Current developments in the use of stem cell for therapeutic neovascularisation: is the future therapy “cell-free”?
DOI: https://doi.org/10.4414/smw.2010.13130
Summary
The plasticity and self-regenerative properties of stem cells have opened new avenues in regenerative medicine. Greater understanding of the biology of stem cells is followed by growing expectations of a rapid translation into alternative therapeutic options. Recent preclinical studies and clinical trials employing stem and progenitor cells from different sources have shown encouraging results. However, their underlying mechanisms are still poorly understood, the potential adverse effects and the discrepancy in efficacy remain to be further investigated.
Their essential role in vessel regeneration has made endothelial progenitor cells (EPC) a suitable candidate for therapeutic applications aiming at tissue revascularisation. Recent evidence suggests that EPC contribute to neovascularisation not only by direct participation in tissue homeostasis but mainly via paracrine mechanisms. In future, novel therapeutic strategies could be based on EPC paracrine factors or synthetic factors, and replace cell transplantation.
Authors contributed equally.
Introduction
Atherosclerotic cardiovascular diseases are increasing in prevalence and a leading cause of mortality and morbidity in the industrialised world [1]. Peripheral arterial disease (PAD) is one of the major manifestations of systemic atherosclerosis affecting the lower extremities and often culminating in critical limb ischaemia (CLI). CLI is characterised by a more than 50% risk of major amputation within one year without revascularisation [2] and a particularly poor prognosis with regard to survival [3, 4]. A substantial number of patients with CLI are negatively affected as they remain refractory to pharmacological therapies [5] and are unsuitable candidates for endovascular or surgical revascularisation [6].
The development of novel therapies to stimulate neovascularisation, a strategy known as therapeutic angiogenesis based on the use of angiogenic factors or stem cells, may represent an option to promote revascularisation and remodelling of collaterals, with the aim of ameliorating symptoms, promoting the regeneration of damaged tissues and preventing amputation [7, 8]. Tissue repair pro-cesses are in fact intimately associated with effective vascular network formation. In a number of cell therapy approaches it has been observed that vascularisation of the ischaemic areas after myocardial infarction or stroke usually anticipates functional improvement of the damaged tissue [9, 10].
This review will briefly outline current clinical developments and discuss the use of stem cell therapy for tissue revascularisation.
|
List of abbreviations
|
| Bone marrow |
BM |
| Bone marrow mononuclear cells |
BM-MNC |
| Circulating angiogenic cells |
CAC |
| Circulating progenitor cells |
CPC |
| Colony-forming unit endothelial cells |
CFU-EC |
| Critical limb ischaemia |
CLI |
| Endothelial cells |
EC |
| Endothelial colony-forming cells |
ECFC |
| Endothelial progenitor cells |
EPC |
| Haematopoietic stem cells |
HSC |
| Late outgrowth endothelial cells |
OEC |
| Mesenchymal stem cells |
MSC |
| Multipotent adult progenitor cells |
MAPC |
| Peripheral artery disease |
PAD |
| Peripheral blood mononuclear cells |
PB-MNC |
| Pain-free walking distance |
PFWD |
| Side population cells |
SP |
| Transcutaneous tissue oxygen tension |
TcPO2
|
Stem and progenitor cell therapy
Endothelial stem and progenitor cells for therapeutic neovascularisation: sources and populations
Preclinical studies have documented the fact that stem and progenitor cells possess the capability of self-renewal and differentiation into organ-specific cell types [11]. When placed in vivo, these cells are provided with the proper milieu in which to help reconstitute organ systems. Interestingly, there appears to be no clear dose response in the augmentation of neovascularisation, indicating that the
apparent promotion of new blood vessels and tissue function does not solely rely on homing and engraftment of the administered cells, but is related to paracrine effects with local secretion of cytokines and growth factors which may inhibit apoptosis and support migration and proliferation of resident differentiated endothelial cells (EC) [12].
For autologous cell transplantation in humans, bone marrow (BM) currently represents the most frequent source of cells used in clinical trials [13]. One reason is that BM is easy to obtain and no complex purification steps are required. Another advantage is that it contains a variety of stem and progenitor cells, including haematopoietic stem cells (HSC), side population (SP) cells [14], mesenchymal stem cells (MSC) [15] and multipotent adult progenitor cells (MAPC) [16]. This mixture of differentiated and less differentiated cells suggests superiority over one selected type of progenitor cell. The clinical advances with BM transplantation appear not to be better than culture expanded precursors of the EC (termed endothelial progenitor cells, EPC) isolated from peripheral blood, confirming the importance of using cell lines committed to endothelial lineage for new blood vessel formation [17]. However, a major limitation of
primary cell transplantation is the fundamental scarcity of progenitor cells in peripheral blood.
With the many different cell types that can be used for stem cell therapy, it is not yet clear which are the most promising. Experimental data suggest that more undifferentiated progenitor cells carrying the CD34 antigen possess a higher potential for regeneration of ischaemic cardiac tissue after acute myocardial infarction than non-selected mononuclear cells [18]. However, no experimental systematic comparison evaluating the different potential of stem or progenitor cell populations has been published. Also, the question remains whether cells need to be extracted from the body and later reinjected, or whether mobilisation of stem cells,
including resident stem cells in the different target organs, will be sufficient.
Among various stem and progenitor cells investigated, EPC have received particular attention as candidates for cell-based therapeutic options for enhancement of revascularisation in ischaemic tissues. The role of EPC in vessel growth and repair is documented in an increasing number of preclinical studies and clinical trial studies [19–21]. However, the mechanisms of action underlying the regenerative potential of EPC are not completely understood.
Current knowledge on EPC: characterisation, trafficking and mechanisms of action
The phenotypic characterisation of the different types of EPC is currently an open issue and a matter of scientific debate [22]. At present there is no general consensus on the definition of an EPC. Rather, the term EPC encompasses a heterogeneous group of cells that exist in a variety of stages ranging from haemangioblast to fully differentiated EC with distinct function, separate origin, and different protein expression profiles [21]. The generally accepted definition of circulating EPC is based on the expression of surface markers including CD34, CD133 and KDR [23]. Later studies have suggested that the actual cell population enriched in the CD34+, CD133+, KDR+ fraction is of haematopoietic lineage and does not form endothelium in vivo [24, 25], although the methodology and implication of such studies were soon questioned [26]. However, further studies attempting to purify and define “genuine” EPC have been difficult due to the lack of cell surface antigens or markers that distinguish these cells from mature EC and from subsets of haematopoietic cells [27, 28]. Four types of EPC have been generated under different ex vivo culture conditions: (1) colony-forming unit endothelial cells (CFU-EC) are derived from CD133+ EPC [29]; (2) colony-forming unit-Hill cells (CFU-Hill) are generated from non-adhesive peripheral blood mononuclear cells (PB-MNC) after two days’ culture [30]; (3) circulating angiogenic cells (CAC) or early EPC appear early in PB-MNC cultures and have limited proliferation and colony-forming
capacity [11]; and, (4) clonogenic expansion of
endothelial colony-forming cells (ECFC) or late outgrowth endothelial cells (OEC) appearing at late stages of in vitro culture and display potent
31].
In order to exert their vascular regenerative actions, EPC are mobilised from the bone marrow into the bloodstream and are recruited to the sites of nascent vessels. Tissue ischaemia is one of the strongest signals initiating a coordinated sequence of adhesive and signalling events leading to recruitment and incorporation of EPC [32]. The initial step of homing of EPC to ischaemic tissue involves adhesion, and transmigration occurs in response to a variety of cytokines and integrins activated by hypoxia [33–36]. VEGF and SDF-1, whose level is elevated in ischaemic tissue, are the strong chemo-attractive factors to EPC [37–39]. The involve-ment of SDF1/CXCR4 and selectin/selectin-ligand in EPC recruitment processes has been emphasized in various studies [40–42]. Other ligand/receptor pairs such as ICAM-1/CD18, fibronectin-1, or VCAM-1/integrin α4 also play a role in modulating EPC recruitment and engraftment [43]. Finally, cytokines, chemokines, and proteases such as MCP-1, interleukins, and MMPs in the ischaemic tissue may
be involved in modulation of EPC trafficking in
ischaemic tissue as well [44].
To date, two main mechanisms are postulated as contributing to the functional activity of EPC, (1) physical incorporation and differentiation into matured EC, and (2) secretion of paracrine angiogenesis enhancing factors. First reports addressed mainly the capacity of EPC to differentiate into mature EC and to physically integrate into newly formed vascular structures [11]. However, there is currently a lack of consensus concerning the incorporation rate of BM-derived cells, with a wide range from 0 to 90% incorporation of the transplanted cells [32]. Indeed, in a number of animal studies BM-derived EPC were found only adjacent to but not incorporated into the vessels [45, 46]. It has therefore been suggested that the angiogenic activity of EPC does not rely solely on their homing and engraftment, but is related to their capacity to secrete growth factors similar to the role of monocytes/macrophages [47]. This hypothesis is corroborated by the fact that EPC are able to elaborate relevant growth factor and cytokines like VEGF, SDF-1, and GM-CSF [47]. Furthermore, recent research has added new evidence of the central importance of the paracrine actions of EPC in the modulation of several vascular functions [48, 49]. However, despite recognition of the tissue-regenerative capacity driven by EPC-soluble factors [50, 51], the spectrum of paracrine effectors and their mechanism of action are only explored in recent studies. Proteomics analysis [52], large scale cytokine array [53] and multiplex assay [54] are chief approaches to revealing the composition and identifying key angiogenic factors. In contrast to early EPC, which contribute to neovascularisation mainly by paracrine secretion of trophic factors that support the viability and functions of the resident vascular cells, late EPC participate in angiogenesis by virtue of their proliferative and transdifferenti-ating properties [55, 56].
Clinical experience with cell therapy for therapeutic neovascularisation
Randomised, controlled clinical trials using BM- or peripheral blood-derived progenitor cells
The Therapeutic Angiogenesis by Cell Transplantation (TACT) study investigators performed a randomised controlled trial in patients with CLI [57]. Following a pilot study in 25 patients, 22 patients with bilateral CLI were randomised to receive intramuscular injections of bone marrow mononuclear cells (BM-MNC) as active treatment in one leg and PB-MNC as placebo in the other leg. A significant increase in TcPO2 (13 [9–17]; p <0.0001), rest pain (–0.85 [–1.6 to –0.12]; p = 0.025), and pain-free walking distance (PFWD) at 4 weeks after the injection was observed in the active treatment group (1.2 [0.7–1.7]; p = 0.0001). These results were sustained to the 24-week follow-up. Notably, freshly isolated PB-MNC exerted no effect [57]. Recently the authors assessed the 3-year safety and clinical outcomes of this angiogenic cell therapy by investigating the mortality and leg amputation-free interval as primary end points [58]. It was shown that cell therapy leads to an extension of amputation-free interval and improvement in the ischaemic pain, ulcer size, and PFWD. The severity of ischaemic pain and the need for repeated bypass surgery were depicted as major determinants negatively affecting the amputation-free interval.
Higashi and colleagues demonstrated that BM-MNC therapy improves endothelial function in patients with PAD (n = 7) [59]. At 4 and 24 weeks after BM-MNC implantation the beneficial effect on vascular function was selective in endothelium-dependent vasodilation (induced by acetylcholine) but not in endothelium-independent vasodilation (induced by sodium nitroprusside) [59]. Since endothelial dysfunction is the initial step in the pathogenesis of atherosclerosis [60], regeneration of the endothelial monolayer by progenitor cells within the fraction of the BM and consecutive improvement of endothelial function may prevent or at least delay the progression of atherosclerosis.
Lenk et al. investigated the safety and potentially beneficial effects of an intra-arterial application of autologous circulating progenitor cells (CPC) in patients with infrapopliteal PAD and CLI. Seven patients with CLI were treated with an intra-arterial infusion of autologous CPCs isolated after granulocyte colony-stimulating factor (G-CSF) stimulation from peripheral blood. At 3 months’ follow-up an increase in the PFWD, a significant increase in the ankle-brachial index and TcPO2 was observed, as well as improvements in endothelial function [61].
Bartsch et al. reported in 2007 (The TAM-PAD study) that combined intramuscular and intra-arterial injection of autologous BM-MNC in PAD patients with chronic ischaemia (Fontaine stage IIb; n = 13) achieved significant improvements in PFWD, ankle-brachial index, capillary-venous oxygen saturation and venous occlusion plethysmography after 2 and 13 months’ follow-up [62]. Since BM-MNC rarely build new vessels by themselves, but operate effectively as a kind of conductor for monocyte cells by secreting cytokines and chemo-kines [63], the authors concluded that the main reason for the improvement is increased angiogenesis [62].
In a prospective, controlled clinical trial Huang et al. reported that intramuscular transplantation of autologous G-CSF-mobilised PB-MNC for CLI improved the outcome of lower limb pain, PFWD, foot ulcers, arterial-brachial index, and angiographic scores in diabetic patients [64]. Furthermore, in a randomised study conducted in 2007, Huang et al. investigated the advantage of intramuscular autologous transplantation of BM-MNC over G-CSF-mobilised PB-MNC for patients with limb ischaemia (n = 150) [65]. There was no significant difference between two groups for PFWD, TcPO2, ulcers, and rate of lower limb amputation. Comparative analysis indicated that mobilised PB-MNC should be more practical in comparison with BM-MNC in the treatment of limb ischaemia.
To date, results from larger, randomised controlled studies using selected stem cells or subfractions are still lacking. At present, around 10 clinical trials are recruiting patients with intermittent claudication or CLI in the USA, Germany and Japan for clinical trials investigating the safety and efficacy of autologous BM cells [66, 67] or CD34 positive cells after G-CSF stimulation isolated via leukapheresis [68–70].
Adverse effects of cell therapy
The current enthusiasm should not preclude careful consideration of predominantly experimental studies implicating adverse effects of progenitor cell therapy. For example, in animal models for transplantation atherosclerosis, BM-derived progenitor cells have been shown to contribute to enhanced blood vessel formation in atherosclerotic plaque with potential to facilitate plaque instability and rupture [71]. Experimental observations in atherosclerosis research indicate that incorporation of BM-derived progenitors in plaque vessel depends on the concomitant presence of ischaemia [72]. Other pre-clinical studies suggest that the contribution of smooth muscle progenitors to the progression of atherosclerosis may temper the positive impact of EPC therapy [73]. At present there exist no human studies indicating a negative influence on atherosclerotic lesion size or plaque instability after cell therapy. To the contrary, major human clinical trials have clearly demonstrated that high EPC levels are associated with reduced cardiovascular event rates underlining the vasculoprotective effect of EPC [74–78].
However, further results are awaited on the long-term effects of progenitor cell therapy. In addition, it remains to be investigated whether crude, non-selected BM may induce muscle calcification as shown in animal models after intramuscular transplantation [79].
“Cell-free” strategy based on paracrine secretome of endothelial progenitor cells
Despite the encouraging results of recent trials, technical and practical limitations such as the invasive methods of harvesting and low abundance are major hurdles for the adoption of direct stem/progenitor cell transplantation into clinical applications. In the meantime, extensive research is currently under way to unravel how the paracrine functions of stem and progenitor cells integrate modulation of angiogenesis [47, 52–54]. Several lines of evidence suggest that the collective array of EPC soluble factors may find a clinical application for the treatment of ischaemic diseases [80]. Use of “cell-free” products may indeed represent an alternative to therapies based on cell transplantation. In our study we exploited the remarkable capacity of EPC to secrete growth factors (EPC secretome) in developing a novel cell-free strategy for therapeutic angiogenesis [54]. Conditioned media harvested from peripheral blood-derived EPC (EPC-CM) supported the survival of mature EC and enhanced the formation of capillary structures in vitro. Using an experimental model of hindlimb ischaemia [81] serial injections of EPC-CM into ischaemic muscles of rats ameliorated the limb ischaemia by promoting neovascularisation and vascular maturation (fig. 1). Moreover, EPC-CM restored muscle functionality. The angiogenic and tissue-regenerative capacity of EPC-CM was preceded by a systemic effect documented by a transient increase in progenitor cell number (CD34+ cells) in the BM and in peripheral blood, as well as augmented recruitment of stem cells within the ischaemic muscle. Remarkably, the therapeutic capacity of EPC-CM was in general comparable to EPC transplantation. It is of note, however, that the number of cells necessary to generate an equivalent therapeutic dose was much lower for EPC-CM production compared to the quantity of cells employed for EPC transplantation.

Figure 1
EPC-CM and EPC transplantation improve blood perfusion in the rat ischaemic hindlimb with increased capillary density. Using a chronic hindlimb ischaemia model in athymic nude rats [81], serial intramuscular injections of EPC secretome (EPC-CM) triggered substantial revascularisation of the ischaemic muscles accompanied by the recovery of blood perfusion [54]. (A) Representative images of hindlimb blood flow measured by laser Doppler before and 35 days after intramuscular injection of EPC-CM, EPC or placebo (control medium). The increase in blood flow in the ischaemic area was equivalent in both groups. (B) Representative immunofluorescent images of healthy (non-operated) and ischaemic hindlimb muscle treated with EPC-CM, EPC or placebo. Thirty-five days after treatment, the improvement of blood flow was accompanied by an augmented number of capillaries in both, the EPC and EPC-CM group indicative of increased neovascularisation.Images adapted from Di Santo S, et al., PLoS ONE, 2009. 4(5): p. e5643, under the terms of the Creative Commons Attribution License.
A number of recent reports suggest that the therapeutic properties of paracrine factors are a common feature of stem cells [82]. Conditioned media obtained from BM stromal cells has been shown to be beneficial in treating oxygen-induced lung injury through a cyto-protective effect on alveoli and vascular cells [83]. Moreover, reports have described how soluble factors secreted by CD133 cells isolated from BM are neuroprotective in a murine model of brain ischaemia [84]. Similarly to our observations, the therapeutic potential of conditioned medium of BM-derived CD133 cells against stroke is equal or superior to cell transplantation. Also, factors secreted by adipose tissue and BM-derived MSC exhibited the capacity to promote vascularisation [85–87], exert anti-apoptotic effects and promote tissue regeneration on heart and brain (for reviews see Bai, X. et al. [88] and Salgado, A. J. et al. [89]).
Thus there is strong evidence that in interventions based solely on stem cells and progenitor cells secretome may replace cell transplantation to enhance therapeutic neovascularisation and tissue regeneration. This cell-free strategy seems to be free from the limitations and problems observed with transplantation of fresh or in vitro cultured cells. In particular, the relative scarcity of circulating EPC and their limited proliferative potential preclude the possibility of expanding these cells in sufficient numbers for effective therapeutic applications. Moreover, there is compelling evidence that the presence of cardiovascular risk factors impairs some fundamental functional properties of EPC such as mobilisation, survival and capacity to differentiate or secrete paracrine factors [90–93].
Thus the use of heterologous cells appears to be a more promising option to circumvent the disadvantages of homologous EPC in patients with cardiovascular disease. However, this type of treatment is hampered by immunotolerance concerns and technical as well as practical difficulties. In contrast, a cell-free medium containing the paracrine secretome from EPC may reduce the risk of adverse immunological reactions and simplify the process of production (fig. 2).
Conclusion
The stimulation of therapeutic neovascularisation mediated by stem cell administration in patients with peripheral arterial disease remains an attractive goal in regenerative medicine [94]. Although efficacy has been demonstrated in animal models and safety in phase I human studies, unequivocal evidence of efficacy has not been demonstrated in placebo-controlled trials. Given the findings that progenitor function and mobilisation are impaired in certain disease states [76], it is reasonable to consider strategies that may include genetic modification of EPC to overexpress angiogenic growth factors, enhance signalling activity of the angiogenic response and rejuvenate the bioactivity and/or extend the life span of progenitor cells aiming to alleviate the potential dysfunction of stem cell populations in ischaemic disorders with ageing, diabetes or hypercholesterolaemia.
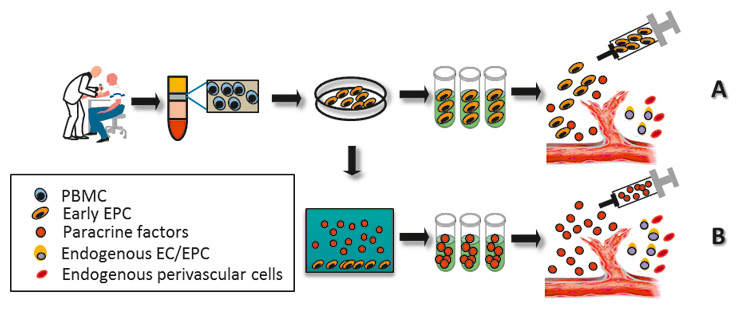
Figure 2
EPC-based cell therapy vs. secretome therapy. In conventional EPC-based cell therapy, EPC are generated from mononuclear cells isolated from the peripheral blood (PMBC) and used directly for transplantation via local (intramuscular) or systemic delivery (intra-arterial and intravenous) routes (A). To avoid immune rejection and achieve effective benefits, EPC therapy usually requires the use of a substantial amount of autologous cells. Alternatively, these ex vivo-derived early EPC can be subjected to hypoxic conditions for the secretion of an angiogenic protein. This culture supernatant-obtained secretome can be prepared and injected into the patients (B). The “cell-free” strategy may offer an ideal solution with higher efficacy, easy reproducibility, ready availability and no risk of immune rejection.
Recent studies have proposed a different therapeutic concept based on paracrine factors secreted by progenitor and stem cells [81, 95]. Therapeutic strategies utilising soluble factors secreted by EPC either as physiological or as synthetic forms might be used as adjuvant of conventional medical therapies or even replace cell transplantation. The advantage of this approach is compelling due to its potential freedom from the limitations and problems observed with cell transplantation. A cell-free medium such as EPC-CM significantly reduces the risk of adverse immunological reactions and simplifies the process of production. It is, therefore, reasonable to imagine that EPC secretome or an equivalent synthetic preparation which mimics physiological EPC secretome will in future find application in regenerative medicine.
|
Table 1Randomised, controlled clinical trials using BM- or peripheral blood-derived progenitor cells for therapeutic neovascularisation. CLI, critical limb ischaemia; PAD, peripheral artery disease; BM-MNC, bone marrow mononuclear cells; CPC, circulating progenitor cells; G-CSF, granulocyte colony-stimulating factor; PB-MNC, peripheral blood mononuclear cells; TcPO2, transcutaneous tissue oxygen tension; PFWD, pain free walking distance. |
|
Disease
|
Reference
|
Cells source
|
Follow-up period
|
Administration route
|
Outcomes
|
| CLI |
Tateishi-Yuyama E, et al. [57] |
BM-MNC |
4 weeks |
Intramuscular |
Increase in TcPO2, rest pain, and PFWD |
| CLI |
Matoba S, et al. [58] |
BM-MNC |
3 years |
Intramuscular |
Extension of amputation-free interval; improvement in ischaemic pain, ulcer size, and PFWD |
| PAD and CLI |
Lenk et al. [61] |
CPC |
3 months |
Intra-arterial |
Increase in the PFWD, ankle-brachial index and TcPO2; improvements in endothelial function |
| CLI |
Huang et al. [64] |
G-CSF-mobilized PB-MNC |
3 months |
Intramuscular |
Improved lower limb pain, PFWD, foot ulcers, arterial-brachial index, and angiographic scores in diabetic patients |
| CLI |
Huang et al. [65] |
Comparison of autologous
G-CSF-mobilised PB-MNC vs. BM-MNC |
3 months |
Intramuscular |
No significant difference between two groups for PFWD, TcPO2, ulcers, and rate of lower limb amputation |
Correspondence:
Stefano Di Santo PhD
Institute of Neurosurgery
Inselspital
University of Bern
CH-3010 Bern
Switzerland
vascmed.unibe@gmail.com
References
1 Lloyd-Jones D, et al. Heart disease and stroke statistics – 2010 update: a report from the American Heart Association. Circulation. 2010;121(7): p. e46-e215.
2 Second European Consensus Document on chronic critical leg ischemia. Circulation. 1991;84(4 Suppl): p. IV1–26.
3 Steg PG, et al. One-year cardiovascular event rates in outpatients with atherothrombosis. JAMA. 2007;297(11):1197–206.
4 Norgren L, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg. 2007;45(1 Suppl):S5–S67.
5 Baumgartner I, Schainfeld R, Graziani L. Management of peripheral vascular disease. Ann Rev Med. 2005;56:249–72.
6 Adam DJ, et al. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet. 2005;366(9501):1925–34.
7 Henry TD. Therapeutic angiogenesis. Br Med J. 1999;318:1536–9.
8 Isner JM, Asahara T. Angiogenesis and vasculogenesis as therapeutic strategies for postnatal neovascularization. J Clin Invest. 1999;103(9):1231–6.
9 Chen J, et al. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke. 2001;32(11):2682–8.
10 Zhang ZG, et al. Bone marrow-derived endothelial progenitor cells participate in cerebral neovascularization after focal cerebral ischemia in the adult mouse. Circ Res. 2002;90(3):284–8.
11 Kalka C, et al. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci USA. 2000;97(7):3422–7.
12 Zhang Y, et al. Release of Pro-Inflammatory Mediators and Expression of Pro-Inflammatory Adhesion Molecules by Endothelial Progenitor Cells. Am J Physiol Heart Circ Physiol, 2009;296(5):1675–82.
13 Aranguren XL, Verfaillie CM, Luttun A. Emerging hurdles in stem cell therapy for peripheral vascular disease. J Mol Med. 2009;87(1):3–16.
14 Jackson KA, et al. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest. 2001;107:1395–402.
15 Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 2004;95(1):9–20.
16 Reyes M, et al. Origin of endothelial progenitors in human postnatal bone marrow. J Clin Invest. 2002;109(3):337–46.
17 Assmus B, et al. Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI). Circulation. 2002;106(24):3009–17.
18 Kawamoto A, et al. CD34-positive cells exhibit increased potency and safety for therapeutic neovascularization after myocardial infarction compared with total mononuclear cells. Circulation. 2006;114(20):2163–9.
19 Pearson JD. Endothelial progenitor cells – hype or hope? J Thromb Haemost. 2009;7(2):255–62.
20 Kalka C, Di Santo S. Endothelial progenitor cells: from bench to bedside. Future Cardiol. 2006;2(4):455–66.
21 Kalka C, Baumgartner I. Gene and stem cell therapy in peripheral arterial occlusive disease. Vasc Med. 2008;13(2):157–72.
22 Prater DN, et al. Working hypothesis to redefine endothelial progenitor cells. Leukemia. 2007;21(6):1141–9.
23 Peichev M, et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95(3):952–8.
24 Case J, et al. Human CD34+AC133+VEGFR-2+ cells are not endothelial progenitor cells but distinct, primitive hematopoietic progenitors. Exp Hematol. 2007;35(7):1109–18.
25 Timmermans F, et al. Endothelial outgrowth cells are not derived from CD133+ cells or CD45+ hematopoietic precursors. Arterioscler Thromb Vasc Biol. 2007;27(7):1572–9.
26 Fadini GP, Avogaro A, Agostini C. Critical assessment of putative endothelial progenitor phenotypes. Exp Hematol. 2007;35(10):1479–80; author reply 1481–2.
27 Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med. 2003;9(6):702–12.
28 Masuda H, Asahara T. Post-natal endothelial progenitor cells for neovascularization in tissue regeneration. Cardiovasc Res. 2003;58(2):390–8.
29 Gehling UM, et al. In vitro differentiation of endothelial cells from AC133-positive progenitor cells. Blood. 2000;95(10):3106–12.
30 Hill JM, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348(7):593–600.
31 Lin Y, et al. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest. 2000;105(1):71–7.
32 Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95(4):343–53.
33 Vajkoczy P, et al. Multistep nature of microvascular recruitment of ex vivo-expanded embryonic endothelial progenitor cells during tumor angiogenesis. J Exp Med. 2003;197(12):1755–65.
34 Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76(2):301–14.
35 Carlos TM, Harlan JM Leukocyte-endothelial adhesion molecules. Blood. 1994;84(7):2068–101.
36 Muller WA. Leukocyte-endothelial cell interactions in the inflammatory response. Lab Invest. 2002;82(5):521–33.
37 Shintani S, et al. Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation. 2001;103(23):2776–9.
38 Kalka C, et al. Vascular endothelial growth factor(165) gene transfer augments circulating endothelial progenitor cells in human subjects. Circ Res. 2000;86(12):1198–202.
39 Lee SH, et al. Early expression of angiogenesis factors in acute myocardial ischemia and infarction. N Engl J Med. 2000;342(9):626–33.
40 Abbott JD, et al. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004;110(21):3300–5.
41 Ceradini DJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10(8):858–64.
42 Biancone L, et al. Role of L-selectin in the vascular homing of peripheral blood-derived endothelial progenitor cells. J Immunol. 2004;173(8):5268–74.
43 Wu Y, et al. Essential role of ICAM-1/CD18 in mediating EPC recruitment, angiogenesis, and repair to the infarcted myocardium. Circ Res. 2006;99(3):315–22.
44 Fujiyama S, et al. Bone marrow monocyte lineage cells adhere on injured endothelium in a monocyte chemoattractant protein-1-dependent manner and accelerate reendothelialization as endothelial progenitor cells. Circ Res. 2003;93(10):980–9.
45 De Palma M, et al. Targeting exogenous genes to tumor angiogenesis by transplantation of genetically modified hematopo46 Ziegelhoeffer T, et al. Bone marrow-derived cells do not incorporate into the adult growing vasculature. Circ Res. 2004;94(2):230–8.
47 Rehman J, et al. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107(8):1164–9.
48 Urbich C, et al. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J Mol Cell Cardiol. 2005;39(5):733–42.
49 He T, et al. Angiogenic function of prostacyclin biosynthesis in human endothelial progenitor cells. Circ Res. 2008;103(1):80–8.
50 Jujo K, M. Ii, Losordo DW. Endothelial progenitor cells in neovascularization of infarcted myocardium. J Mol Cell Cardiol. 2008;45(4):530–44.
51 Murayama T, et al. Determination of bone marrow-derived endothelial progenitor cell significance in angiogenic growth factor-induced neovascularization in vivo. Exp Hematol. 2002;30(8):967–72.
52 Pula G, et al. Proteomics identifies thymidine phosphorylase as a key regulator of the angiogenic potential of colony-forming units and endothelial progenitor cell cultures. Circ Res. 2009;104(1):32–40.
53 Yang Z, et al. Paracrine factors secreted by endothelial progenitor cells prevent oxidative stress-induced apoptosis of mature endothelial cells. Atherosclerosis. 2010;211(1):103–9.
54 Di Santo S, et al. Novel cell-free strategy for therapeutic angiogenesis: in vitro generated conditioned medium can replace progenitor cell transplantation. PLoS ONE. 2009;4(5): p. e5643.
55 Hur J, et al. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol. 2004;24(2):288–93.
56 Yoon CH, et al. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: the role of angiogenic cytokines and matrix metalloproteinases. Circulation. 2005;112(11):1618–27.
57 Tateishi-Yuyama E, et al. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet. 2002;360(9331):427–35.
58 Matoba S, et al. Long-term clinical outcome after intramuscular implantation of bone marrow mononuclear cells (Therapeutic Angiogenesis by Cell Transplantation [TACT] trial) in patients with chronic limb ischemia. Am Heart J. 2008;156(5):
1010–8.
59 Higashi Y, et al. Autologous bone-marrow mononuclear cell implantation improves endothelium-dependent vasodilation in patients with limb ischemia. Circulation. 2004;109(10):1215–8.
60 Ross R. Atherosclerosis – an inflammatory disease. N Engl J Med. 1999;340(2):115–26.
61 Lenk K, et al. Therapeutical potential of blood-derived progenitor cells in patients with peripheral arterial occlusive disease and critical limb ischaemia. Eur Heart J. 2005;26(18):1903–9.
62 Bartsch T, et al. Transplantation of autologous mononuclear bone marrow stem cells in patients with peripheral arterial disease (the TAM-PAD study). Clin Res Cardiol. 2007;96(12):891–9.
63 Iwase T, et al. Comparison of angiogenic potency between mesenchymal stem cells and mononuclear cells in a rat model of hindlimb ischemia. Cardiovasc Res. 2005;66(3):543–51.
64 Huang P, et al. Autologous transplantation of granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cells improves critical limb ischemia in diabetes. Diabetes Care. 2005;28(9):2155–60.
65 Huang PP, et al. Randomised comparison of G-CSF-mobilized peripheral blood mononuclear cells versus bone marrow-mononuclear cells for the treatment of patients with lower limb arteriosclerosis obliterans. Thromb Haemost. 2007;98(6):1335–42.
66 NCT00282646: Intraarterial Progenitor Cell Transplantation of Bone Marrow Mononuclear Cells for Induction of Neovascularization in Patients With Peripheral Arterial Occlusive Disease (Provasa). National Institute of Health, Bethesda, MD, USA. 2006.
67 NCT00434616: Security and Effectiveness of Autologous Bone Marrow Stem Cell Transplantation to Avoid Amputations in Patients With Limb-Threatening Ischemia: A Multicentric Randomized Placebo-Controlled Double-Blind Study (bonmot). National Institute of Health, Bethesda, MD, USA. 2007.
68 NCT00311805: Injection of Autologous CD34-Positive Stem Cells for Neovascularization and Symptom Relief in Patients With Severe Intermittent Claudication. National Institute of Health, Bethesda, MD, USA. 2006.
69 NCT00616980: Injection of Autologous CD34-Positive Cells for Improved Symptomatic Relief and Ischemic Wound Healing in Subjects With Moderate or High-Risk Critical Limb Ischemia. National Institute of Health, Bethesda, MD, USA. 2008.
70 NCT00221143: Phase I / II Clinical Trial Regarding Vascular Regeneration Therapy by Transplantation of Autologous Peripheral Blood Endothelial Progenitor Cells (CD34+ Cells) in No-Option Patients With Chronic Critical Limb Ischemia. National Institute of Health, Bethesda, MD, USA. 2005.
71 Hu Y, et al. Endothelial replacement and angiogenesis in arteriosclerotic lesions of allografts are contributed by circulating progenitor cells. Circulation. 2003;108(25):3122–7.
72 Silvestre JS, et al. Transplantation of bone marrow-derived mononuclear cells in ischemic apolipoprotein E-knockout mice accelerates atherosclerosis without altering plaque composition. Circulation. 2003;108(23):2839–42.
73 Sata M. Circulating vascular progenitor cells contribute to vascular repair, remodeling, and lesion formation. Trends Cardiovasc Med. 2003;13(6):249–53.
74 Werner N, et al. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353(10):999–1007.
75 Vasa M, et al. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89(1):E1–7.
76 Heeschen C, et al. Profoundly reduced neovascularization capacity of bone marrow mononuclear cells derived from patients with chronic ischemic heart disease. Circulation. 2004;109(13):1615–22.
77 Schmidt-Lucke C, et al. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: proof of concept for the clinical importance of endogenous vascular repair. Circulation. 2005;111(22):2981–7.
78 Fadini GP, et al. Circulating endothelial progenitor cells are reduced in peripheral vascular complications of type 2 diabetes mellitus. J Am Coll Cardiol. 2005;45(9):1449–57.
79 Yoon YS, et al. Unexpected severe calcification after transplantation of bone marrow cells in acute myocardial infarction. Circulation. 2004;109(25):3154–7.
80 Tongers J, Roncalli JG, Losordo DW. Therapeutic angiogenesis for critical limb ischemia: microvascular therapies coming of age. Circulation. 2008;118(1):9–16.
81 Yang Z, et al. Call for a reference model of chronic hind limb ischemia to investigate therapeutic angiogenesis. Vascul Pharmacol. 2009;51(4):268–74.
82 Gnecchi M, et al. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103(11):1204–19.
83 Aslam M, et al. Bone marrow stromal cells attenuate lung injury in a murine model of neonatal chronic lung disease. Am J Respir Crit Care Med. 2009;180(11):1122–30.
84 Bakondi B, et al. CD133 identifies a human bone marrow stem/progenitor cell sub-population with a repertoire of secreted factors that protect against stroke. Mol Ther. 2009;17(11):1938–47.
85 Estrada R, et al. Secretome from mesenchymal stem cells induces angiogenesis via Cyr61. J Cell Physiol. 2009;219(3):563–71.
86 Bhang SH, et al. Locally delivered growth factor enhances the angiogenic efficacy of adipose-derived stromal cells transplanted to ischemic limbs. Stem Cells. 2009;27(8):1976–86.
87 Rubina K, et al. Adipose stromal cells stimulate angiogenesis via promoting progenitor cell differentiation, secretion of angiogenic factors, and enhancing vessel maturation. Tissue Eng Part A. 2009;15(8):2039–50.
88 Bai X, Alt E. Myocardial regeneration potential of adipose tissue-derived stem cells. Biochem Biophys Res Commun. 2010;401(3):321–6.
89 Salgado AJ, et al. Adipose tissue derived stem cells secretome: soluble factors and their roles in regenerative medicine. Curr Stem Cell Res Ther. 2010;5(2):103–10.
90 Vasa M, et al. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circulation Res. 2001;89(1):E1–7.
91 Hill JM, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348(7):593–600.
92 Tepper OM, et al. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106(22):2781–6.
93 Rauscher FM, et al. Aging, progenitor cell exhaustion, and atherosclerosis. Circulation. 2003;108(4):457–63.
94 Losordo DW, Dimmeler S. Therapeutic angiogenesis and vasculogenesis for ischemic disease: part II: cell-based therapies. Circulation. 2004;109(22):2692–7.
95 Webber MJ, et al. Capturing the stem cell paracrine effect using heparin-presenting nanofibres to treat cardiovascular diseases. J Tissue Eng Regen Med. 2010 (Epub ahead of print).

