
Figure 1
Reprogramming human patient samples can generate patient-specific human induced pluripotent stem (iPS) cells. iPS cells share extensive defining characteristics with embryonic stem cells.
DOI: https://doi.org/10.4414/smw.2011.13144
The most anticipated clinical application of stem cells is in customised cell therapies where cells, or tissues derived from them, are introduced into a patient’s body [1]. While possible in principle, these applications have, up to this point, been largely limited to the hematopoietic system using adult stem cells. Significantly more research is required for application to other tissues and physiological systems, particularly for therapies derived from embryonic stem cells. A much more immediate application of stem cell biology is to generate in vitro disease models [2]. Within the category of stem cells, embryonic stem cells hold special status as they can generate all tissues of the body (i.e., they are “pluripotent”) [3]. It is for this reason that embryonic stem cells are thought to have the potential both to treat a wide variety of human diseases and to faithfully model disease development in vitro.

Figure 1
Reprogramming human patient samples can generate patient-specific human induced pluripotent stem (iPS) cells. iPS cells share extensive defining characteristics with embryonic stem cells.
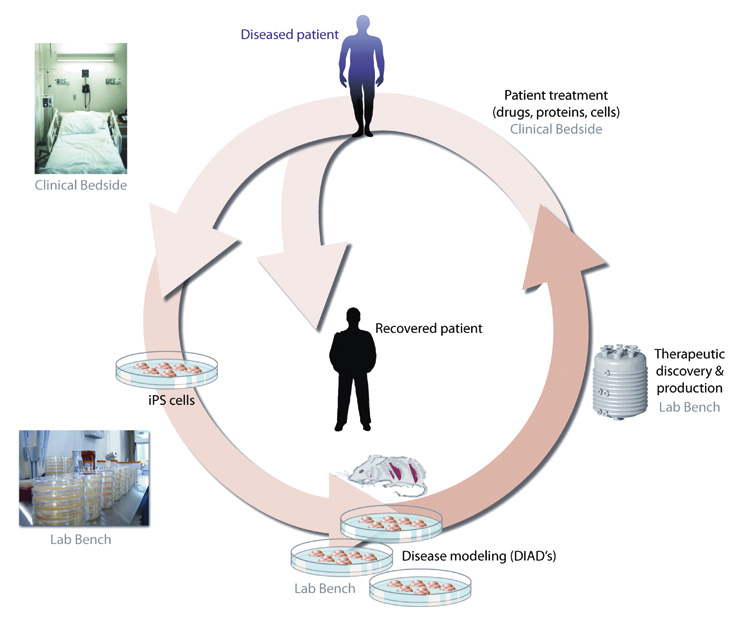
Figure 2
A schematic of the nonlinear connections between the laboratory and clinic. iPS cells derived from patients can be used to model disease and can also potentially feedback to the same patient through work in the laboratory to treat their disease.
In 2006, a technique was developed to “reprogram” adult skin cells to generate embryonic-like stem cells through a simple procedure involving four transcription factors [4] (fig. 1). Such cells, dubbed “induced pluripotent stem (iPS) cells,” have now been readily produced from a wide variety of human tissue biopsies and blood samples [2]. Samples include those from healthy, diseased, aged and young individuals [5]. Cell lines are increasingly being generated from patients afflicted by specific diseases, such as Parkinson’s, anaemia and schizophrenia, as well as rare conditions like LEOPARD Syndrome [5, 6]. These sets of human, patient-specific iPS cells are often called “disease-in-a-dish” (DIAD) models [2]. Some see DIAD as a biological surrogate for patients, faithful to the disease in the body but experimentally expendable. For instance, one researcher characterised the use of DIAD to screen pharmaceutical compound libraries as “clinical trials in a dish” [7].
The research community sees DIAD as a technology for preserving the natural, biological systems that are present in actual patients, while removing the scientific and ethical complexities of patient participation – and, thus, clinical involvement – in characterising human disease (fig. 2). DIAD can be used as a surrogate for patients’ bodies; the cells are a renewable and expendable experimental tool that ostensibly represent the same biological system as is present in the patient. Furthermore, it has been conceived as a tool for generating more precise and less mediated representations of the biology of disease. As one scientist has put it, DIAD is a tool for “drilling down” to fundamental disease mechanisms that are neither immediately evident nor accessible to study in the clinical presentation of the disease [8]. As such, DIAD is also a tool of standardisation and purification: DIAD models provide a stable, singular biological model of disease, controlling for the inevitable genotypic, phenotypic and behaviour/environmental variation between human beings.
Notwithstanding the promise of DIAD to deepen molecular understanding of pathology, there are several technical complexities that may represent pitfalls and artefacts, complicating direct translation to the clinic [2, 9]. There are substantial challenges to capturing the kinetics of disease onset and progression as well as the spatial localisation of disease in the patient’s body in a simple laboratory culture dish [2]. In addition, there are concerns about subtle epigenetic differences between iPS cells and embryonic stem cells that may have a meaningful and functional significance in disease modelling [9, 10].
While DIAD is conceived as a basic research technology, as a means to culture, model and expose the cellular and molecular basis of human disease, it is at the same time a translational tool, a pathway for generating clinically relevant knowledge, diagnostics and therapeutics. In both respects, as a basic technology or as a translational tool, it is a powerful platform for innovation. As a technology for modelling disease, it has the potential to enormously expand the possibilities for examining the biology of disease on the cellular and molecular level and to augment clinical understanding. DIAD models will, ideally, preserve genetic contributions to disease by maintaining an intact functional human genome, something that cannot be straightforwardly accomplished in animal models, in part because they typically lack conservation of gene order with humans and differ in the co-linearity of their genomes [2]. Given that cell lines are expandable and cells are expendable, DIAD models can be put through virtually any biochemical and physical assay in the lab and can be tracked dynamically as the embryonic-like cells mature into adult somatic cells. The expendable and plastic properties of such cells also give the DIAD project great translational potential as it may play a central role in future approaches to drug discovery and to the development of cell-based therapeutics [1].
DIAD is conceived as a platform technology that is not limited to specific disease or disease categories. The technology potentially offers a new tool and new methodologies for the study and treatment of human disease across the board. Given its broad scope and its dual potential in basic biology and translational innovation, DIAD positions non-clinical, laboratory research as a primary space for the production of knowledge and technologies with direct clinical application. In other words, DIAD is conceived as a tool for both studying and treating disease in the laboratory.
This prioritisation of laboratory-based research imagines translation as unidirectional from “bench to bedside,” from the esoteric knowledge of basic biology to applied tools of clinical medicine [2]. This linear model of translation is by no means unique to DIAD. However, given the expectation that DIAD will serve as both a model system for basic research on disease and as an experimental tool for producing and testing therapeutics, there is a tendency to re-imagine the laboratory not merely as a wellspring of new knowledge with potential clinical applications, but as a source of magic bullets – a space in which the problems of clinical medicine can be definitively understood and resolved.
This vision of laboratory-derived innovation obscures the complex of social ingredients that are always already embedded in disease concepts and in the practice of clinical medicine. As DIAD represents a powerful vein of innovation and a platform for reorienting the study of human disease, it is imperative that its significance for the social and normative ordering of biomedicine be attended to in advance. Ethical and legal commentary on cellular reprogramming has primarily focused on issues of consent, privacy, property, and on the moral status of iPS cells [11–13]. In our view, it is the technology of translation itself, and not the biological materials it employs, that deserve more ethical attention, and here the clinical community must play a significant role.
Twenty-five hundred years ago, the Hippocratics observed that medicine “consists in three things, the disease, the patient and the physician” [14]. The natural phenomenon of disordered physiology, the patient’s expressive account of suffering, and the interpretive and remediation role of the physician were each seen as essential components of medical practice, each in some sense constitutive of the other. Patients’ accounts have always been an essential ingredient in the characterisations of disease produced by clinicians. At the same time, patients’ experiences of disease are shaped by the forms of diagnosis and explanation offered by physicians, both in terms of their narration of their own illness, and in terms of their interactions – therapeutic and bureaucratic – with medicine [15–17]. In the complex structures of modern biomedicine, patients have continued to find ways to assert an active role in determining how their diseases are made sense of – clinically, morally and as social priorities. Indeed even the production of medical knowledge, ostensibly a task for experts, has, in some cases, been an important locus for patient agency. For instance, since the 1960s, clinical research has become an increasingly important means for some patients to engage with their illness, to express hope and to exert influence over how a disease is studied, understood and treated [18, 19].
The notion of a unidirectional, translational pathway that ushers from the experimental and ethical simplicity of DIAD obscures a set of social realities that are integral to the production, application and meaning of biomedical knowledge. DIAD purports to produce an in vitro model that can replace the patient body as a site of experimental interrogation. However, this move alters the sorts of questions that can be asked, and, more importantly, the sorts of interactions that can take place between researcher and research subject, and the meanings that derive from them. Though much is gained through a tool like DIAD, something is simultaneously lost. Put simply, DIADs cannot speak the way patients do, nor do bench researchers hear the accounts of disease experience that are necessarily given in the clinic. In providing unmediated access to disease at the level of the cell, DIAD potentially produces much greater mediation between the investigation of disease by researchers and the experience of disease by patients.
The relevance of forms of medical knowledge for clinical action and for the scientific, social and moral meanings of disease are primarily negotiated between patient and physician though the dynamics of the clinic (fig. 2). Because in the clinical setting the sick body and the sick person are one and the same, negotiations of the social and moral meaning of disease cannot be disentangled from medical knowledge or therapeutic approaches. In purporting to “drill down” past clinical presentation to molecular mechanism, DIAD, and the translational model that it represents, inscribes an altered set of relationships between patients and the structures of biomedicine: the problem of disease is re-imagined as a technical matter to be understood (and resolved) in the laboratory.
It is important to note that these altered meanings of disease are not contingent on DIAD producing therapeutic results. Rather, they are already incorporated in the structure of the research program itself: they are built into the ways research questions are asked and to what ends. DIAD is a powerful tool for seeing pathology at the cellular and molecular level, but potentially to the neglect of disease at the level of lived experience. That said, neither are these altered meanings of disease a necessary, deterministic outcome of DIAD research. Concepts of disease are never purely technologically defined or determined. Rather, they emerge out of the social, epistemological and institutional spaces in which illness is made sense of. Yet if the DIAD project treats disease as a problem to be resolved in the laboratory, it engenders a rebalancing of these spaces regardless of what the technology is actually capable of accomplishing on a technical level. Thus, it is not the technology of DIAD per se that invites such change, but the epistemic and therapeutic expectations placed thereon.
The history of medicine has shown that it is often the way questions about disease are asked – by what sorts of experts and in what sorts of institutional settings – that most profoundly reshape the way diseases are understood, treated and, experienced, and thus the sorts of meanings, identities and hopes that come to be associated with them. Such changes need not result from new medical knowledge; they can occur simply when investigators begin to look at disease in new ways [20–23]. Such changes are generally accompanied by, and often precipitated by, changes in social order, particularly in relation to morally laden ideas of where responsibility for health lies, and what models of medical intervention serve the public well. We offer two historical examples by way of illustration.
In the late 18th century, the French Revolution precipitated a fundamental alteration in the way medicine was practiced and medical knowledge was produced. Medicine at the bedside was seen as an elitist vestige of the ancien régime, and the focus of medical care shifted to the structured setting of the hospital. The hospital was reconceived as an egalitarian space for treating the masses [24]. Efficient, standardised routines of examination were developed, as were techniques of bureaucratic management and medical record-keeping. Patients were grouped into like cases and each case was examined in the same way. This form of social organisation of patients – motivated primarily by a utopian political ideology of egalitarianism – generated a large volume of standardised, statistical data. The idiosyncrasies of the individual case, which had previously been the primary focus of medical examination, gave way to vast quantities of standardised data, and the individual patient dissolved into a massive pool of cases [25]. The hospital setting also gave physicians easy and virtually unlimited access to bodies for post-mortem examination, and autopsies became routine [26].
These changes in the social and institutional ordering of medicine shifted the focus of pathology from holistic examination of the patient toward aetiological explanation that focused on a limited set of anatomical lesions. The practices and the knowledge they generated produced a regime in which diagnosis of a specific patient was a matter of assimilating the individual case to pre-existing diagnostic categories, with the diagnosis later confirmed through autopsy. Thus the shift in the social organisation of medical care generated a profound alteration in models of medical knowledge, in the practices of diagnosis and treatment, and in patient experience. The hospital, informed by the ideology of the revolution, transformed the sick person into a sick body [24–27]. These changes did generate important new knowledge and clinical innovations, many of which remain core features of 21st century medicine. Yet these were (and remain) inseparably linked with social, institutional and political changes in how disease was approached and experienced. Indeed, here technical and epistemic change was a result rather than a cause of changes in social order [24, 28].
The politics of patient advocacy in the late 20th century provides a second and in many respects opposite example, yet one that reveals similar social dynamics. Sociologists of medicine have described how patient groups have been politically organised around disease diagnoses – including highly unstable and tentative diagnoses – to simultaneously transform medical knowledge, research priorities, therapeutics and the moral and political meaning of the disease. For instance, Callon and Rabeharisoa have described how the Association Française contre les Myopathies (AFM) – the French muscular dystrophy association – effectively shifted muscular dystrophy (MD) from scientific and social marginality to a politically powerful disease identity and a major focus of biomedical research [29]. The AFM accomplished this by radically transforming the MD patient’s status on two levels. AFM advanced research on MD through advocacy, funding and by making patient bodies available to researchers. At the same time, the organisation affected a transformation in the political and moral identity of the MD patient, from a deformed figure at the margins of humanity to a citizen deserving of concern, care and social support. These dual pathways – which AFM described as the “path to cures” and the “path to citizenship” – were inseparably linked; the social, moral and scientific identity of the French MD patient were remade simultaneously and by the same network of agents. Most important for our purposes, however, is that the concept of disease was the scientifically, socially and morally fertile ground upon around which the AFM was organised. Indeed the success of this organisation has led to greater refinement in the understanding of (and, ironically, to diagnostic fragmentation of) MD, and this has risked dissolving the disease category around which the group is organised. In response to this, the AFM has worked to classify families of genetically distinct conditions under “model disease” categories which are defined in both social and therapeutic terms: by similar patient experiences and by response to similar therapeutic interventions. Thus the organisation has sought to maintain the stability of the hybrid scientific-political disease concept lest its fragmentation destabilise the foundation of AFM itself.
These anecdotes illustrate the following: first, they demonstrate how social dynamics that are ostensibly external to medical science shape the ways in which medical knowledge is generated, understood and applied. Both cases generated novel technical approaches to disease – the clinico-anatomical method in the former, and the diagnostic refinements of MD aetiology in the latter. Yet these technical approaches were driven by social dynamics that were less concerned with generating knowledge than with generating a particular form of politics. Second, they demonstrate that disease is not merely a biological reality to be understood as such; rather, it emerges out of a more complex interaction between disease, patient and physician, one which shapes and is shaped by forms of social organisation, by moral orientations and by institutions, in addition to scientific approaches. The hospital, imbued with the egalitarian spirit of the revolution, transformed the sick individual into one more body to be examined, catalogued and, ultimately, dissected, thereby transforming disease into a problem of bureaucratic classification and administration. The political efforts of the AFM transformed MD from a condition of sub-humanity, neglected in medicine, to the foundation for a hybrid scientific-political disease identity. A class of stakeholder-citizens used the disease identity to simultaneously shape the institutions and priorities of biomedical research and the social and legal position of the disabled in France. Finally, each case demonstrates the profound social effect of reorienting how disease is approached, by whom, in what institutional structures, and to what end.
DIAD represents an analogous (if less revolutionary) project to shift the study of disease from one institutional space to another. As such, it has the potential to concomitantly alter notions of the sorts of problems that disease represents, and the sorts of solutions that should be given priority. Specifically, it constructs disease as a biological problem to be rectified through technological innovation. A pit-fall lies not in the technical approach itself, but in how the technical approach, in claiming disease as a problem for the laboratory, engenders a reorientation to the problem of disease, privileging its scientific elements over its social and moral features. The collective social commitment to the laboratory approach – investing it with hopes and expectations – is at once a collective commitment to the idea of disease (and the imperative of innovation) that the technology represents. While we in no way wish to suggest that the DIAD project of therapeutic innovation is an unworthy one, we insist that it can be much enhanced by attending to the concomitant social reorientations that the project itself can engender. By appreciating these features, we can attend to the social and scientific in a coordinated manner rather than attending to social reorientations as an unanticipated aftermath.
As custodians not only of the biological state of the patient’s body, but also of the psychological and moral meanings of illness, physicians are trained to be sensitive to the complex social and moral elements of disease that are simply not represented in a cell-based laboratory model. For these reasons, clinicians can play an important role in attending to these more complex, social dimensions of disease and can engender more careful reflection about what forms of innovation – social and technological – serve the project of medicine. We wish to highlight three dimensions in particular:
Disease is always both a technical and a social reality, since disease meanings are derived not only from an account of natural mechanism, but also from the institutional structures that undergird the act of diagnosis and from the lived experience of illness. Given that DIAD provides a radically different way of making sense of disease, it has the potential to perturb these social orderings for patient and physician alike. Physicians should be aware of this possibility.
The effort to model a disease in a dish frequently involves researchers coming to clinicians to discuss potential aetiologies of disease. Clinicians are involved in selecting the most interesting (i.e., experimentally promising) patients who then participate by donating tissue for DIAD studies. Cells cultured from several or one patient will ultimately be used in place of the genetically heterogeneous patient pool. As tools of disease modelling, they necessarily generate a standardised form of the disease. As such they prioritise certain aspects of the disease phenotype, constructing, in effect, a “model disease.” Yet unlike in the case of AFM, the question of what variation included within (or excluded from) the disease category is treated as a technical problem, whereas for the AFM it is simultaneously a scientific and a social problem. Furthermore, judgments about the fidelity of a DIAD model to a clinical condition emphasise molecular mechanism, excluding the narratives of illness – of what it is to have a vulnerable body and to suffer from a disease – that are central to clinical practice. While this form of standardisation can generate reproducible experimental results, it may fail to capture important variation. It also emphasises certain features of the disease over others, thereby shaping downstream knowledge, clinical therapeutics and the politics of disease identity. If the question of what counts as the disease is treated as purely technical and purely molecular, researchers risk missing the most important phenotypic features of disease as it is experienced, both socially and biologically.
Biochemical and biological phenotypes modelled by DIAD researchers in the lab are typically interpreted through the help of clinicians. This provides an opportunity for clinicians to ensure that researchers attend to the ways disease is experienced as an illness and seek ways to treat the patient, rather than merely curing the disease in the dish.
Clinical research requires the willing participation of research subjects. This enterprise is built on hope – hope that in subjecting themselves to risk, patients may benefit themselves or others. Translational efforts like DIAD requires patient inputs at different moments than other forms of clinical research. DIAD depends on patients as tissue donors and as consumers of downstream technologies, rather than as active participants in research. Work at the laboratory bench therefore potentially truncates patient involvement and delegates greater authority to the bench researcher to interpret what disease really is without reference to the experiences of patients and the social and moral orders that have emerged around the disease category. While this can be a highly beneficial arrangement that shifts risk of harm from patients to cells, it at the same time limits the pathways whereby patients can exert control over how their diseases are understood and approached. The move from clinic to laboratory potentially transforms patients from partners in the research enterprise to tissue donors and consumers of the products of the laboratory. Therefore, as the domain of biomedical research expands beyond the clinic, it is necessary to attend to the accompanying alterations in social order and in forms of patient agency. Given their experience-based knowledge of the dynamics of patient participation, agency and hope, clinicians can play an important mediational role between laboratory research and patients.
Expressions of hope, as well as the politics, institutions and practices that they engender, are altered by the “basic research” orientation of DIAD research. The stem cell biology research community frames DIAD as a once-and-for-all, fundamental, more real, less mediated picture of disease, and re-orientation from clinical palliation to laboratory-derived “cure” is often part of this framing. However, treatment in medicine depends on what works for the patient, and thus the patient traditionally has had some role in saying what constitutes a successful therapy. As our historical examples demonstrate, approaches to disease inform the normative position of the patient vis-à-vis the political collective. What is at stake in the study of disease is not merely biological knowledge, but the social and moral identities of patients and the meaning of their suffering. DIAD research, with its explicit therapeutic orientation, could benefit from recognising this fundamental dynamic of medicine in its research design, such that its development is sensitive to differing perspectives on how to define health and disease. The goal of medicine is to alleviate suffering and promote health, but there is no laboratory assay for human flourishing.
Thus DIAD researchers must attend to the sorts of hope, the varieties of suffering and the forms of flourishing that well-performed medicine makes its goal. Disease can no more be divorced from these dynamics than it can be separated from the bodies it inflicts. Since on one level the DIAD project attempts to achieve precisely this separation, it must at once recognise how this new technical construction has an ethics of medical care embedded in it that prioritises technological innovation. In seeking to solve the problems of the bedside at the laboratory bench, the DIAD project should appreciate that the suffering observed at the bedside has always been, and will always be, the place where medicine begins. If disease is re-imagined as merely a technical problem to be resolved in the laboratory – an imagination that is engendered in making innovation the repository of hope – something essential about the meaning of disease and the project of medicine is denigrated, if not lost. The promise of the laboratory shifts the focus of medicine from contending with human frailty to a search for magic bullets. Given the promise of this technology, therapeutic progress will no doubt come. Yet in spite of this promise, it is urgent that the social complexities of disease not be imagined merely as a problem of the interim that will disappear as the technical fruits of the laboratory ripen. Notwithstanding the many accomplishments of medicine, the vulnerability of the body, manifest as disease, is an inevitable element of human life. In this sense, we are always in the interim.
For these reasons, the ethics of innovation, implicit in the imagined promise of projects like DIAD, should not supplant an ethics of care. The tremendous promise of the laboratory should be seen for what it is – a rich space for the generation of tools and techniques that can augment the work of the clinic – and not as a wellspring of technical solutions for the deficiencies of clinical care. Since DIAD is conceived as a platform technology – applicable not merely to certain diseases but to the study of disease as such – particular urgency should be given to this set of concerns. An appreciation of how the goals of medicine are (or are not) embedded in translational projects like DIAD can help direct research in more clinically productive directions, thereby serving medicine in both its scientific and its ethical dimensions.
Stem cell research and DIAD are altering approaches to health and disease, making the laboratory bench a primary site in the emergence of an innovation and translation-focused medicine. The emphasis on the laboratory as the site for characterising and solving the problem of disease ignores the complex social and moral fabric that is always a feature of clinical medicine. Excessive allocation of hope to the laboratory distracts from this richer view of medicine, rendering the patient little more than a consumer of laboratory-derived technology, and the clinic little more than a dispensary. The tremendous potential of the laboratory to augment medical care will only be realised if care in the face of disease remains recognised as a great achievement of medicine rather than a marker of its impotence to deliver cures.
Rather than simply wait for new knowledge and technologies to flow from the lab, clinicians should be aware of the active role that they can and should play in the initial stages of DIAD projects by helping to ensure that they serve appropriate clinical goals in both their research design and in the forms of innovation they claim to anticipate. Though ostensibly a basic research enterprise, in vitro disease modelling is necessarily enmeshed in the larger, familiar dynamics of clinical medicine, simply by virtue of its focus on the problem of disease. Clinicians can play an important role in the DIAD enterprise by shaping the assays and phenotypes that laboratory researchers identify as faithful reflections of human disease, by enriching molecular aetiologies with narratives of illness, and by tempering the imagination among laboratory researchers, patients, and the public in general that the problems of medicine can be solved once and for all in the laboratory. The clinic is the primary place where the meanings of disease are shaped, where applications of techniques and technologies are mediated, and where hope and despair are expressed. In short, it is the space where the dynamics of medicine – between patient, physician and disease – are most directly manifest. It is where ambiguities are navigated, ambivalences are articulated and uncertainties are faced. Clinicians should remain mindful that these dynamics are features of disease itself, regardless of where it is experienced and how it is framed, and they should seek to remind others of the same.
For these reasons, clinicians can contribute to emerging domains of innovation like DIAD by attending to the complex social elements that underlie notions of disease. They should serve as conduits for patients to inform translationally oriented research priorities and they should resist – and help others resist – the imagination that given the right tools, disease is a problem that can and will be solved in the laboratory. In short, they should remind researchers, patients and the larger community that the proper goal of medicine is not curing the disease in the dish, but treating patients. In so doing, clinicians can help to guarantee a more robust development – scientific and social – of this promising technology.
We thank Sheila Jasanoft and members of the STS fellows program at Harvard University for helpful discussions. Further, we gratefully acknowledge Tom Di Cesare for his assistance with the illustrations.
1 Daley GQ, Scadden DT. Prospects for stem cell-based therapy. Cell. 2008;132(4):544–8.
2 Saha K, Jaenisch R. Technical challenges in using human induced pluripotent stem cells to model disease. Cell Stem Cell. 2009;5(6):584–95.
3 Rossant J. Stem cells and early lineage development. Cell. 2008;132(4):527–31.
4 Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76.
5 Park IH, Arora N, Huo H, Maherali N, Ahfeldt T, Shimamura A, et al. Disease-specific induced pluripotent stem cells. Cell. 2008;134(5):877–86.
6 Carvajal-Vergara X, Sevilla A, D’Souza SL, Ang Y-S, Schaniel C, Lee D-F, et al. Patient-specific induced pluripotent stem-cell-derived models of LEOPARD syndrome. Nature. 2010;10;465(7299):808–12.
7 Johnson C. Stem cells allow drug trials in a dish. South San Francisco, CA: KGO-TV; 2009.
8 CIRM. Stem Cells Accelerating Basic Research. Available from: http://www.cirm.ca.gov/StemCellBasics_BasicResearch.
9 Kim K, Doi A, Wen B, Ng K, Zhao R, Cahan P, et al. Epigenetic memory in induced pluripotent stem cells. Nature. 2010;467(7313):285–90.
10 Hanna JH, Saha K, Jaenisch R. Pluripotency and Cellular Reprogramming: Facts, Hypotheses, Unresolved Issues. Cell 2010;143(4):508-25. doi:10.1016/j.cell.2010.10.008.
11 Caulfield T, Scott C, Hyun I, Lovell-Badge R, Kato K, Zarzeczny A. Stem cell research policy and iPS cells. Nat Methods. 2010;7(1):28–33.
12 Simon BM, Murdoch CE, Scott CT. Pluripotent patents make prime time: an analysis of the emerging landscape. Nature Biotechnology. 2010;28(6):557–9.
13 Zarzeczny A, Scott C, Hyun I, Bennett J, Chandler J, Chargé S, et al. iPS cells: mapping the policy issues. Cell. 2009;139(6):1032–7.
14 Hippocrates. Of The Epidemics: Kessinger Publishing; 2004.
15 Kleinman A. The illness narratives: suffering, healing, and the human condition: Basic Books; 1989.
16 Rosenberg CE. The tyranny of diagnosis: specific entities and individual experience. The Milbank Quarterly 2002;80(2):237–60.
17 Hacking I. The social construction of what?: Harvard University Press; 1999.
18 Epstein S. Impure Science: AIDS, Activism, and the Politics of Knowledge: University of CaliforniaPress; 1996.
19 Epstein S. Inclusion: The Politics of Difference in Medical Research: University Of Chicago Press; 2009.
20 Feudtner JC. Bittersweet: diabetes, insulin, and the transformation of illness: UNC Press; 2003.
21 Marks HM. The Progress of Experiment: Science and Therapeutic Reform in the United States, 1900–1990: Cambridge University Press; 2000.
22 Weisz G. Divide and Conquer: A Comparative History of Medical Specialization: Oxford University Press, USA; 2005.
23 Rosenberg CE. Framing Disease: Studies in Cultural History: Rutgers University Press; 1992.
24 Foucault M. The Birth of the Clinic: An Archaeology of Medical Perception: Vintage; 1994.
25 Jewson ND. The Disappearance of the Sick-Man from Medical Cosmology, 1770–1870. Sociology. 1976;10(2):225–44.
26 Ackerknecht EH. Medicine at the Paris Hospital, 1794–1848. Baltimore: Johns Hopkins Press; 1967.
27 Albury WR. Corvisart and Broussais: Human Individuality and Medical Dominance. Constructing Paris Medicine. Amsterdam: Editions Rodopi B. V.; 1998. p. 221–50.
28 Weisz G. The Medical Mandarins: the French Academy of Medicine in the nineteenth and early twentieth centuries: Oxford University Press US; 1995.
29 Callon M, Rabeharisoa V. The Growing Engagement of Emergent Concerned Groups in Political and Economic Life: Lessons from the French Association of Neuromuscular Disease Patients. Science, Technology & Human Values. 2008;33(2):230–261.
KS is supported by a Society in Science: Branco-Weiss fellowship. JBH was partially supported by NSF Award #0724133.