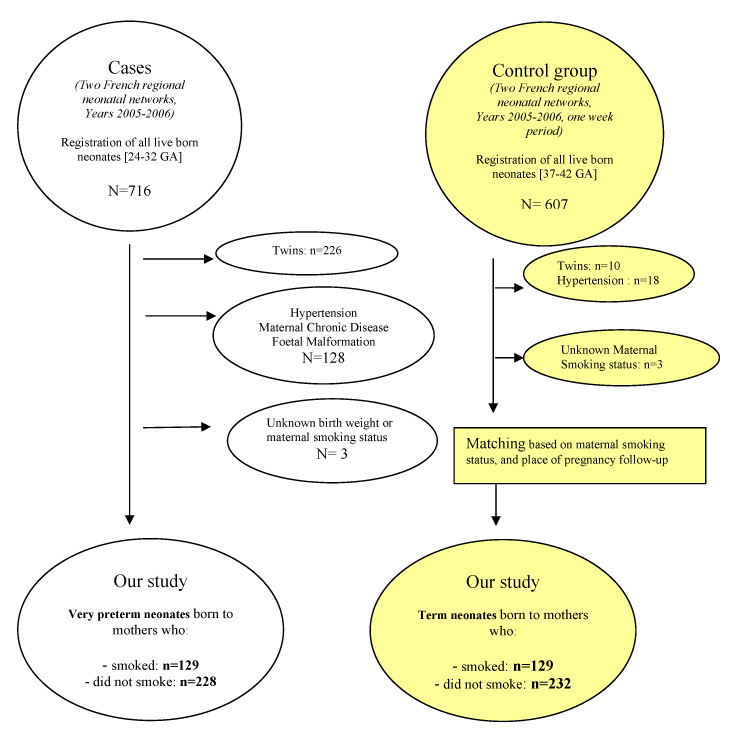
Figure 1
Inclusion and exclusion of cases and control group.
DOI: https://doi.org/10.4414/smw.2010.13139
Effects in preterm infants of gestational age less than 33 weeks
Normal foetal growth and development are determined by the genetic potential of the foetus and by several other factors that may be maternal and placental. Genetically predetermined growth may, however, be influenced by environmental factors which can exert either inhibitory or stimulatory effects. Nutrient supply to the foetus depends both on maternal nutritional status as well as on the capacity of the placenta to transport nutrients to the foetus. Among other factors, maternal genetic constitution, the existence of certain pathological conditions such as preeclampsia and diabetes, as well as toxic consumption also play an important role in foetal growth [1, 2].
Foetal chromosomal abnormalities and infections and poor socio-economic conditions also interfere with normal foetal growth and lead to restriction. Foetal growth restraint and consequent low birth weight are major health problems with long term epidemiological and metabolic complications, so that determination of causes and knowledge of patho-physiology are necessary if prevention policies are contemplated.
Among the maternal factors that interfere with foetal growth, maternal cigarette smoking during pregnancy represents one of the commonest, yet most preventable causes of low birth weight, at least in theory.
In France, national data from ANAES [3] are consistent with an overall reduction in the number of women who smoke from 35% of the population in 1984–1986 to 25% in 2002–2003. This same report however noted that smoking remains high in the youngest women of childbearing age. Although women stop smoking around the age of 25–34 years, 37% do smoke before pregnancy and as many as 27% continue smoking throughout their pregnancy.
Smoking during pregnancy not only leads to foetal growth restriction but is also associated with cases of retro-placental haematoma, placenta praevia and in-utero death. Some studies have related smoking in pregnancy to increased incidence of preterm births, which, combined with intrauterine growth restriction (IUGR), leads to a double insult to the perinatal outcome of infants born to mothers who smoke during pregnancy [4].
In the post neonatal period, maternal smoking increases the likelihood of sudden infant deaths in those children who were exposed in utero [5, 6].
Since the first report by Simpson et al. [7] maternal smoking during pregnancy is a recognised cause of IUGR characterised by a reduction in body length, as well as birth weight and head circumference. The majority of published research reports on outcome from the growth standpoint have been in term born infants [8, 11] with very few addressing preterm infants [4, 12].
Although not particularly designed to assess the effects of smoking during pregnancy on growth in preterm infants some reports have shown that smoking cessation early in pregnancy spared foetal growth and reduced infants morbidity [12–15].
Generally it has been suggested that maternal smoking during pregnancy leads to an increased risk of preterm delivery although foetal size does not appear to be compromised in some [12, 16–19] but not all studies [20, 21].
Very few studies have evaluated these observations in a prospective manner, which raises the possibility of reporting bias in the ascertainment of the effect of smoking.
The main aim of this study was, therefore, to evaluate in a prospective manner the effects of smoking during pregnancy on foetal growth using anthropometric measures (birth length, weight and head circumference) and comparing the effects in a group of preterm infants of gestational age (GA) less than 33 weeks versus term-born infants.
This is an observational cross-sectional study from an ongoing cohort of a Prospective Regional Survey of Preterm Births in two French perinatal networks (Poitou-Charentes & Franche-Comté) over a period of two years (2005–2006). The study population was extracted from the database and divided into two groups cases and controls. All participants gave their consent prior to inclusion.

Figure 1
Inclusion and exclusion of cases and control group.
We included all live-born preterm infants (GA 24–32 weeks) delivered in all maternity unit (n = 30) located in our two regional perinatal networks (Poitou-Charentes & Franche-Comté), they served as cases. Infants were further subdivided into those born by mothers who did (129 infants) or did not smoke during pregnancy (229 infants).
A group of term neonates was also recruited during a week long period, and served as controls. This group included term infants (GA 37–42 weeks) born immediately after the case (preterm infant) in the same maternity unit that followed up the preterm pregnancy prior to in-utero transfer to level III.
These were selected on the basis of term of delivery and maternal smoking status. They served as a comparison group with reference to the effects of maternal smoking on foetal growth. We included equal number of term and preterm infants. Adjustment in recruitment was undertaken on the basis of smoking status (one control smoker recruited for each preterm smoker to avoid tobacco exposure disparity between cases and controls).
The term born infants group also was further subdivided, as were the preterm infants group, into the offsprings of smokers and non-smokers.
The following exclusion criteria were applied to both groups, twin and triplet pregnancies, infants whose mothers had hypertension (pregnancy-induced or pre-existing), gestational diabetes or pre-existing diabetes, newborn infants with congenital malformations and/or chromosomal abnormalities. Summary of inclusion and exclusion shown in flow diagram, figure 1.
We sought to demonstrate a 7% reduction in BW among live-born preterm infants in relation to maternal smoking. The sample size was calculated with a “priori” estimated BW of 1500 g among the global preterm cohort, and an “a priori” rate of smokers equal to 33% among mothers with pregnancy complicated with spontaneous labour and/or premature rupture of membranes. 130 very preterm infants born to smoking mothers and 230 very preterm infants born to non-smokers were needed to reach the statistical significance levels (alpha risk 5% and beta risk 80%). An inclusion period of two years was estimated necessary to attain this population sample size.
– Gestational age (GA) was calculated according to the date of last menstrual period and/or first obstetrical ultrasonography.
– Smoking during pregnancy was defined as the consumption of at least one cigarette/day by self-reporting. The information on maternal smoking status was obtained by the midwives in a questionnaire filled out at booking, at time of delivery or shortly after. Details were gathered concerning daily cigarette consumption during the year before current pregnancy, any changes that took place during the actual pregnancy and the date of such a change along with current smoking habits were recorded. No biological assay was performed. Smoking quantification was done by classifying mothers into three groups as follow: non-smokers (0 cigarette/day), moderate smokers (1 to 9 cigarettes/day) and heavy smokers (>10 cigarettes/day).
– Foetal growth was assessed by measuring three anthropometric parameters: birth weight (BW) in kilograms recorded with electronic scale (Scale Neonatometer, Sud-Ouest Hospitalier, Matériel Médical, Merignac, France), birth length (BL) in centimetres measured with a Neonatometer (Scale Neonatometer, Sud-Ouest Hospitalier, Matériel Médical, Merignac, France), with the precaution taken that difference between two measures be <0.5 cm and head circumference (HC) in centimetres measured with a metal tape measure. The three anthropometric variables were recorded in the labour room or immediately upon admission to either the Neonatal Intensive Care Unit or the Special Care Baby Unit.
– Foetal growth restriction was defined by a BW, BL and HC below 10th centile of normal for gestational age according to AUDIPOG French curves [22]. These curves take several parameters into account such as GA, BW, BL, HC, birth rank and gender, maternal age and height as well as pre-pregnancy body weight, to predict the infant’s genetic growth potential. Ponderal Index (PI) was derived using the formula P.I = 100 x BW (g)/BL3.
For each mother, in addition to smoking status, we also recorded data on age, height, weight before present pregnancy, body mass index (BMI), level of education, past obstetrical history, profession and country of origin (recorded as European or non-European).
Statistical analyses were performed with STATA (9) Software (College Station, Texas, Stata Corp LP). Analysis of variance, non parametric tests (Kruskall-Wallis), Pearson chi-square test and Fisher exact test were used as necessary for bivariate analysis, with level of significance at P <0.05. We used Logistic regression and multiple linear regression analyses for multivariate analysis.
The first analysis compared principal risk factors of foetal growth restriction as defined by AUDIPOG (GA, mother’s height and weight, parity, foetal sex, birth rank) between preterm infants (GA 24–32 weeks) smokers versus non-smokers on one side, and term infants (GA 37–42 weeks) smokers versus non-smokers on the other side. The second analysis studied the effects of smoking on anthropometric parameters at birth. Lastly, multivariate linear regression analysis was performed after adjustment for GA, sex of the baby, maternal height and weight, parity and socio-economic conditions to compare the effects of maternal smoking during pregnancy in the preterm infants group (smokers versus non-smokers) and the term-born infants group (smokers versus non-smokers).
We were able to recruit a cohort of 719 live-born infants into the study, of which 358 were preterm (GA 24–32 weeks) and 361 term infants (GA 37–42 weeks). In the preterm group 129 were born to mothers who smoked during their pregnancy, the remaining 229 were born to non-smokers. In the control group 129 infants were born to smokers and 232 were born to non-smokers. We were able to collect all the required data for all participants.
In case group (table Ia), mothers who smoked were younger (P <0.001), with similar BMI (P = 0.74) in comparison to non-smokers, but a greater proportion had BMIs in the lower and higher distribution than the non-smokers, BMI <18.5 kg/m² (P <0.01), normal BMI (P <0.01), and BMI >25 kg/m² (P <0.01). Mothers who smoked had a lower education level (P <0.001) and were more often unemployed in comparison to non-smokers (P <0.001). No difference was found between groups as far as mothers’ country of origin was concerned.
In the control group (table Ib), there were no significant differences in maternal age, pre-pregnancy BMI was comparable between smokers and non-smokers. Within the BMI category there was a greater preponderance of smoking mother at the extremes of the BMI range: BMI <18.5 kg/m² (P = 0.03), normal BMI (P = 0.03), BMI >25 kg/m² (P = 0.03). Mothers who smoked were less educated in comparison to non-smokers and were likely to be unemployed (P <0.001). Mothers’ country of birth was not different between cases and controls (P = 0.23).
| Table 1a:Maternal characteristics and fœtal gender in Preterm Infants group (term 24–32 weeks). | |||
| Smokers % (n = 129) | Non smokers % (n = 229) | P value | |
| Maternal age (yrs) Mean ± SD | 27.6 ± 6.4 (n = 129) | 29.7± 5.4 (n = 229) | 0.001 |
| Maternal Height (cm) Mean ± SD | 162.6 ± 6.0 (n = 122) | 162.6 ± 6.7 (n = 226) | 0.93 |
| Pre pregnancy weight (kg) Mean ± SD | 58.9 ± 14.3 (n = 124) | 58.3 ± 11.8 (n = 226) | 0.66 |
| Pre-pregnancy BMI (kg/m²) Mean ± SD | 22.3 ± 5.4 (n = 121) | 22.1 ± 4.3 (n = 225) | 0.74 |
| Pre-pregnancy BMI groups <18.5 18.5–24.9 >= 25.0 kg/m² | 22.3 54.6 23.1 (n = 121) | 13.8 71.1 15.1 (n = 225) | 0.01² |
| Parity Primiparous | 39.5 (n = 129) | 49.8 (n = 229) | 0.06² |
| Foetal Gender Male | 55.0 (n = 129) | 56.8 (n = 229) | 0.75² |
| Ethnic origin Born out of Europe | 5.6 (n = 121) | 6.6 (n = 225) | 0.71² |
| Education status Secondary school A level & more | 60.8 39.2 (n = 120) | 29.2 70.8 (n = 216) | 0.001² |
| Profession during gestation Employees Workers Jobless | 24.2 4.8 61.3 (n = 124) | 40.0 6.7 29.3 (n = 225) | 0.001² |
| BMI: Body Mass Index; p non parametric test, p² chi2 Pearson | |||
| Table 1b:Maternal characteristics and fœtal gender in term born Infants group (term 37–41 weeks). | |||
| Smokers % (n = 129) | Non Smokers % (n = 232) | P value | |
| Maternal age (yrs) Mean ± SD | 29.7 ± 6.1 (n = 127) | 30.4 ± 5.2 (n = 228) | 0.23 |
| Maternal Height (cm) Mean ± SD | 163.0 ± 6.1 (n = 127) | 164.4 ± 6.3 (n = 221) | 0.05 |
| Pre pregnancy weight (kg) Mean ± SD | 60.9 ± 13.8 (n = 127) | 62.4 ± 12.4 (n = 223) | 0.29 |
| Pre-pregnancy BMI (kg/m²) Mean ± SD | 23.0 ± 4.9 (n = 127) | 23.0 ± 4.4 (n = 224) | 0.88 |
| Pre-pregnancy BMI groups <18.5 18.5–24.9 >=25.0 kg/m² | 15.8 59.1 25.2 (n = 127) | 8.0 71.0 21.0 (n = 224) | 0.03² |
| Parity Primiparous | 44.5 (n = 128) | 49.1 (n = 228) | 0.40² |
| Foetal Gender Male | 51.6 (n = 128) | 47.8 (n = 228) | 0.50² |
| Ethnic origin Born out of Europe | 0.8 (n = 126) | 2.6 (n = 228) | 0.23² |
| Education status Secondary school A level & more | 27.3 72.7 (n = 120) | 16.2 83.8 (n = 222) | 0.01² |
| Profession during gestation Employees Workers Jobless | 19.0 40.5 36.4 (n = 121) | 45.9 4.6 16.4 (n = 220) | 0.001² |
| BMI: Body Mass Index; p non parametric test, p² chi2 Pearson | |||
There were more heavy smokers (>10 cigarettes/day) among mothers who gave birth to very preterm infants as compared to smoking mothers of term infants (P = 0.01).
To evaluate the effects of maternal tobacco consumption during pregnancy on foetal growth we compared anthropometric parameters at birth (BW, BL, HC) in infants (both preterm and term infants) born to smokers and those born to non-smokers. In both cases and controls there were no significant differences between mothers who smoked and those that did not in terms of GA at delivery, maternal height, pre-pregnancy weight, parity and sex of the offspring (Tables IIa and IIb).
Preterm infants born to smoking mothers were comparable to those born to non-smoking mothers with regard to BW (P = 0.52), BL (P = 0.44) and HC (P = 0.81). No significant difference was found between preterm infants whether the mother smoked or not during gestation. Ponderal index was also comparable between the two sub-groups (smokersversus non-smokers, P = 0.30) (table IIa).
Comparison of anthropometric measures in term born infants revealed a significant difference according to maternal smoking status (smokers versus non-smokers): mean BW (P <0.001), mean BL (P <0.001), and head circumference was smaller in infants born to mothers who smoked in comparison to that of infants born to non-smoking mothers (P = 0.01) (table IIb).
| Table 2a: Comparison of fœtal growth parameters in preterm infants (GA 24–32 weeks) born to smokers versus those born to non smokers. | |||
| Neonates due mothers that smoked (n = 129) | Due to mothers who did not smoke (n = 228) | P | |
| BW (g) Mean (SD) Median min-max (n = 357) | 1403 (449) 1400 600–3500 (n = 129) | 1433 (412) 1420 490–2760 (n = 228) | 0.35 |
| Foetal growth retardation (Audipog) <10th centile | 4.7% (n = 6) (n = 129) | 6.1% (n = 14) (n = 228) | 0.55² |
| BL (cm) Mean (SD) Median min-max (n = 292) | 38.8 (4.0) 39.0 31.0–49.0 (n = 97) | 39.1 (3.8) 39.0 29.5–48.0 (n = 196) | 0.37 |
| HC (cm) Mean (SD) Median min-max (n = 303) | 27.6 (2.4) 28.0 22.0–33.0 (n = 103) | 27.6 (2.5) 28.0 20.2–32.5 (n = 200) | 0.65 |
| Miller’s index Mean (SD) Median min-max (n = 303) | 2.46 (0.44) 2.42 1.61–5.40 (n = 97) | 2.41 (0.36) 2.38 1.31–4.45 (n = 196) | 0.37 |
| p non parametric test , p² chi2 Pearson BW: birth weight; BL: birth length and HC: head circumference. | |||
| Table IIb: Comparison of fœtal growth parameters in term born infants (GA 37–42 weeks) born to smokers versus those born to non smokers. | |||
| Neonates born to mothers who smoked (n = 129) | Born to mothers who did not smoke (n = 232) | P | |
| BW (g) Mean (SD) Median min-max (n = 357) | 3210 (492) 3195 2130–4520 (n = 129) | 3358 (389) 3390 2330–4370 (n = 232) | 0.001 |
| Foetal growth retardation (Audipog) <10th centile | 15.5% (n = 20) (n = 129) | 4.3% (n = 10) (n = 232) | 0.001² |
| BL (cm) Mean (SD) Median min-max (n = 292) | 49.0 (2.5) 49.0 40.0–54.0 (n = 122) | 49.9 (2.0) 50.0 43.0–55.0 (n = 227) | 0.001 |
| HC (cm) Mean (SD) Median min-max (n = 303) | 34.0 (1.5) 34.0 30.0–39.0 (n = 126) | 34.4 (1.3) 34.0 31.0–38.0 (n = 227) | 0.01 |
| Miller’s index Mean (SD) Median min-max (n = 303) | 2.73 (0.27) 2.74 2.06–3.40 (n = 122) | 2.69 (0.21) 2.68 2.15–3.47 (n = 227) | 0.04 |
| p non parametric test, p² chi2 Pearson | |||
We performed multivariate linear regression analysis after adjusting for confounding factors (gestational age, foetal gender, maternal pre-pregnancy body weight and height, parity, and socio-economic conditions). Analyses were performed for each group separately (term and preterm infants), with BW, BL and HC as dependent variables respectively (tables IIIa to Vb).
In term infants, BW (P <0.0001), BL (P <0.001) and HC (P = 0.005) were lower in smokers in comparison with non-smokers; BW increased, however, with gestational age (P <0.0001), foetal gender (P <0.0001) and maternal pre-pregnancy weight (P <0.0001). In this group, BL increased with foetal gender (P <0.003), gestational age (P <0.0001), maternal height (P <0.007) and maternal pre-pregnancy body weight (P = 0.005). Lastly, HC was positively influenced by gestational age (P <0.001), foetal gender (P <0.0001) and maternal pre-pregnancy weight (P <0.0001).
In preterm infants, factors that correlated with a rise in BW were gestational age (P <0.0001), foetal gender (P = 0.013) and to a lesser extent, mother’s age (P = 0.032).
The only factor that had positive correlation with BL was the birth term (P <0.0001). However, foetal HC correlated positively with both gestational age (P <0.0001) and foetal gender (P = 0.015).
Maternal smoking negatively influenced BW, BL and HC only in term infants, whereas these anthropometric parameters correlated positively only with the gestational age in preterm infant group. We found not statistic correlation between these factors and maternal smoking.
| Table 3a:The impact of maternal smoking on the birth weight of very preterm neonates. | |||||
| Coefficient | Standard error | z | p| | 95% Confidence interval | |
| Maternal smoking | 14.94 | 30.54 | 0.49 | 0.63 | (–44.92 to 74.80) |
| Gestational age (week) | 144.07 | 6.54 | 22.01 | 0.001 | (131.25 to 156.90) |
| Male gender | 80.81 | 29.64 | 2.73 | 0.01 | (22.70 to 138.91) |
| Maternal height (cm) | 1.48 | 2.35 | 0.63 | 0.53 | (–3.12 to 6.10) |
| Maternal weight (kg) | 1.15 | 1.18 | 0.98 | 0.33 | (–1.15 to 3.46) |
| Parity (0 vs 1 & more) | 11.68 | 29.80 | 0.39 | 0.70 | (–46.72 to70.09) |
| Multiple linear regression, n = 343, r square of the model = 0.69 | |||||
| Table 3b:The impact of maternal smoking on the birth weight of term neonates. | |||||
| Coefficient | Standard error | z | p| | 95% Confidence interval | |
| Maternal smoking | –170.33 | 42.04 | –4.05 | 0.001 | (–252.72 to –87.94) |
| Gestational age (week) | 121.19 | 17.51 | 6.92 | 0.001 | (86.86 to 155.52) |
| Male gender | 177.98 | 40.00 | 4.45 | 0.001 | (99.57 to 256.38) |
| Maternal height (cm) | 6.34 | 3.53 | 1.79 | 0.07 | (–0.58 to 13.26) |
| Maternal weight (kg) | 4.86 | 1.67 | 2.90 | 0.01 | (1.57 to 8.15) |
| Parity (0 vs 1 & more) | –55.57 | 40.48 | –2.79 | 0.17 | (–134.93 to 23.78) |
| Multiple linear regression, n = 352, r square of the model = 0.22 | |||||
| Table IVa:The impact of maternal smoking on the length of very preterm neonates. | |||||
| Coefficient | Standard error | z | p| | 95% Confidence interval | |
| Maternal smoking | –0.26 | 0.33 | –0.80 | 0.43 | (–0.91 to 0.38) |
| Gestational age (week) | 1.35 | 0.07 | 17.87 | 0.001 | (1.20 to 1.50) |
| Male gender | 0.38 | 0.31 | 1.22 | 0.22 | (–0.23 to 1.00) |
| Maternal height (cm) | 0.15 | 0.25 | 0.58 | 0.55 | (–0.03 to 0.06) |
| Maternal weight (kg) | –0.08 | 0.12 | –0.71 | 0.48 | (–0.04 to 0.02) |
| Parity (0 vs 1 & more) | –0.20 | 0.32 | –0.64 | 0.53 | (–0.82 to 0.42) |
| Multiple linear regression, n = 288, r square of the model = 0.55 | |||||
| Table IVb:The impact of maternal smoking on the length of term neonates. | |||||
| Coefficient | Standard error | z | p| | 95% Confidence interval | |
| Maternal smoking | –1.07 | 0.20 | –5.29 | 0.001 | (–1.46 to -0.67) |
| Gestational age (week) | 0.59 | 0.08 | 7.10 | 0.001 | (–1.40 to -0.65) |
| Male gender | 1.02 | 0.19 | 5.40 | 0.001 | (0.66 to 1.40) |
| Maternal height (cm) | 0.06 | 0.17 | 3.39 | 0.001 | (0.02 to 0.09) |
| Maternal weight (kg) | 0.02 | 0.01 | 2.48 | 0.01 | (0.00 to 0.03) |
| Parity (0 vs 1 & more) | –0.27 | 0.19 | –1.41 | 0.16 | (–0.65 to 0.10) |
| Multiple linear regression, n = 346, r square of the model = 0.28 | |||||
| Table Va:The impact of maternal smoking on the head circumference of very preterm neonates. | |||||
| Coefficient | Standard error | z | p| | 95% Confidence interval | |
| Maternal smoking | –0.05 | 0.19 | –0.28 | 0.78 | (–0.43 to 0.33) |
| Gestational age (week) | 0.89 | 0.07 | 20.2 | 0.001 | (0.80 to 0.98) |
| Male gender | 0.43 | 0.19 | 2.35 | 0.01 | (0.07 to 0.80) |
| Maternal height (cm) | –0.004 | 0.15 | –0.27 | 0.79 | (–0.03 to 0.02) |
| Maternal weight (kg) | –0.002 | 0.007 | –0.33 | 0.74 | (–0.12 to 0.17) |
| Parity (0 vs 1 & more) | –0.16 | 0.18 | –0.87 | 0.38 | (–0.52 to 0.20) |
| Multiple linear regression, n = 298, r square of the model = 0.58 | |||||
| Table Vb:The impact of maternal smoking on the head circumference of term neonates. | |||||
| Coefficient | Standard error | z | p| | 95% Confidence interval | |
| Maternal smoking | –0.48 | 0.14 | –3.29 | 0.001 | (–0.76 to –0.19) |
| Gestational age (week) | 0.31 | 0.06 | 5.10 | 0.001 | (0.19 to 0.42) |
| Male gender | 0.69 | 0.14 | 4.97 | 0.001 | (0.41 to 0.95) |
| Maternal height (cm) | 0.02 | 0.01 | 1.35 | 0.18 | (–0.01 to 0.04) |
| Maternal weight (kg) | 0.007 | 0.005 | 1.33 | 0.52 | (–0.004 to 0.02) |
| Parity (0 vs 1 & more) | –0.09 | 0.14 | –0.64 | 0.52 | (–0.36 to 0.18) |
| Multiple linear regression, n = 348, r square of the model = 0.16 | |||||
This study was designed to assess the effects of maternal smoking during pregnancy on foetal growth in preterm infants of gestational age less than 33 weeks, by comparing anthropometric measures of these infants to those of term infants (GA 37 to 42 weeks) serving as controls.
Our results suggest that maternal smoking during pregnancy does not impair foetal growth as reflected in the parameters of BW, BL and HC and derived measures of PI in very preterm infants of gestational age <33 weeks.
Mothers who smoked were younger, more likely to be unemployed and to have undergone less school education confirming other reports [9, 23].
Factors that influence foetal growth such as GA and gender were common to both groups, whereas mother’s pre-pregnancy weight influenced BW only in term infants born to smokers.
Smoking appeared to be an important determinant of preterm labour but whether this is a direct effect or a phenomenon associated with other socio-economic factors is unclear from this study. Our study objective being different, we did look any further into this.
Our results corroborate those recently reported by Fitzgerald et al. [24] for whom smoking during pregnancy increased Odd ratio for IUGR only in infants of GA >32 weeks, but contradict those by Vielwert et al. [21] and Jaddoe et al. [25] who reported that foetal growth restraint occurs earlier than the third trimester of gestation.
Similar findings on foetal growth and smoking have been reported in the literature. The body of evidence suggests that maternal smoking during pregnancy tends to interfere with foetal growth towards the end of gestation (third trimester) rather than earlier [12, 16, 18–20, 26]. These observations are reinforced by Lieberman et al. [17], who noted that mothers who started smoking from the second through the third trimester, or started smoking during the third trimester had the same risk factors for small babies as those mothers who smoked during their entire gestation. MacArthur et al narrowed this critical window for exposure, as infants whose mothers stopped smoking before GA 16 weeks had similar BW with those born to non-smokers, whereas in those that stopped smoking between 16 and 30 weeks GA, the effects on BW were weak [20]. These findings are supported by recent studies that show that giving up smoking early in pregnancy leads to improved foetal growth comparable to that seen in non-smokers [4, 13–15].
The exact mechanism(s) through which smoking in pregnancy alters foetal growth is/are unclear. Cigarette consumption during gestation places the foetus in a state of chronic hypoxia, interferes with placental-foetal nutrient and gas exchanges, as well as reducing the circulating concentrations of growth factors such as IGF-I and IGF-BP3 that are essential for normal foetal growth [9, 27] and adipokines such as leptin [28–30]. Despite these observations the lack of an effect on foetal growth in very preterm infants is not clear.
Jauniaux et al. [31] have shown, in a comparative study of placental cotinine transfer between maternal and foetal circulations, that cotinine transfer increases with advancing gestation. Foeto-maternal cotinine ratio is low during the second trimester, and became almost identical near term. This may reflect progressive damage to the placenta so that increased permeability occurs with time. Hence, foetal growth restriction takes place late in pregnancy when the placenta has lost much of its role as filter and compensatory changes are exhausted. Alternatively, the impact may simply be related more to the periods when organ growth is at its fastest, which given that most is towards the end of the second/early third trimesters, might simply mean that this is the time when it becomes most obvious.
It would appear that the effects of maternal smoking are manifest in two ways. Firstly, as an increase in preterm delivery and secondly by reduced organ growth during the third trimester of pregnancy. Both situations are detrimental to the offspring – prematurity in terms of mortality and neurodevelopmental handicap and altered organ growth in terms of the subsequent association with poor cognitive function, cardiovascular disease and type 2 diabetes mellitus.
Variations in reported effects of maternal smoking in different studies may probably be explained by the pharmacogenomics of maternal tobacco use, so that the results of the gene-environment interaction vary according to individual genetic susceptibility as shown by Hui-Ju Tsai et al. [1].
Our study adds to the body of evidence that point to the adverse effects of smoking during pregnancy. The study strengths are the comprehensive prospective data collection with case-control type selection in an unbiased manner. As a formalised RCT in this area is not possible this is probably the best method available to reduce this bias.
Some authors have suggested that studies evaluating correlation between a risk factor and its effects on foetal growth should be based on reference charts that combine weight of both live births and foetuses in utero [32]. Although our study population was classified with reference to a chart that is solely based on measures performed in live births, we did adjust infant biometric data for other confounders known to influence both antenatal and postnatal anthropometry in attempt to minimize possible bias. Besides, this allows better differentiation between infants who are small as result of in utero growth restriction and those who are small in accordance with their genetic potential [33].
As always in large studies there is a compromise in ascertainment and it is accepted that urinary cotinine measures would have complemented the study. However, both Burguet et al. [34] and Klebanoff [35] have demonstrated that self-reporting smoking status is sufficiently reliable.
The data suggest that ongoing public awareness programmes to highlight the problems of smoking during pregnancy are required, perhaps targeted at the population most difficult to engage. Adaptation of measures such as those reported by Bolliger et al. to women of child-bearing age could be attempted [36]. It is advisable to stop smoking during the whole gestation as severe side effects due to maternal smoking seem to be preventable if smoking is stopped early in pregnancy. More studies are needed to understand the mechanisms involved in growth effects of tobacco consumption in both term and preterm infants.
1 Hui-Ju Tsai, Xin Liu, Karen Mestan, Yunxian Yu, Shanchun Zhang, Yaping Fang, et al. Maternal smoking, metabolic gene polymorphisms and preterm delivery: a new insights on GXE interactions and pathogenic pathways. Hum Gen. 2008;123:359–69.
2 Desforges M, Sibley CP. Placental nutrients supply and foetal growth. Int J Dev Biol. 2010;54:377–90.
3 Conférence de consensus ANAES 2004: grossesse et tabac.
4 McCowan LME, Dekker GA, Chan E, Stewart A, Chappell LC, Hunter M, et al. Spontaneous preterm birth and small for gestational age infants in women who stop smoking early in pregnancy: prospective cohort study. BMJ. 2009;338:1–6.
5 Lewis KW, Bosque EM. Deficient hypoxia awakening response in infants of smoking mothers: possible relationship to sudden infant death syndrome. J Pediatr. 1995;127:691–9.
6 Ueda Y, Stick SM, Hall G, Sly PD. Control of breathing in infants born to smoking mothers. J Pediatr. 1999;135:226–32.
7 Simpson WJ, Linda LA. A preliminary report in cigarette smoking and the incidence of prematurity. Am J Obstet Gynecol. 1957;73:808–15.
8 Chiolero A, Bovet P, Paccaud F. Association between maternal smoking and low birth weight in Switzerland: the EDEN study. Swiss Med Wkly. 2005;135:525–30.
9 Pringle PJ, Geary PP, Rodeck CH, Kingdom JCP, Kayamba-Kay’s S, Hindmarsh PC. The influence of cigarette smoking on antenatal growth, birth size, and the insulin-like growth factors axis. J Clin Endocrinol Metab. 2005;90:2556–62.
10 Ingvarsson R, Bjarnason AO, Dagbjartsson A, Hardardottir H, Haraldsson A, Thorkelsson T. The effects of smoking in pregnancy on factors influencing foetal growth. Acta Paediatr. 2007;96:383–6.
11 Anderson GD, Blidner IN, McClemont S, Sinclair JC. Determinants of size at birth in a Canadian population. Am J Obstet Gynecol. 1984;150:236.
12 Vik T, Jacobsen G, Vatten L, Bakkeitig LS. Pre- and post-natal growth in children of women who smoked in pregnancy. Early Hum Dev. 1996;45:245–55.
13 Ahlsten G, Cnattingius S, Lindmark G. Cessation of smoking during pregnancy impoves foetal growth and reduces infant morbidity in the neonatal period. A population-based prospective study. Acta Paediatr. 1993;82:177–81.
14 Suzuki K, Tanaka T, Kondo N, Minai J, Sato M, Yamagata Z. Is maternal smoking during early pregnancy a risk factor for all low birth weight infants? J Epidemiol. 2008;18:89–96.
15 Jaddoe VWV, Troe EWM, Hofman A, Mackenbach JP, Moll HA, Steegers EAP, Witteman JCM. Active and passive maternal smoking during pregnancy and preterm birth: the generation R study. Paediatr Perinat Epidemiol. 2008;22:162–71.
16 Ohmi H, Hirooka K, Mochizuki Y. Foetal growth and timing of exposure to maternal smoking. Pediatr Int. 2002;44:55–9.
17 Lieberman E, Gremy I, Lang JM, Cohen AP. Low birthweight at term and the timing of Foetal exposure to maternal smoking. Am J Public Health. 1994;84:1127–31.
18 Hebel JR, Fox NL, Sexton M. Dose-response of birth weight to various measures of maternal smoking during pregnancy. J Clin Epidemiol. 1988;41:483.
19 Lang JM, Cohen A, Lieberman E. Risk factors for small-for- gestational age at birth in a preterm population. Am J Obstet Gynecol. 1992;166:1374–8.
20 Macarthur C, Knox EG. Smoking in pregnancy: effects of stopping at different stages. Br J Obstet Gynaecol. 1988;95:551–5.
21 Vielwerth SE, Jensen RB, Larsen T, Greisen G. The impact of maternal smoking on foetal and infant growth. Early Hum Dev. 2007;83:491–5.
22 Mamelle N, Munoz F, Grandjean H. Croissance fœtale à partir de l’étude AUDIPOG, l’établissement de courbe de référence. J Gynecol Obstet Biol Reprod. 1996;25:61–70.
23 Bernstein IM, Plociennik K, Stahle S, Badger GJ, Secker-Walker R. Impact of maternal smoking on foetal growth and body composition. Am J Obstet Gynecol. 2000;183:883–6.
24 Fitzgerald K, Cai J, Hoff G, Dew P, Okah F. Clinical manifestation of small-for-gestational-age risk pregnancy from smoking is gestational age dependent. Am J Perinatol. 2007;24:519–24.
25 Jaddoe VW, Verburg BO, De Ridder MA, Hofman A, Mackenbach JP, Moll HA, et al. Maternal smoking and foetal growth characteristics in different periods of pregnancy: the generation R study. Am J Epideiol. 2007;165:1207–15.
26 Lindey AA, Becker S, Gray RH, Herman AA. Effects of continuing or stopping smoking during pregnancy on infant birth weight, crown-heel length, head circumference, ponderal index, and brain: body weight ratio. Am J Epidemiol. 2000;152:219–25.
27 Klauwer D, Blum WF, Hanitsch S, Bascher W, Lee PD, Kiess W. IGF-I, IGF-II, free IGF-I and IGF-BP-1, -2 and –3 levels in venous cord blood: relationship to birth weight, length and gestational age in healthy newborns. Acta Paediatr. 1997;86:826–33.
28 Kayemba-Kay’s S, Geary MP, Pringle J, Rodeck CH, Kingdom JC, Hindmarsh PC. Gender, smoking during pregnancy and gestational age influence cord leptin concentrations in newborn infants. Eur J Endocrinol. 2008;159:217–24.
29 Mantzoros CS, Varvarigou A, Kaklamani VG, Beratis NG, Flier JS. Effects of birth weight and maternal smoking on cord blood leptin concentrations of full-term and preterm newborns. J Clin Endocrinol Metab. 1997;82:2856–61.
30 Helland IB, Reseland JE, Saugstad OD, Drevon CA. Smoking related to plasma leptin concentration in pregnant women and their newborn infants. Acta Paediatr. 2001;90:282–7.
31 Jauniaux E, Burton GJ. Morphological and biological effects of maternal exposure to tobacco smoke on feto-placental unit. Early Hum Dev. 2007;83:699–706.
32 Hutcheon JA, Platt RW. The missing data problem in birth weight percentiles and thresholds for small for gestational age. Am J Epidemiol. 2008;167:786–92.
33 Gardosi J, Chang A, Kalyan B, Sahota D, Symonds EM. Customised antenatal growth charts. Lancet. 1992;339:283–7.
34 Burguet A, Berard M, Woronoff AS, Roth P, Menetrier M, Vanlemmens P, Schaal JP, Menget A. An appreciation of maternal smoking with high-performance liquid chromatographic determination of maternal and neonatal urinary cotinine. J Gynecol Obstet Biol Reprod. (Paris). 2001;30:166–73. French.
35 Klebanoff M, Levine R, Clemens JD, DerSimonian R, Wilkins DG. Serum cotinine concentration and self-reported smoking during pregnancy. Am J Epidemiol. 1998;148(3):259–62.
36 Bolliger CT, Van Biljon X, Humair JP, El Fehri V, Cornuz J. Promoting hospital-based smoking cessation services at major Swiss hospitals: a before and after study. Swiss Med Wkly. 2008;138:427–31.
No funding; no competing interests.