of patients. Thirty three percent (30/91) of all deaths were caused by cardiac events, followed by malignant diseases (8%) and infections (7%). The cause of death could not be determined in 37 (41%) patients.
During the observational period 23 out of 266 (9%) patients terminated dialysis. The median survival after the termination of dialysis was five days (range 2–381). However, three patients survived between 228 and 381 days after the termination of dialysis. Patients` characteristics including reasons for withdrawal are summarized in table 6.
| Table 5:Baseline characteristics stratified according to survival status. | |||
| Non-Survivors (n = 91) | Survivors (n = 175) | p-Wert° | |
| Female | 38 (42) | 80 (46) | 0.31** |
| Age at initiation of dialysis | 69 [41–88] | 60 [15–90] | <0.01* |
| BMI | 23 [12–34] | 23 [13–51] | 0.16* |
| Underlying Kidney Disease | |||
| Diabetic nephropathy Type 1 | 3 (3) | 7 (4) | 0.54** |
| Diabetic nephropathy Type 2 | 16 (18) | 19 (11) | 0.90** |
| Glomerulonephritis | 12 (13) | 24 (14) | 0.53** |
| Interstitial nephropathy | 0 (0) | 4 (2) | 0.19** |
| Vascular nephropathy | 15 (17) | 25 (14) | 0.38** |
| Analgesic nephropathy | 10 (11) | 6 (3) | 0.02** |
| ADPKD | 3 (3) | 21 (12) | 0.01** |
| Other nephropathies§ | 23 (25) | 45 (26) | 0.53** |
| Unknown | 9 (10) | 24 (14) | 0.24** |
| Co-Morbidities | |||
| Diabetes mellitus Type 1 | 2 (2) | 8 (5) | 0.27** |
| Diabetes mellitus Type 2 | 35 (39) | 45 (26) | <0.01** |
| Coronary artery disease | 38 (42) | 36 (21) | <0.01** |
| Peripheral artery disease | 42 (46) | 32 (18) | <0.01** |
| Cerebrovascular disease | 20 (22) | 23 (13) | 0.05** |
| Obstructive pulmonary disease | 18 (20) | 14 (8) | <0.01** |
| Autoimmune disease | 8 (9) | 11 (6) | 0.30** |
| Malignancies | 28 (31) | 42 (24) | 0.15** |
| Data are displayed as counts and percentages (%) or median plus range [r], *Mann-Whitney-U-test; **Fisher-exact-test; °p-values: comparing patients who survived or who died during the observation period; §includes among others nephrectomy due to renal cell carcinoma (n = 12), primary focal segmental glomerulosclerosis (n = 11), reflux nephropathy (n = 9). | |||
| Table 6:Characteristics of 23 patients withdrawing haemodialysis: | |
| Age at death, years | 75 [63–91] |
| Duration of dialysis | 2.2 [0.02–9.1] |
| Reason for withdrawal of dialysis | 23 (100) |
| advanced stage of malignancy | 4 (17) |
| advanced cardiovascular disease (CAD, CVD, gastrointestinal ischaemia) | 10 (44) |
| missing quality of life | 3 (13) |
| others (acute pancreatitis, calciphylaxy, imminent amputation, dementia, new diagnosis of cancer) | 6 (26) |
| Days to death after withdrawal of dialysis | 23 (100) |
| 2–3 days | 11 (48) |
| 4–6 days | 5 (22) |
| 7–15 days | 4 (17) |
| >200 days | 3 (13) |
| Cause of death | 23 (100) |
| unknown | 8 (35) |
| cardiac event | 6 (26) |
| uraemia | 4 (17) |
| other (respiratory failure, bowel ischemia, bleeding, carcinoma) | 5 (22) |
| Autopsy | 2 (9) |
| Died at hospital / at home (%) | 18 / 5 (78 / 22) |
| Data are displayed as counts and percentages (%) or median plus range [r]. CAD: coronary artery disease; CVD: cerebrovascular disease | |
In this study we specifically examined the survival of ESRD patients undergoing chronic haemodialysis at a Swiss dialysis centre between 1995 and 2006. Most importantly we found the median survival on chronic haemodialysis to be 4.25 years. One, three and five year survival rates were 88%, 68% and 46% respectively.
These results are in agreement with the only other recently published study evaluating survival of Swiss haemodialysis patients. Saudan and his colleagues at the Western Switzerland Dialysis Study Group found three year survival on chronic haemodialysis to range from 54% to 79% depending on transplantation waiting list status [6]. The overall three year survival rate reached 61% in their cohort. The slightly lower three year survival rate of the Saudan cohort is probably caused by the lower percentage of incident dialysis patients, as well as the relatively large number of late referrals in their study. Furthermore, our findings are in concurrence with the results of the latest European Renal Association Annual Report [2]. Pooling data from 28 European countries this report found overall one, two and five year survival rates to be 84%, 72% and 46% respectively. Unfortunately, despite the substantial differences in age, co-morbidities and transplantation frequencies observed throughout the 28 countries, regional survival analyses of this report are presently not available.
Of note, an older study evaluating survival on haemodialysis between 1978 and 1990 previously described a five year survival rate of 87% [7]. This astonishing result is probably caused by the relatively young patient age and the higher than usual weekly time on dialysis (3 x 8 hours per week on average). Dialysis session length has repeatedly been found to be associated with survival [8]. In a recent study of 8552 American patients Brunelli and colleagues found dialysis sessions under 4 hours to be associated with a 42% increase in all cause mortality [9]. Similarly, an Australian registry study suggested the lowest mortality risk in patients undergoing dialysis sessions over 4.5 hours [10]. Furthermore, a recent randomized trial found patients undergoing in-centre haemodialysis six times per week to be less likely to experience a combined endpoint of death or increased ventricular hypertrophy after 12 months compared to patients undergoing only thrice weekly haemodialysis [11]. A time-dependent trend to lower mortality was present even in patients undergoing short daily haemodialysis [12]. It has recently been speculated, that the lower incidence of dialysis induced myocardial stunning in patients undergoing longer dialysis sessions might contribute to the improved outcomes in these patients [13]. Patients in our study generally underwent 3 x 4 hours of dialysis per week.
Additionally, this study showed no significant effect of gender on survival. Similarly, no gender differences in mortality were observed in a DOPPS sub-analysis [4], a French community-based study [14] and an American registry study [15]. However, in contradiction to the results presented here diabetes mellitus has previously been described as carrying an increased mortality risk [4, 16]. Interestingly, a recent study even found HbA1c levels in non-diabetic patients to predict mortality [17]. Despite not observing an increased mortality in diabetic patients in this study, we did find a trend towards shorter survival in male diabetics. This trend, however, did not reach significance, probably due to the small sample size analysed in this study.
The present paper further confirms cardiac death to be the most important cause of death in haemodialysis patients. In this analysis a third of all attributable deaths were caused by cardiac reasons. In agreeance with our results, cardiac death accounts for 41% of all-cause mortality in the USRDS database [18], while in the HEMO, the 4D and the AURORA study, cardiac death accounted for up to 50% of the observed mortality of dialysis patients [19–21]. These observations characterise the cardiorenal syndrome type IV, in which primary kidney disease has been postulated to cause decreased cardiac function and an increased risk of adverse cardiovascular events [22, 23]. The slightly lower number of cardiac deaths observed in the present study are probably due to the retrospective analysis of our data and the exclusion of sudden death in the definition of cardiac death. Hence, the cause of death was not attributable in 37 patients. We expect that with more autopsy results the number of cardiac deaths would have been even higher.
Dialysis was terminated in 9% of all patients and contributed to death in one fourth of all deaths. Recently a “Dialysis Outcomes and Practice Patterns Study” sub-analysis described considerable regional differences for the frequency of dialysis withdrawal [24]. While withdrawal from dialysis was uncommon in Germany and Italy, it appeared more prominent in France, the United Kingdom and the United States of America. Withdrawal from dialysis was most common in the United States of America with 3.5 terminations occurring per 100 patient years on chronic haemodialysis. This rate is comparable to the rate observed during our study. The higher rate of withdrawal from haemodialysis observed in the present study might be a consequence of our unit’s emphasis on the principle of autonomy, i.e. the right of patients to make choices about their own lives. Additionally, in Switzerland there is an open national debate on “end-of-life decisions” with discussions about the need for and the adequate format of living will`s being broadcasted on national television [25]. Furthermore, two nationwide agencies are battling for the right to medically assisted suicide in terminally ill patients. Consequently, this topic has been widely debated in the media and the general population. We hypothesize that due to these circumstances, Swiss patients are more likely to consider the option of withdrawal from live saving therapies such as haemodialysis and to approach their families and physicians with this wish. Additionally, differences between the legislative and judicial systems may partially explain the regional differences observed in the DOPPS sub-study.
In the course of the study period a malignant disease was diagnosed in 70 patients (26%). Since we selectively enrolled patients undergoing chronic haemodialysis, we cannot comment on the relative risk of certain malignoma in our cohort. Additionally, in contrast to our results, previous studies mainly described the prevalence of malignoma at study enrolment [19–21]. However, some ten years ago, a large international registry analysis demonstrated a significant increase in cancer mortality for dialysis patients [26]. Among European patients, the most frequent malignancies were located in the genitourinary (kidney: HR 3.60, 95%CI [3.45–3.76], bladder: HR 1.50, 95%CI [1.42–1.57]) [26], and endocrine system (HR 2.28, 95%CI [2.03–2.54]). Importantly, the incidence of cancers of the lung, colorectum, prostate, breast, and stomach was not consistently increased. Hence, general carcinoma screening is not recommended in patients on chronic haemodialysis [27]; instead screening efforts should be initiated according to the individual patient`s risk as well as the estimated lifetime remaining.
During the observational period 69 (26%) patients underwent kidney transplantation, equalling a transplantation rate of 10.8 per 100 patient years on chronic haemodialysis. This compares favourably to European transplantation rates, which vary widely among countries, from 3.3 kidney transplants per 100 patient years on dialysis in Italy to 11.6 in Spain [5]. These considerable differences are largely due to regional practices and legal ramifications in living donor and deceased donor transplantation and organ allocation. Spain’s high transplantation rate is based on a very efficient deceased donor organ allocation system, while our unit traditionally favours living organ transplantations.
It needs to be noted that patients undergoing kidney transplantation during the observational period were significantly younger and suffered from less co-morbidities compared to patients not receiving transplantation. This observation is backed by a report from the USRDS database [28] and a recent DOPPS sub-study, which also described transplant recipients as being younger, predominantly male and suffering from fewer co-morbidities [29]. These baseline differences between the dialysis and the transplant population need to be remembered and adjusted for when comparing mortality figures. They can largely explain the almost 50% lower adjusted mortality of patients on the transplantation waiting list compared to non-wait-listed dialysis patients [28].
Our study has several limitations. Firstly, we retrospectively analysed a relatively small number of patients enrolled at a single dialysis centre. Secondly, we did not evaluate data concerning the adequacy of dialysis or the treatment of secondary complications of ESRD (i.e. anaemia control, calcium and phosphate product, serum albumin, pre-dialysis blood pressure, type of vascular access and spKt/V). However, all data were collected prospectively at the time of dialysis. Furthermore baseline characteristics and mortality rates were similar to those observed in other national and international dialysis studies as well as the European Renal Association Annual Report.
Survival on chronic haemodialysis treatment in Switzerland has not been analysed in previous international multicentre trials. However, indirect comparisons show favourable results in relation to international reference values. A negative selection of people in respect to age and burden of cardiovascular diseases due to the activity of transplant programs has to be considered in the interpretation of survival analysis. Additionally, it is important to note that the policy regarding withdrawal of dialysis may vary considerable between centres and countries with implications on mortality data.
The authors thank Catherine Haenlin and Carla Hertel from the nursing team and Thomas Voegele, transplantation coordinator for their help during the data collection process.
1 2002 Albert Lasker Award for Clinical Medical Research. J Am Soc Nephrol. 2002;13:3027–30.
2 European Renal Association EDaTA. Annual Report. 2007.
3 Held PJ, Brunner F, Odaka M, Garcia JR, Port FK, Gaylin DS. Five-year survival for end-stage renal disease patients in the United States, Europe, and Japan, 1982 to 1987. Am J Kidney Dis. 1990;15:451–7.
4 Goodkin DA, Bragg-Gresham JL, Koenig KG, et al. Association of co morbid conditions and mortality in haemodialysis patients in Europe, Japan, and the United States: the Dialysis Outcomes and Practice Patterns Study (DOPPS). J Am Soc Nephrol. 2003;14:3270–7.
5 Rayner HC, Pisoni RL, Bommer J, et al. Mortality and hospitalization in haemodialysis patients in five European countries: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant. 2004;19:108–20.
6 Saudan P, Kossovsky M, Halabi G, Martin PY, Perneger TV. Quality of care and survival of haemodialysed patients in western Switzerland. Nephrol Dial Transplant. 2008;23:1975–81.
7 Charra B, Calemard E, Ruffet M, et al. Survival as an index of adequacy of dialysis. Kidney Int. 1992;41:1286–91.
8 Kurella M, Chertow GM. Dialysis session length (“t”) as a determinant of the adequacy of dialysis. Semin Nephrol. 2005;25:90–5.
9 Brunelli SM, Chertow GM, Ankers ED, Lowrie EG, Thadhani R. Shorter dialysis times are associated with higher mortality among incident haemodialysis patients. Kidney Int. 77:630–6.
10 Marshall MR, Byrne BG, Kerr PG, McDonald SP. Associations of haemodialysis dose and session length with mortality risk in Australian and New Zealand patients. Kidney Int. 2006;69:1229–36.
11 In-Center Hemodialysis Six Times per Week versus Three Times per Week. New England Journal of Medicine;0.
12 Kjellstrand C, Buoncristiani U, Ting G, et al. Survival with short-daily haemodialysis: Association of time, site, and dose of dialysis. Hemodial Int. 14:464–70.
13 Jefferies HJ, Virk B, Moran J, Schiller B, McIntyre CW. Frequent haemodialysis schedules are associated with reduced levels of dialysis-induced cardiac injury (myocardial stunning). Clin J Am Soc Nephrol in Press.
14 Kessler M, Frimat L, Panescu V, Briancon S. Impact of nephrology referral on early and midterm outcomes in ESRD: Epidemiologie de l’Insuffisance Renal chronique terminale en Lorraine (EPIREL): results of a 2-year, prospective, community-based study. Am J Kidney Dis. 2003;42:474–85.
15 Miskulin DC, Meyer KB, Martin AA, et al. Comorbidity and its change predict survival in incident dialysis patients. Am J Kidney Dis. 2003;41:149–61.
16 Hayashino Y, Fukuhara S, Akiba T, et al. Diabetes, glycaemic control and mortality risk in patients on haemodialysis: the Japan Dialysis Outcomes and Practice Pattern Study. Diabetologia. 2007;50:1170–7.
17 Chen KH, Lin JL, Lin-Tan DT, et al. Glycated Hemoglobin Predicts Mortality in Nondiabetic Patients Receiving Chronic Peritoneal Dialysis. Am J Nephrol. 32:567–74.
18 National Institute of Health US Renal Data System: USRDS 2008 Annual Data Report. 2008.
19 Cheung AK, Sarnak MJ, Yan G, et al. Cardiac diseases in maintenance haemodialysis patients: results of the HEMO Study. Kidney Int. 2004;65:2380–9.
20 Wanner C, Krane V, Marz W, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing haemodialysis. N Engl J Med. 2005;353:238–48.
21 Fellstrom BC, Jardine AG, Schmieder RE, et al. Rosuvastatin and cardiovascular events in patients undergoing haemodialysis. N Engl J Med. 2009;360:1395–407.
22 Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol. 2008;52:1527–39.
23 Breidthardt T, Mebazaa A, Mueller C. Predicting progression in nondiabetic kidney disease: the importance of cardiorenal interactions. Kidney Int. 2009;75:253–5.
24 Fissell RB, Bragg-Gresham JL, Lopes AA, et al. Factors associated with “do not resuscitate” orders and rates of withdrawal from haemodialysis in the international DOPPS. Kidney Int. 2005;68:1282–8.
25 http://www.puls.sf.tv . Puls SF. 9.11.2009.
26 Maisonneuve P, Agodoa L, Gellert R, et al. Cancer in patients on dialysis for end-stage renal disease: an international collaborative study. Lancet. 1999;354:93–9.
27 Holley JL. Screening, diagnosis, and treatment of cancer in long-term dialysis patients. Clin J Am Soc Nephrol. 2007;2:604–10.
28 Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725–30.
29 Satayathum S, Pisoni RL, McCullough KP, et al. Kidney transplantation and wait-listing rates from the international Dialysis Outcomes and Practice Patterns Study (DOPPS). Kidney Int. 2005;68:330–7.
No funding; no competing interests.