Pacemakers and magnetic resonance imaging
DOI: https://doi.org/10.4414/smw.2011.13147
D
Weishaupt, H
Hoppe, P
Votik
Summary
Electromagnetic fields arising from magnetic resonance imaging (MRI) can cause various clinically relevant functional disturbances in patients with cardiac pacemakers. Consequently, an implanted pacemaker is generally considered a contraindication for an MRI scan. With approximately 60 million MRI scans performed worldwide per year, MRI may be indicated for an estimated majority of pacemaker patients during the lifetime of their pacemakers. The availability of MR conditional pacemakers with CE labelling is of particular advantage since they allow the safe use of pacemakers in MRI. In this article the current state of knowledge on pacemakers and MR imaging is discussed. We present the results of a survey conducted among Swiss radiologists to assess current practice in patients with pacemakers.
Current status and survey in Switzerland
Introduction
Magnetic resonance imaging (MRI) is currently considered the imaging method of choice for a wide spectrum of diseases and pathologies. Due to its excellent soft-tissue contrast, MRI is useful in particular for imaging of the brain, the head and neck, the spine and the musculoskeletal system. In recent years MRI has also proven useful for the imaging of other body areas including the abdomen, pelvis, breast, heart and vascular system. Apart from imaging morphology MRI also provides insights into tissue function and metabolism. In combination with novel contrast agents MRI makes it possible to selectively visualise certain cell lines enabling cell-specific imaging. Another advantage of MRI is the lack of potentially harmful irradiation. The number of MRI scans is still increasing annually, and the trend towards increasing use of MRI will continue in the future, due to the nearly unlimited potential of the method.
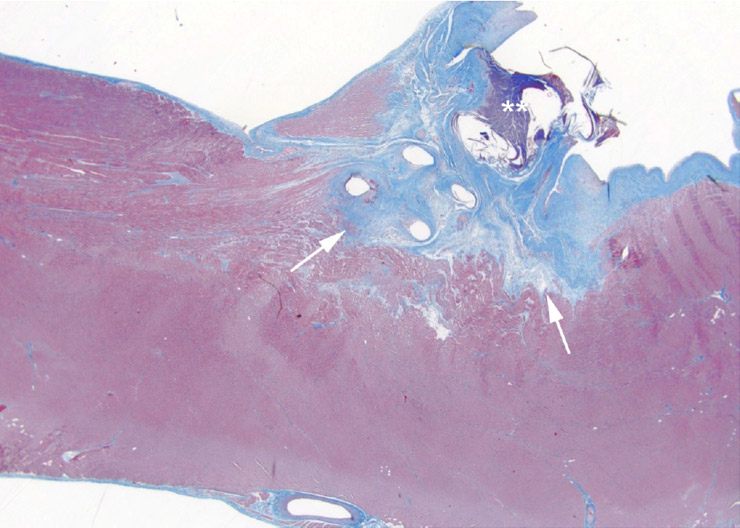
Figure 1
Histological specimen of the heart muscle obtained in a pig with implanted conventional pacemaker lead after imaging with a high power RF MRI scan. Tissue damage due to heating around the lead tip is visible (arrow). Note: The location of the lead tip is highlighted by asterisks.

Figure 2
A Axial post-contrast MR image in patient with an MRI SureScan pacemaker.
All anatomical details including left atrial appendage, left pulmonary vein and the main stem of the left coronary artery (arrowhead) are visualised well. The lead of the pacemaker causes only a small artifact (arrow).
B MR image in short-axis view in a patient with a SureScan pacemaker. The proximal portion of the right ventricle is well visualised. The pacemaker lead creates an artifact at the posterior aspect of the tricuspid valve annulus (arrow). RCA = right coronary artery; PA = pulmonary artery; TVP = tricuspid-valve plane; LV = left ventricle.
(images courtesy of Prof. J. Schwitter, CHUV, Lausanne/CH).
The growing problem
Parallel to the growth and evolution of MR technology an increasing number of patients are benefiting from cardiac pacemakers [1–2]. In 2009, 4085 initial implantations of cardiac pacemakers were recorded in Switzerland. In total, 31 825 patients in Switzerland have a pacemaker implanted and are monitored by cardiologists [3]. With the growing proportion of the elderly population it can be expected that the number of pacemaker patients will increase even more in the next few years. The trend towards implantation of pacemakers in older age groups is also accompanied by an increasing number of co-morbidities in those patients. A recently published study by Kurtz et al. [4] has shown that the co-morbidity index in Medicare patients with newly implanted pacemakers increased in the period 1993–2006.
Considering the prevalence of frequently occurring diseases in the older age group, it is evident that diseases affecting the neurological, cardiovascular and musculoskeletal system are particularly frequent. Precisely in this disease spectrum MRI is a very powerful method not only for diagnosis but also for monitoring of disease. Although there are imaging alternatives for a wide spectrum of diseases, there are some diseases where MRI is the only imaging method to establish the diagnosis. Reflecting all these issues, it has been estimated that in 50–75% of all pacemaker patients an MRI examination is indicated at least once in the lifetime of their device [5]. Since there is an increasing need for MR imaging in such patients, MRI-compatible pacemakers are a desideratum [1].
MRI and conventional pacemakers
In this paper we use the term “conventional pacemakers” when discussing pacemaker-related issues concerning devices which are not specifically designed for use with MRI scanning. Conventional pacemakers encompass nearly all pacemakers which are currently on the market, with the exception of the newly available MR compatible pacing systems with the trade names MRI SureScan (Medtronic International, Tolochenaz, Switzerland) and ProMRI (Biotronik GmbH & Co., Berlin, Germany).
MRI in patients with conventional cardiac pacemakers may be hazardous due to the possible interaction of the device with the different electromagnetic field used for MRI [6–8]. It is important to underline that both the device itself and the leads may interact with the different electromagnetic fields used in MRI. MRI uses three different electromagnetic fields to produce images: the main magnetic field; the time-varying magnetic gradient fields, and the pulsed radiofrequency (RF) field. The main magnetic field is used to align protons and is always on. Currently, MR imaging is performed at magnetic field strengths between 0.5 and 3.0 Tesla (T), whereas most MR scanners work at 1.5 T. The time-varying magnetic gradient fields are used for spatial localisation and change their strength along different orientations. The pulsed RF-field is generated by the body coil or send/receive coils and is used to change the energy state of the protons as well as elicit MRI signals from tissue.
Pacemakers can interact with all of the three types of electromagnetic field used during MRI. The theoretical interactions between conventional pacemakers and the electromagnetic fields affecting the device and the leads are listed in table 1.
The most important risk posed by MR imaging in patients with pacemakers is a transient or permanent influence or definite damage to the electric components of the pacemaker device (i.e., activation of the reed-switch, pacemaker resets of reprogramming). This interaction may lead to asynchronous or unpredictable pacing, which may result in tachycardia or asystole [9]. Also, animal testing has demonstrated that the temperature at the lead tip increased up to 20 °C during MRI scanning of the heart, thus possibly resulting in tissue damage [10] (fig. 1). The risk of pacemaker displacement by a possible interaction with the static magnetic field is considered to be of minor importance. Another disadvantage of conventional pacemakers is the fact that the device and the leads produce metallic artifacts which may hamper imaging interpretation at the level of the heart and the mediastinum. First experience with MRI SureScan devices did indicate that the artifacts induced by the leads and the device itself may not limit the MR scan’s diagnostic value (fig. 2).
The potentially hazardous complications for patients with a conventional pacemaker in whom MRI were accidentally performed are underlined by the reported lethal consequences in these patients. In Germany alone there have been six fatalities relating to MR examinations in pacemaker patients [11]. In three of these cases there was suggestive evidence that death may have occurred due to induced ventricular fibrillation [11]. It may be assumed that the complication rate in patients with pacemakers accidentally undergoing MR imaging is higher than is reflected by the number of cases published in the literature.
The potentially lethal outcome from the interaction of MR-generated electromagnetic interference on pacemakers provides the medical basis for the widely held view that MRI is absolutely contraindicated in patients with cardiac pacemakers.
Several reports in the literature have questioned this strict viewpoint with regard to the use of MRI in pacemaker patients. In these studies more or less large series of pacemaker patients are described who have safely undergone MR scanning [8, 12–16]. The common feature of these studies is that they all were performed under dedicated safety precautions (such as patient selection, monitoring, reanimation preparedness, prior and subsequent examinations), and that imaging should be performed in body areas more distant from the chest, such as the brain and knee. In addition, special pacemaker program settings (such as the OFF mode; i.e., sensing (monitoring)-only mode (0A0, 0V0, 0D0)) are recommended in order to avoid arrhythmias when MR imaging is performed [15].
It is against the background of these controversial data that the safety guidelines of professional societies of medical physicists, cardiologists and radiologists regarding MR safety of conventional pacemakers have to be seen. Although the recommendations of the ACR Blue Ribbon panel on MR Safety, the European Heart Rhythm Association and Working Group on Cardiovascular Magnetic Resonance of the European Society of Cardiology (EuroCMR), the American College of Cardiology Foundation, the North American Society for Cardiac Imaging differ slightly from each other, it is commonly agreed that MRI in pacemakers is not clinical routine and MRI should only be performed under dedicated safety precautions and by experienced personnel from medical physics, cardiology and radiology [17–19]. It is evident that the indication to perform MRI in these patients must be very strict, and there should be consensus between the referring clinician and the radiologist on the clinical need for the exam, meaning that no other imaging method is available to answer the relevant clinical question.
Data on the management of patients with conventional pacemakers who are referred for MRI in clinical practice are scarce in the literature. In a 2001 survey from the Cleveland Clinic [20] academic cardiologists and radiologists were asked whether they would scan a pacemaker patient. Responses from radiologists suggested that 97% would not do so, whereas 34% of the cardiologists said they would do so under certain circumstances.
Swiss survey on the use of MR in pacemaker patients
We performed a survey designed to explore the current practice and knowledge of radiologists with regard to MRI in patients with a conventional pacemaker.
The purpose of this survey of radiologists in Switzerland was twofold: first, to provide data on the use of MR imaging in pacemaker patients, and second, to determine the potential use of MRI conditional pacemakers.
|
Table 1:Potential interactions of MRI on a pacemaker device (from [9]). |
|
|
Pacemaker
|
Lead
|
|
Main magnetic field
|
Magnetic forces1
|
Magnetic forces1
|
|
|
Magnetic torque1
|
Magnetic torque1
|
|
|
Interaction with the reed-switch2
|
|
|
|
Destruction of the device in case of high voltage charging2
|
|
|
Gradient field
|
Inhibition/Fast pacing2
|
Stimulation of the heart2
|
|
|
Pacemaker resetting2
|
|
|
|
Vibrations1
|
|
|
RF field
|
Destruction of device circuits2
|
Heating effects at the tip of the leads2
|
|
|
Pacemaker reprogramming2
|
|
|
|
Pacemaker resetting2
|
Stimulation of the heart2
|
|
|
Inhibition/Fast pacing2
|
|
|
1 Interactions of theoretical concern but without clinical relevance in humans.
2 Reported interactions of conventional pacemakers during MRI in humans. |
Material and methods
Based on the records of a dedicated company acting in health care consulting (IMS Health GmbH, Hergiswil, Switzerland) a total of 576 board-certified radiologists working in public and private hospitals and in private practice in all parts of Switzerland were contacted in writing. All radiologists were asked to complete a fully-structured online questionnaire available in German and French. All participants received a personal password along with the access information. Only one questionnaire could be completed per password. In addition to the personal details (name, position, institution), the 25-question questionnaire gathered institution-specific information, such as the frequency and handling of cases in which patients were referred for MR examination despite their having pacemakers. The questions also addressed the potential use of MR conditional pacemakers and the practical procedure or administration of MR examinations in patients with MR conditional pacemakers. The survey took place within a period of six weeks from March to April 2009, months before the first MRI conditional pacemaker was commercially available.
Results
Demographics of participants and radiological equipment
A total of 70 Swiss radiologists participated in the survey (total participation rate of 12%). All participants fully completed the questionnaire. Participants were from 56 radiological institutions (radiological institutes in public hospitals, 37 (53%); radiological institutes in private hospitals, 15 (21%) and private practice not in a hospital setting, 18 (26%). Fifty-five of 56 institutions (98%) of which a radiologist took part in the survey were equipped with at least one MR scanner. 83% of the participating radiology institutes located either in private or public hospitals had permanent access to a cardiology department. Among the radiologists working in private practice, 61% had no access to a permanently employed cardiologist.
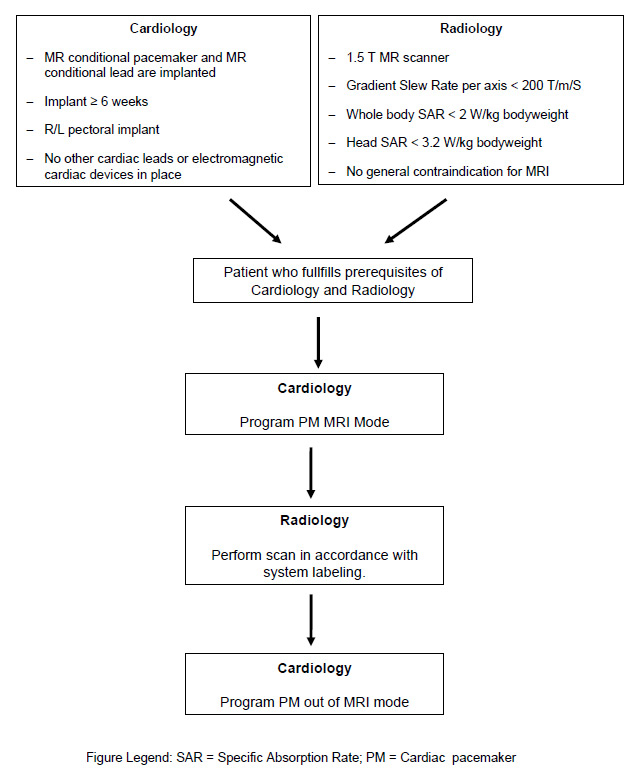
Figure 3
Prerequisites and patient workflow of a patient with a conditional cardiac pacemaker (PM) who needs an MR scan.
The distribution of MR scanners according to magnetic field strength was as follows: 76 1.5 Tesla (T) MR scanners, 37 3.0 T MR scanners, and 17 MR scanners with a magnetic field strength below 1.0 T. The mean number of MR examinations performed per centre was 5516 (SD = 3581). The majority of the radiologists (67%) described their interest in the topic of MRI and pacemakers as “great”, 29% as “moderate”, and 4% as “little” or “no interest”.
Current practice in pacemaker patients with regard to MR imaging
A patient with an implanted pacemaker is considered an absolute contraindication for MR examination by 93% of the radiologists. Approximately 7% of the radiologists interviewed reported that the presence of a pacemaker was considered a relative contraindication in their institution. In 2008 in each institution approximately 30 pacemaker patients on average were referred for MR examination. In 2008, in only one patient with a conventional MR pacemaker was an MR scan performed among all institutions in Switzerland. An alternative imaging method was used in all other patients with conventional pacemakers who were initially referred for possible MR scanning. Approximately 58% of responders consulted the referring physician where the referral was of a patient with a pacemaker. Only a minority subsequently performed the requested scan, usually after consultation and under supervision, and/or with prior or subsequent examination by a cardiologist.
Radiologists were asked to estimate the proportion of patients who, because of the pacemaker contraindication, are probably not referred for an MR examination at the radiological institution at all, and examined instead by other imaging methods. The proportion of MR examinations not carried out at the survey participants’ workplaces (because refused) was estimated at a mean of 1.9% (SD 1.8%) of all MR examinations.
This survey underlines the careful approach of radiologists working in Switzerland when patients with conventional pacemakers are referred for MRI. MRI in patients with conventional pacemakers is currently considered an absolute contraindication for the majority of patients, and is only performed when there is no alternative imaging method and under strict safety conditions. If an MR examination in a patient with a conventional pacemaker is performed, the procedure is done under strict safety precautions and in close cooperation with a cardiologist and a medical physicist. We acknowledge the following limitations of the study: first, with the exception of one single institution, all radiologists who took part in the survey worked in institutions which were equipped with at least one MR scanner. Second, the response rate of the radiologists initially contacted was relatively low.
To summarise, the survey has demonstrated that clinical practice does not follow the recommendations of the professional societies which consider patients with conventional pacemakers a relative contraindication for MR examinations. The clinical practice in Switzerland is stricter in this patient group.
MR conditional pacemakers
An MR conditional pacemaker is a specially designed device for safe use in the MR environment. MR conditional pacemakers are dedicated pacing systems designed to avoid potential interactions with the electromagnetic fields of MR imaging, and to avoid complications of conventional pacemakers including arrhythmias, inhibition of the pacing output, triggered stimulations and radiofrequency heating of the pacing leads with potential thermal damage to the electrode/tissue interface. Technically the MR compatibility of these conditional pacemakers is achieved by minimising the energy discharged at electrodes and by ensuring reliable operation while MRI is active. Special design of the leads is necessary to reduce lead tip heating from RF. However, since MR is a rapidly evolving imaging method and as long as a pacing system contains wires, the term “MR safe” will never be applied, and in particular also considering the legal aspects. Therefore, any MR compatible cardiac pacemaker will be called MR conditional. The term “conditional” means that the device can undergo an MR examination under conditions provided by the pacemaker manufacturer, such as maximum specific absorption rate (SAR) levels, allowed field strength, excluded body regions etc.
In the above-mentioned survey the radiologists were also asked for their opinion on MR conditional pacemakers. The usefulness of MR conditional pacemakers was considered by survey responders to be “great”. On a scale of 0 (“not useful”) to 6 (“very useful”), the average score was 4.97. Their usefulness in patients with cerebral diseases or injuries and spinal cord diseases or injuries was rated highest.
Clinical experience with MR conditional pacemakers
The first MR conditional pacemaker system has been on the market since 2010 (the Enrythm® MRI SureScan™ pacemaker). The Enrythm MRI SureScan pacing system has been successfully tested pre-clinically and clinically in 1.5 T MR scanners [21–22]. In two trials MR scans of the brain and lumbar spine were successfully performed without complications in patients with MR conditional pacemakers [21–22]. Based on the positive results of the aforementioned studies as well as on other unpublished safety data, the MRI SureScan device was initially authorised for use in MR scanners with a magnetic field strength of up to 1.5 T and in all body regions but with a restriction on imaging of the chest. In the meantime the MRI SureScan system has been extensively tested in computer models and in vivo canine studies for the entire MR imaging spectrum including MR imaging of the chest with the heart (internal unpublished safety data of Medtronic). Based on these additional safety data the MRI Surescan pacemaker system received the CE mark of approval for the full body scan indication (fig. 3). FDA approval will be forthcoming in 2011. Another MR conditional pacemaker system has recently been marketed (ProMRI pacing system). Similar to the first generation of SureScan devices, the ProMRI pacing system is restricted to scanning outside the chest.
The common feature of all currently available MR conditional pacemakers is that these devices should not undergo MR imaging at any other magnetic field strengths than 1.5 T.
Clinical experience with MR compatible pacemakers is so far limited. In a prospective trial Forleo et al. [23] compared the safety and effectiveness of an MR conditional pacemaker system with a conventional DDD implant. The investigators reported no difference between the two groups with regard to implantation or technical success rates. The prerequisites and workflow of a patient with an MR compatible pacemaker who needs an MR scan is shown in figure 3. It is of practical importance that a special programming modus (MRI mode) must be set on the MR conditional pacemaker before the MR scanner. After scanning this MRI mode must be turned off. Usually both the setting and turning-off of the MRI mode are done by the cardiologist.
Which patients are candidates for implantation of MR conditional pacemakers?
With the introduction of MR conditional pacemakers the discussion regarding the patients in whom the device should be implanted is launched. Clearly at this time there are not enough data available to conclude that MR conditional pacemakers are equal to conventional pacemakers with regard to technical success, functionality and long term results. However, assuming that MR conditional pacemakers and conventional pacemakers are equal with regard to technical success, efficacy and outcome, selection of the device should be evaluated carefully. At the present time, patients who need a pacemaker and who have a pre-existing co-morbidity of an oncological, neurological, orthopaedic or cardiovascular disease should be proposed for implantation of an MR conditional pacemaker system. Alternatively, the patients should at least be considered for implantation of MR compatible leads. Although this seems to be a short term strategy, since almost all device manufacturers are announcing MR conditional pacemakers, it is very likely that MR conditional pacemakers will become standard of care in the near future.
Outlook
Currently available MR conditional pacing systems meet an increasing demand for imaging in a growing number of patients with pacemakers. New generations of MR pacemakers with fewer restrictions and more automaticity can be expected, resulting in faster and easier procedures. Future research will also be directed to development of MR compatible implantable cardioverter defibrillator (ICD) devices. However, at the time of writing there is no ICD product on the horizon which meets the demand for an MR conditional ICD.
Future generations of MR compatible pacemakers will also be approved for 3.0 T scanning, although at this time this is not a real limitation since MR imaging at 1.5 T is adequate for most imaging requirements.
Study funding / potential competing interests
The results of the survey published in this article were supported by a grant from Medtronic (Schweiz) AG.
Correspondence:
Professor Dominik Weishaupt
Institut für Radiologie
Stadtspital Triemli
Birmensdorferstrasse 497
CH-8063 Zürich
Switzerland
dominik.weishaupt@triemli.zuerich.ch
References
1 Auricchio A, Moccetti T. Electronic cardiac medicine: present and future opportunities. Swiss Med Wkly. 2010;140:w13052.
2 Schaer B, Kuhne M, Koller MT, Sticherling C, Osswald S. Therapy with an implantable cardioverter defibrillator (ICD) in patients with coronary artery disease and dilated cardiomyopathy: benefits and disadvantages. Swiss Med Wkly. 2009;139(45-46):647–53.
3 SGK AHuEd. Statistik für Herzschrittmacher, ICD und Katheterablationen 2009. SGK; 2009 [cited 2009 1.7.2009]; http://www.pacemaker.ch/de/statistik/].
4 Kurtz SM, Ochoa JA, Lau E, Shkolnikov Y, Pavri BB, Frisch D, et al. Implantation trends and patient profiles for pacemakers and implantable cardioverter defibrillators in the United States: 1993–2006. Pacing Clin Electrophysiol. 2010;33(6):705–11.
5 Kalin R, Stanton MS. Current clinical issues for MRI scanning of pacemaker and defibrillator patients. Pacing Clin Electrophysiol. 2005;28(4):326–8.
6 Bhachu DS, Kanal E. Implantable pulse generators (pacemakers) and electrodes: safety in the magnetic resonance imaging scanner environment. J Magn Reson Imaging. 2000;12(1):201–4.
7 Duru F, Luechinger R, Scheidegger MB, Luscher TF, Boesiger P, Candinas R. Pacing in magnetic resonance imaging environment: clinical and technical considerations on compatibility. Eur Heart J. 2001;22(2):113–24.
8 Mollerus M, Albin G, Lipinski M, Lucca J. Ectopy in patients with permanent pacemakers and implantable cardioverter-defibrillators undergoing an MRI scan. Pacing Clin Electrophysiol. 2009;32(6):772–8.
9 Luechinger R, Duru F. Do we need MR conditional pacemakers? Kardiovaskuläre Medizin. 2010;13(00):1–5.
10 Luechinger R, Zeijlemaker VA, Pedersen EM, Mortensen P, Falk E, Duru F, et al. In vivo heating of pacemaker leads during magnetic resonance imaging. Eur Heart J. 2005;26(4):376–83; discussion 25–7.
11 Irnich W, Irnich B, Bartsch C, Stertmann WA, Gufler H, Weiler G. Do we need pacemakers resistant to magnetic resonance imaging? Europace. 2005;7(4):353–65.
12 Sommer T, Vahlhaus C, Lauck G, von Smekal A, Reinke M, Hofer U, et al. MR imaging and cardiac pacemakers: in-vitro evaluation and in-vivo studies in 51 patients at 0.5 T. Radiology. 2000;215(3):869–79.
13 Naehle CP, Meyer C, Thomas D, Remerie S, Krautmacher C, Litt H, et al. Safety of brain 3-T MR imaging with transmit-receive head coil in patients with cardiac pacemakers: pilot prospective study with 51 examinations. Radiology. 2008;249(3):991–1001.
14 Martin ET, Coman JA, Shellock FG, Pulling CC, Fair R, Jenkins K. Magnetic resonance imaging and cardiac pacemaker safety at 1.5-Tesla. J Am Coll Cardiol. 2004;43(7):1315–24.
15 Gimbel JR, Bailey SM, Tchou PJ, Ruggieri PM, Wilkoff BL. Strategies for the safe magnetic resonance imaging of pacemaker-dependent patients. Pacing Clin Electrophysiol. 2005;28(10):1041–6.
16 Shinbane JS, Colletti PM, Shellock FG. MR in patients with pacemakers and ICDs: Defining the issues. J Cardiovasc Magn Reson. 2007;9(1):5–13.
17 Kanal E, Barkovich AJ, Bell C, Borgstede JP, Bradley WG, Jr., Froelich JW, et al. ACR guidance document for safe MR practices: 2007. AJR Am J Roentgenol. 2007;188(6):1447–74.
18 Levine GN, Gomes AS, Arai AE, Bluemke DA, Flamm SD, Kanal E, et al. Safety of magnetic resonance imaging in patients with cardiovascular devices: an American Heart Association scientific statement from the Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology, and the Council on Cardiovascular Radiology and Intervention: endorsed by the American College of Cardiology Foundation, the North American Society for Cardiac Imaging, and the Society for Cardiovascular Magnetic Resonance. Circulation. 2007;116(24):2878–91.
19 Roguin A, Schwitter J, Vahlhaus C, Lombardi M, Brugada J, Vardas P, et al. Magnetic resonance imaging in individuals with cardiovascular implantable electronic devices. Europace. 2008;10(3):336–46.
20 Gimbel JR, Lorig RJ, Wilkoff BL. Survey of magentic resonance imaging in pacemaker patients. Available at: http://wwwheartweborg. 2001.
21 Sutton R, Kanal E, Wilkoff BL, Bello D, Luechinger R, Jenniskens I, et al. Safety of magnetic resonance imaging of patients with a new Medtronic EnRhythm MRI SureScan pacing system: clinical study design. Trials. 2008;9:68.
22 Wilkoff BL, Bello D, Taborsky M, Vymazal J, Kanal E, Heuer H, et al. Magnetic Resonance Imaging in Patients with a Pacemaker System Designed for the MR Environment. Heart Rhythm. 2010 Oct 5.
23 Forleo GB, Santini L, Della Rocca DG, Romano V, Papavasileiou LP, Magliano G, et al. Safety and efficacy of a new magnetic resonance imaging-compatible pacing system: early results of a prospective comparison with conventional dual-chamber implant outcomes. Heart Rhythm. 2010;7(6):750–4.



