Suppressive effects of black seed oil on ovalbumin induced acute lung remodelling in E3 rats
DOI: https://doi.org/10.4414/smw.2010.13128
M
Shahzad, X
Yang, Q
Sun, F
Zhang, Y
Han, S
Lu, Asim
Raza
Summary
OBJECTIVE: Black seed oil (BSO) is widely used as a traditional medicine for asthma and other inflammatory diseases. The aim of this study is to evaluate the effects of BSO on ovalbumin (OVA) induced acute lung remodelling in E3 inbred rats.
METHOD:Rats were divided into three groups; Control, OVA and BSO. The rats were intraperitoneally sensitised and challenged intranasally with OVA and treated intraperitoneally with pure BSO for seven days. The collagen deposition and other pathological alteration were determined by Masson’s trichrome, PAS and HE staining. Activity of arginase, ornithine decarboxylase (ODC) and proline level was determined by spectrophotometry, and polyamine by HPLC. The mRNA expression of arginase І, endothelin1 (Edn1), matrix metallopeptidase 3 (MMP3) and growth factors was determined by real time RT-PCR.
RESULTS: Massive inflammation and characteristics of lung remodelling including collagen deposition, goblet cell hyperplasia and proline level were observed in the lungs of OVA exposed rats. Administration of BSO in the OVA exposed rats suppressed the inflammatory cells infiltration, goblet cell hyperplasia and collagen deposition. The activity of total arginase and ODC; proline and polyamine level was decreased in the lung homogenate of BSO treated rats. Furthermore, BSO abrogated the mRNA expression of Edn1, MMP3, transforming growth factor beta (TGF-β), fibroblast growth factor 2 (FGF2) and vascular epidermal growth factor (VEGF) in the lungs of OVA challenged rats.
CONCLUSION: Administration of BSO significantly reduced the level of allergen induced lung remodelling. The effect of BSO on lung remodelling is probably mediated by the inhibition of arginase pathways and the expression of Edn1, MMP3 and growth factors. Our findings suggest that BSO might have useful implications in the treatment and future research into allergen-induced lung remodelling.
Introduction
Allergic asthma is an immune driven inflammatory disease that is associated with the development of various structural alterations in airway wall and smooth muscles of blood vessels in lung tissues, collectively termed lung remodelling. Acute lung remodelling is the initial stage of structural changes, caused by the deposition of different types of collagen around airways and blood vessels, goblet cell hyperplasia and cell proliferation in the lung tissues [1–3]. Presently, gluco-corticosteroids are the most common and effective anti inflammatory medicines being used for the treatment of mild to moderate persistent asthma. However, their use also raises concerns over side effects and compliance issues [4]. Therefore, new medicines are needed. These should be safe, specific and robust. Currently, many herbal medicines are available for the medication of various illnesses and the list is steadily growing. Among natural products, the seeds and oil of Nigella sativa have attracted the interest of medical scientists [5]. Nigella sativa belongs to the Ranunculaceaefamily, which is an annual herbaceous plant with black seeds. The black seeds of Nigella Sativa have been used as a spice and a food preservative in different countries. Emerging studies have demonstrated that black seed oil (BSO) and its different components, such as thymoquinone, manifest anti-inflammatory, anti-tumour, immune stimulatory and healing properties [6, 7]. In addition, oral administration of BSO can decrease the disease scores in patients with allergic rhinitis, bronchial asthma and atopic eczema [8].
In a previous study we found that BSO ameliorates allergic airway inflammation by inhibiting T-cell proliferation in the spleen [9] and others have observed that it can modify leukotriene synthesis and inhibit histamine release [10]. Previously, it was suggested that pure BSO may inhibit the allergen induced airway inflammation [9] and we hypothesised that it might be able to attenuate the acute lung remodelling in an asthma rat model. Therefore, we used ovalbumin (OVA) to induce asthma in rats to order to investigate the effects of black seed oil on lung remodelling in acute asthmatic conditions.
Materials and methods
Animal
E3 inbred rats were obtained from the Section of Medical Inflammation Research, Lund University, Sweden. Male and female rats, approximately 8 to 10 weeks old were maintained in a pathogen free animal house with controlled temperature (24 ± 2 oC), humidity (52 ± 10%) and continuous air renovation environment. Ovalbumin (OVA) free food and water were provided to the rats. All experiments were approved by the Institutional Animal Ethics Committee of the University (Xi’an Jiaotong University).
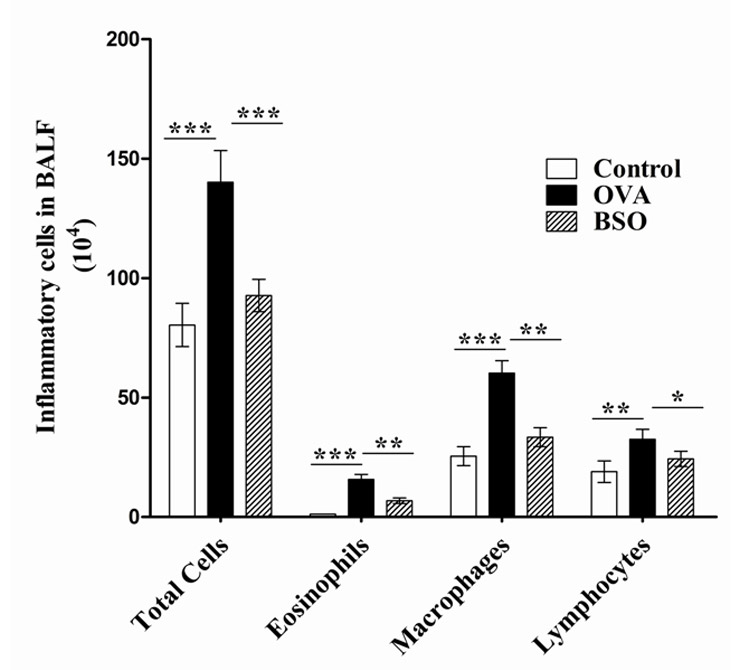
Figure 1
BSO inhibited the recruitment of inflammatory cells in BAL fluid of E3 rats. Rats were divided into 3 groups; Control (immunised and challenged with PBS), OVA (immunised and challenged with OVA) and BSO (immunised and challenged with PBS, and treated with 4 ml/kg of BSO groups). The results were expressed as the means ± S.E.M. for 10 rats in each group. *p <0.05 and **p <0.01, ***p <0.001 were considered statistically significant differences between the control group and OVA group or between the OVA group and BSO group.
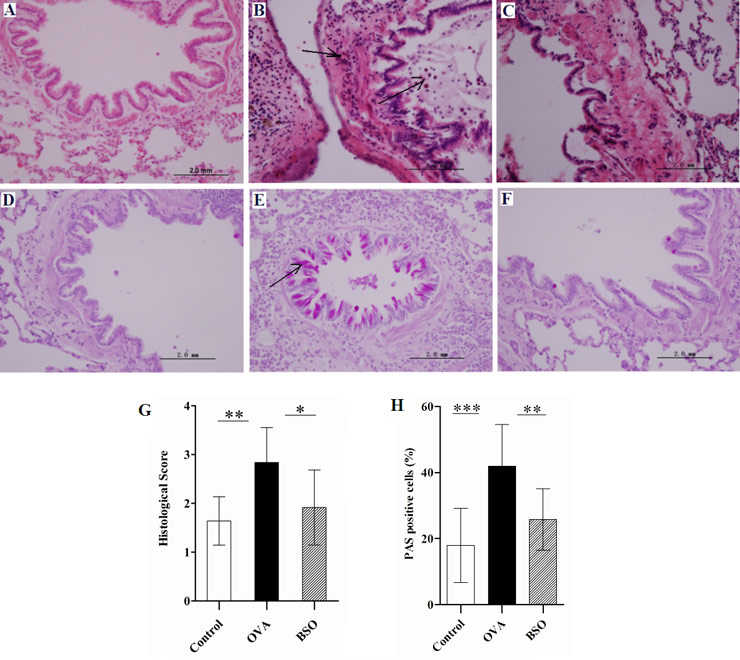
Figure 2
BSO suppressed the airway inflammation and goblet cell hyperplasia. Lung tissue was collected from three groups of rats for the determination of pathological changes. A and D represent lung sections of control rats which do not show pathological changes. B and E indicate lung sections of OVA challenged rats. (B) Infiltration of inflammatory cell around the airways and (E) goblet cells hyperplasia and mucous hyper secretion. C and F show sections from OVA challenged and BSO treated rats. BSO treatment inhibited the infiltration of inflammatory cells (C) and goblet cell hyperplasia and mucous hyper secretion (F) in the lungs. In representative histological images, lung tissues stained with HE (A, B, C; magnification 400X) or PAS (D, E, F; magnification 400X). The scoring data of pathological score (G) and percent of PAS positive cells (H). Arrow indicated the infiltration of inflammatory cells and goblet cells. Data shown as mean ± S.E.M. *P <0.05, **P <0.01 and *** P <0.001 compared between two group.
Black seed oil (BSO)
We used 100% pure BSO obtained from Marhaba Laboratories, Pakistan. To assure the quality of BSO, endotoxin effect was checked by using a Gel Clot TAL (Tachypleus Amebocyte Lysate) kit with a sensitivity of 0.03 E.U/ml and found to be free of endotoxin. A pilot experiment was performed in rats to validate the standard dose of BSO and to elicit the side effects. We maintained and determined the dose of BSO as 4 ml/kg to be that with no adverse effects and maximum tolerance.
Sensitisation and challenge with ovalbumin
Animals were divided randomly into three experimental groups; Control, OVA and BSO group. Each group contained ten rats matched for age and sex. Animals in OVA and BSO groups were sensitised by intraperitoneal injection of 1mg of OVA (Sigma–Aldrich, St. Louis, MO) emulsified in 50 mg aluminium hydroxide (AlumImject; Pierce Biotechnology, Rockford, IL, USA) in a volume of 1ml phosphate buffered saline (PBS) at the day 0. Two weeks after the sensitisation, the rats were subjected to intranasal challenge with OVA (1 mg/ml PBS) once daily for seven days. The animals in the control group were sensitised and challenged with PBS.
Treatment with black seed oil
After one hour of challenge with OVA, animals in the BSO group received black seed oil intraperitoneally at a dose of 4 ml/kg body weight, once daily for seven days preceding the first OVA challenge. The rats from the control and OVA groups were injected with PBS. Twenty four hours after the last challenge, all the animals were euthanized. The lungs were removed and bronchoalveolar lavage fluid (BALF) was collected using cold PBS and stored at –20 °C.
Inflammatory cells in bronchoalveolar lavage fluid (BALF)
BALF was taken from the lungs of experimental rats and the cells counted using a previously described method [9].
Histology
The lungs were infused with 10% neutral buffered formalin and subjected to haematoxylin and eosin (HE), periodic acid-Schiff (PAS) and Masson-trichome staining. Morphological alterations and inflammatory cell infiltration in the airway were evaluated using a scoring method [9]. For the quantification of PAS staining, at least three medium size bronchioles were evaluated on each slide for PAS cells. The results were expressed as the percentage of PAS positive cells per bronchiole, which was determined from the number of PAS positive cells divided by the total number of epithelial cells in each bronchiole by Image-Pro Plus 6 (Media Cybernetics, USA). Masson’s trichome staining was performed to evaluate the collagen deposition in the lung tissues of experimental rats using a previously described method [11].
Proline determination
A previously described method was adopted to determine the level of proline in homogenate of lungs [12].
Arginase activity
Total arginase activity was measured in lung homogenate using a previously described method [13]. The level of total protein in lung homogenate and mitochondria was determined using a BCA kit (Jian Kang Yuan, biomedicine, Zhu Hai China). The absorbance was measured at 562 nm by ELISA reader (Thermo Electron Corporation, Finland). One unit of the enzyme activity was defined as the amount of enzyme that catalyzed the formation of 1 mmol of urea per minute.
Activity of ornithine decarboxylase
The activity of ornithine decarboxylase (ODC) was measured in lung mitochondria as previously described [14]. The absorbance of the organic phase was measured at 426 nm against a reagent blank by ELISA reader (Thermo Electron Corporation, Finland).
Isolation, derivatisation and chromophoric separation of polyamines
The polyamines were isolated and derivatized from lung homogenates [15]. High performance liquid chromatography (HPLC) was performed on a system containing two solvent metering pumps (Shimadzu, model 20AT, Japan) and programmed with a microprocessor controller (Shimadzu, model CBM-20A). The 90 µl of sample with 10 µl internal standard (Hexanediamine) was injected into 20 µl loop for loading into reverse phase C18 column (5 µm particle diameter, 4.6 × 150 mm; Dikma, Technologies, Beijing, China). The samples were eluted in column by using solvent (water: methanol) (v/v). For dansyl polyamines, an excitation wavelength (365 nm) and emission wavelength (510 nm) was used. The peak area and retention time were recorded by LC solution software (Shimadzu, Japan). Calibration was performed by external calibration with putrescine, spermine and spermidine standards.
Extraction of RNA and real time RT-PCR
Total RNA from lung tissue was isolated by using TRIZOL and quantified by measuring the optical density (CECIL Instrument, England). According to the manufacturer’s protocol (RevertAid™, Fermentas Life Sciences, International INC, Canada), total cellular RNA was reverse transcribed by reverse transcription (RT) PCR. Resulting cDNA was amplified by real-time PCR with SYRB Premix Ex Tag II (TaKaRa, Japan). By using the primers for genes such as arginase І, Edn-1, MMP-3, TGF-β, FGF2 and VEGF, real-time RT-PCR was performed at 95 °C for 10 seconds, 62 °C for 15 seconds, and 72 °C for 30 seconds. β-actin was used as reference gene. Primer sequences and their annealing temperatures are depicted in table 1.
Statistical analysis
All the data was expressed as Mean ± S.E.M. We analysed the data using one way ANOVA followed by Bonferroni’s post test. We used Graph pad Prism5 Demo to determine the significant difference among groups. Differences were considered statistically significant when the P value was less than 0.05.
|
Table 1: A list of primer sequences used for real time RT-PCR. |
|
Gene name
|
FP/RP* |
Sequence(5`-3`) |
Size (bp) |
Tm °C |
| β-actin |
FP |
CTATCGGCAATGAGCGGTTCC |
146 |
60 |
| RP |
TGTGTTGGCATAGAGGTCTTTACG |
| Arginase І |
FP |
AGTGTGGTGCTGGGTGGAG |
118 |
60 |
| RP |
GCGGAGTGTTGATGTCAGTGTG |
| Edn1 |
FP |
TTGCTCCTGCTCCTCCTTGATG |
214 |
54 |
| RP |
TCCCTTGGTCTGTGGTCTTTGTG |
| MMP3 |
FP |
GATGATGATGAACGATGGACAGATG |
120 |
54 |
| RP |
GCTACACATTGGTAAGGTCTCAGG |
| TGF-β |
FP |
GGACTACTACGCCAAAGAAG |
294 |
60 |
| RP |
TCAAAAGACAGCCACTCAGG |
| FGF2 |
FP |
TCGCACACACTCCCTTGATGG |
219 |
63 |
| RP |
TCGCACACACTCCCTTGATGG |
| VEGF |
FP |
CAATGATGAAGCCCTGGAGTGC |
287 |
63 |
| RP |
GCGGATCTTGGACAAACAAATGC |
| *FP, forward primer; RP, reverse primer |
Results
BSO decreased inflammatory cell infiltration in BALF
A small number of total cells (80.40 ± 9.04 × 104 cells) were found in BALF of control rats. A few number of eosinophils (1.21 ± .03 × 104 cells), macrophages (25.50 ± 3.99×104cells) and lymphocytes (19.00 ± 4.51 × 104cells) were observed in the BALF of control rats sensitized and challenged with PBS. Administration of OVA for 7 days elicited a massive recruitment of total cell numbers (140.20 ± 13.23 × 104cells), and eosinophils (15.71 ± 2.16 × 104cells), macrophages (60.20 ± 5.22 × 104cells) and lymphocytes (32.53 × 4.23 × 104 cells) in the BALF, indicating the presence of a significant proportion of inflammatory cells. Intraperitoneal treatment of BSO (4ml/kg) significantly decreased the total cells (92.36 ± 6.77 × 104cells) and differential number of eosinophils (6.83 ± 1.14 × 104cells), macrophages (33.40 ± 3.9 × 104cells) and lymphocytes (24.34 ± 3.23 x 104cells) in the BALF of rats sensitised and challenged with OVA (fig. 1). The data indicated that BSO decreases the inflammatory cell infiltration in the airways.
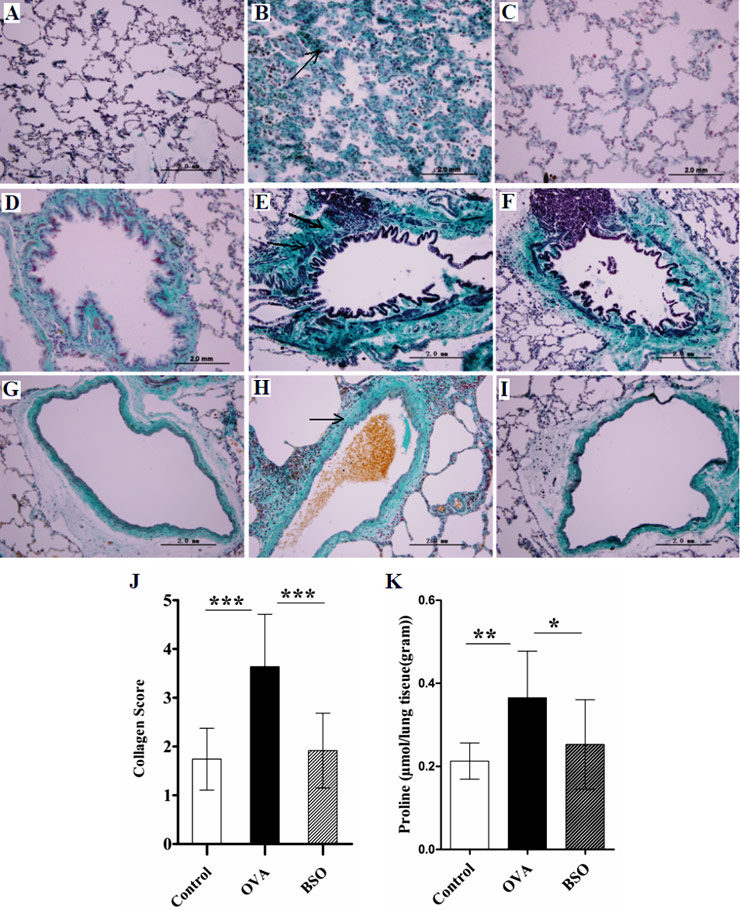
Figure 3
BSO attenuated the collagen deposition and proline level. The lung tissue from experimental rats was stained by Masson’s trichome method. A, D and G represent lung sections of control rats, showing normal level of collagen. B, E and H show lung sections of OVA challenged rats, indicating deposition of collagen around airways, blood vessels and alveolar wall. C, F and I show sections from OVA challenged and BSO treated rats. BSO administration decreased the collagen deposition in the lung. In the representative histological images, A, B and C indicate parenchymal interstitium in alveolar area, D, E and F peribronchial and G, H and I perivascular connective tissues. In all figures, magnification is 400X.
The score for collagen deposition and proline level in the lung homogenate is shown in J and K respectively. Arrows show thickening of alveolar septae and deposition of collagen around the bronchus and blood vessels. Data shown as mean ± S.E.M. *P <0.05, **P <0.01 and *** P <0.001 compared between two group.
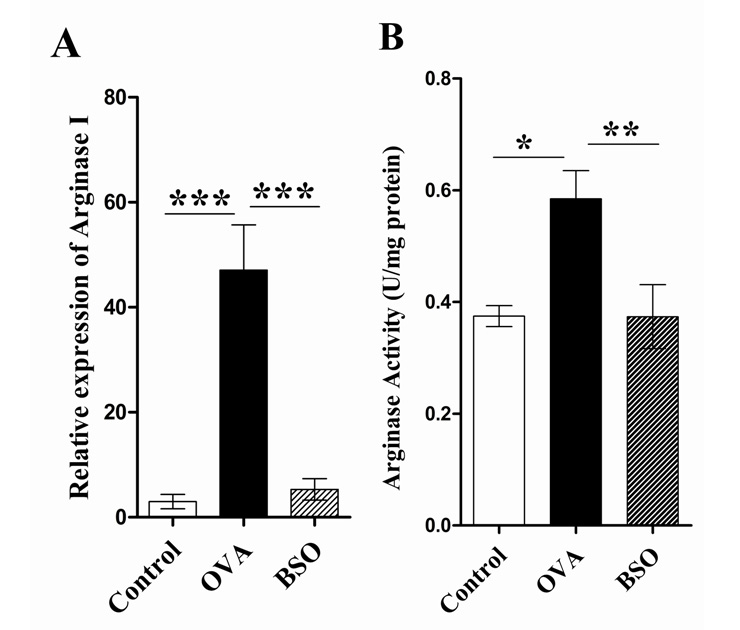
Figure 4
Black seed oil diminished the mRNA expression and activity of arginase. The mRNA expression of arginase І was determined by real time RT-PCR in the lung of experimental rats whereas β-actin gene was amplified as reference (A). Total arginase activity was determined by photometry method in the lung homogenate of lung (B). This experiment used 10 rats for each group. Data shown as mean ± S.E.M. * P <0.05, ** P <0.01, *** P <0.001 compared between two groups.
BSO suppressed the airway inflammation and goblet cell hyperplasia
The effect of BSO on rats sensitised and challenged with OVA was determined through HE, where we observed histological changes (fig. 2). Massive recruitment of inflammatory cells around the airway was observed in the lungs of OVA group rats (fig. 2B) and the same effect was observed in the alveolar spaces and perivascular areas (not shown in data). No obvious pathological abnormalities were found in the lungs of the control rats (fig. 2A). Intraperitoneal administration of pure BSO suppressed the inflammation in the lungs of OVA exposed rats by decreasing the inflammatory cell infiltration in the airways, alveoli and perivascular areas (fig. 2C). The histological score for inflammatory cell infiltration was significantly decreased in the lungs of OVA exposed rats after BSO treatment (fig. 2G). We calculated the percentage of positive goblet cells from bronchioles in the lung tissues of experimental rats using PAS staining (fig. 2D-F). In the lungs of OVA group we found 41.90 ± 4.00 percent positive PAS cells (goblet cells) leading to goblet cell hyperplasia and mucous hyper secretion, which was a significant increased as compared to control (17.90 ± 3.54%). BSO treatment significantly reduced the percentage of positive goblet cells to 25.80 ± 2.94% in the lungs of rats exposed to OVA (fig. 2H).
BSO attenuated the collagen deposition and proline level
To determine the acute lung remodelling, collagen deposition in lung tissue was examined using Masson’s trichome staining. As shown in fig. 3, high collagen deposition was found in parenchymal interstitium of alveolar areas, peribronchial and perivascular connective tissues of OVA group as compared to control.
Moreover, low proline level (0.25 ± 0.01 µmol/g of lung tissues) was found in lung homogenate of PBS sensitised and challenged rats. However, a significantly high proline level (0.36 ± 0.04 µmol/g of lung tissues) in homogenate lung tissues of OVA-sensitised and challenged rats was observed (fig. 3K). The results showed that the score for collagen deposition in lung tissues and proline level (0.25 ± 0.03 µmol/g of lung tissues) was remarkably attenuated after intraperitoneal treatment of BSO.
Expression of arginase І, activity of arginase, ODC and the level of polyamine abolished with BSO treatment
To further investigate whether the arginase pathway also related to the BSO induced protective effect for acute stage of lung remodelling in the OVA induced asthma rat model, we measured the mRNA expression of arginase І in the lung of experimental rats by real time RT-PCR. As shown in Fig. 4A, real time RT-PCR provided a quantitative measure of arginase І mRNA expression in the lungs of experimental rats. The mRNA expression of arginase І predominantly increased in the lungs of OVA group compared with control. By comparison, intraperitoneal treatment with BSO significantly decreased the arginase mRNA expression in OVA-induced rats. We also measured the activity of total arginase in the lung homogenate of experimental rats. Increased activity of total arginase (0.585 ± 0.051 U/mg protein) in the lung homogenate of OVA challenged rats was observed as compared to control rats (0.375 ± 0.019 U/mg protein) (fig. 4B). Total arginase activity in the lung homogenate of rats exposed to OVA was significantly suppressed by the BSO treatment (0.374 ± 0.0573 U/mg protein).
To test whether the suppressive effect of BSO on OVA exposed animals leads to polyamine production, the effects of BSO on OVA exposed ODC activity and polyamine concentration were examined in the lung homogenate of the experimental rats. As shown in fig. 5A-B, the activity of ODC and the level of polyamine especially of spermine were significantly increased (ODC: 2.50 ± 0.67 U/mg protein, Spermine: 0.05 ± 0.01 µmol/L) in the lung of OVA group rats as compared to control (ODC: 0.69 ± 0.13 U/mg protein, Spermine: 0.02 ± 0.003 µmol/L). However, treatment of BSO decreased the ODC activity (0.93±0.21 U/mg protein) and spremine level (0.024 ± 0.0031 µmol/L).
Black seed oil abrogated the mRNA expression of Endothelin1, MMP3 and growth factors
To determine the molecular basis of acute lung remodelling in the OVA induced rat model, we examined the mRNA expression of EdnІ, MMP3, TGF-β, FGF2 and VEGF by real time RT-PCR. As shown in fig. 6A-E, the mRNA expression of EdnІ, MMP3, TGF-β, FGF2 and VEGF in the lungs of rats challenged with OVA was remarkably increased as compared with control rats. By comparison, intraperitoneal administration of BSO normalised the mRNA expression in the lungs of OVA exposed rats.
Discussion
In our study we investigated the hypothesis that pure black seed oil (BSO) of Nigella sativa significantly attenuated lung remodelling induced by repetitive allergen challenge in E3 rats. We also showed potential BSO effects that abate allergen induced collagen deposition in the lungs of OVA exposed rats through inhibiting arginase pathways, expression of growth factor (TGF-β, FGF2 and VEGF), Edn1 and MMP3.
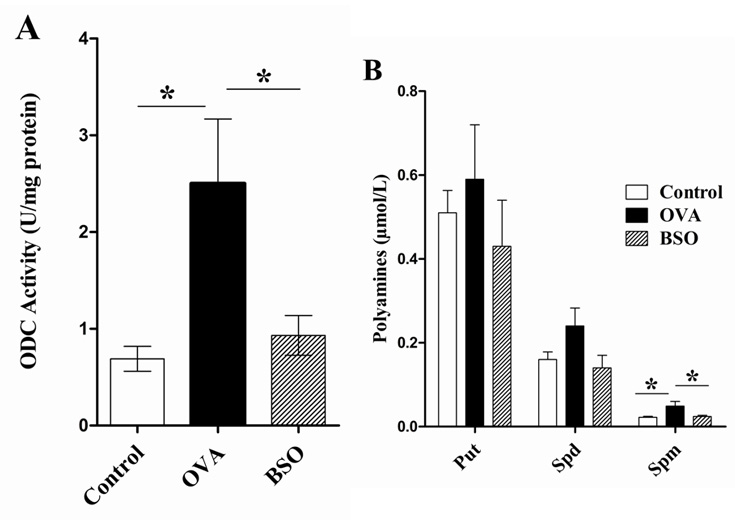
Figure 5
Black seed oil decreased the ODC activity and polyamine production. A, represents the activity of ODC in the lung homogenate of experimental rats. Polyamines such as putrescine (Put), spermidine (Spd) and spermine (Spm) isolated from lung of experimental rats (B). The dansyl derivatives of polyamines were determined by HPLC method. Data shown as mean ± S.E.M for 10 rats each group. * P <0.05 compared between two groups.
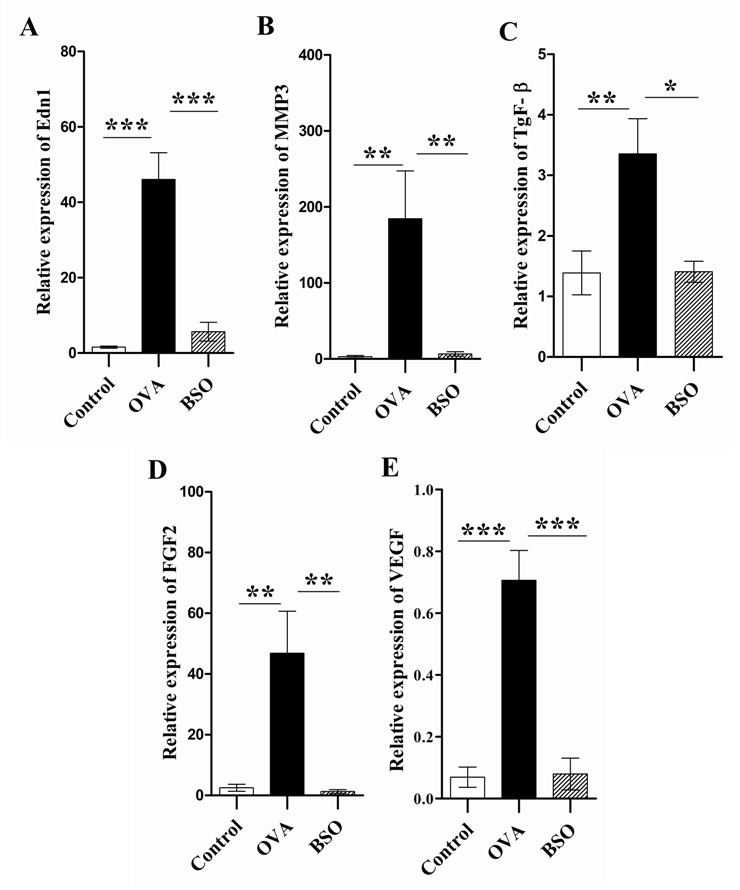
Figure 6
Black seed oil abrogated the mRNA expression of Edn1 and MMP3 and growth factors in the lungs. Total RNA was extracted from the lung of experimental rats and used in real time RT-PCR to determine mRNA expression of Edn1, MMP3 and growth factors, while β-actin was used as the reference gene. Relative mRNA expression of Edn-І (A), MMP3 (B), TGF-β (C), FGF2 (D) and VEGF (E) was expressed as mean ± S.E.M. *P <0.05, **P <0.01 and *** P <0.001 by comparing two groups.
Collagen, as type І, ІІ, ІІІ and ІV, is located in extracellular matrix of connective tissues and is used as a major fibrous and structural protein in animals [2]. We found high intensity of collagen deposition in the alveolar parenchymal interstitium, peribronchial and perivascular connective tissues of the lungs of asthmatic rats. This is consistent with previous observations that found an increase level of total collagen, collagen I, procollagen I, III and α-smooth muscle actin in the lungs of both mice and rats after OVA exposure for few days [16–18]. Proline plays a prime role in collagen biosynthesis and the level of proline positively correlated with collagen level [19]. We observed high proline levels in the lungs of OVA exposed rats compared to control. Arginase is a key enzyme (arginase pathways) in the urea cycle for the production of proline and polyamine. L-proline, a well known precursor of collagen, is synthesized from L-ornithine in a two-step reaction, while ODC initiates the synthesis of polyamines that could be involved in the proliferation of structural cells in the lung tissues [20]. Our studies demonstrated that BSO reduced the downstream products of arginase pathways such as polyamine and proline by inhibition of arginase and ultimately reduced collagen deposition. The upregulation of arginase in cultured airway fibroblasts indicates a possible role of the enzyme in the collagen synthesis and cell proliferation that leads to lung remodelling. Increased levels of polyamines have been observed in mouse lungs after allergen challenge and in serum of asthmatic patients [21–23]. Polyamines can stimulate cell proliferation and structural alteration by promoting histone acetyltransferase activity, resulting in chromatin hyperacetylation [24]. High levels of polyamine was found in human pulmonary artery smooth muscle cells (hPASMC) exposed to oxygen [25]. Our HPLC analysis revealed that polyamines, especially spermine, are strongly increased in the lung of OVA exposed rats. Moreover the activity of ODC also increased in the lung homogenate of asthmatic E3 rats. It has been reported that the high expression of arginase I and ODC mRNA expression increased in the epithelium, fibroblast and smooth muscle bundles of smoking asthmatics [26]. During in vivo and in vitro study, the production of polyamine especially spermine as the result of upregulation of arginase I promoted axonal regeneration in the CNS after the injury [27]. Thus, to inhibit allergen-induced arginase expression as well as its activity, BSO might play a significant role in attenuation of lung remodelling during asthma.
Edn1 is a potent bronchoconstrictor and a profibrotic agent. It is strongly expressed in the epithelium of airways during mild and severe asthma. Edn1 is associated with eosinophilic rather than neutrophilic airways inflammation. It plays an important role in the pathogenesis of airway disease by stimulating collagen deposition and decreasing collagen degradation [28]. In bronchial epithelium a positive correlation was found between the increased Edn1 synthesis and airway smooth muscles in refractory asthmatics [29, 30]. Our data showed that mRNA expression of Edn1 and MMP3 significantly increased in the lungs of OVA challenged rats. The MMPs such as MMP3, MMP9 and MMP13 have relevance to the acute and chronic structural airway changes in asthma. Stromelysin 1(MMP3) is involved in the development of inflammatory acute lung injury and associated with activation of MMP7 and MMP9 [31, 32]. Stromelysin 1 is an extracellular endopeptidase of vertebrate tissues and is homologous with interstitial collagenase. It activates procollagenase after digesting proteoglycan, fibronectin and collagen. These proteinases can be generated by structural and inflammatory cells, and have the ability to degrade the proteoglycans and thus potentially enhance lung fibrosis and smooth muscle proliferation through their ability to release and activate latent matrix bound growth factors [33]. High mRNA expression of MMP3 has been reported in the human myometrium during pregnancy and labour and in human uterine smooth muscle cells (SMCs). Recent studies have shown that cytokine stimulation increases MMP3 level in lung fibroblast [34], which can lead to cell invasion [35]. Thus, the ability of BSO to inhibit allergen-induced mRNA expression of Edn-1 as well as MMP3 expression might play a significant role in reducing lung remodelling in asthmatic rats.
Cytokines, chemokines and growth factors such as TGF-β, FGF2 and VEGF are released from inflammatory and structural cells in the lung. They are considered to play a pivotal role in the development of lung remodelling, fibrosis and pulmonary hypertension by increasing the collagen deposition, cell proliferation and mucous hyper secretion [36]. Our work showed that BSO reduced the level of peribronchial inflammatory cells as well as goblet cell hyperplasia. Interestingly, TGF-ß is a potent profibrotic growth factor that directly or indirectly stimulates the fibroblasts to produce collagen, fibronectin and proteoglycan during pulmonary fibrosis. The role of TGF-β increases the expression of arginase I and II in primary mouse fibroblasts, demonstrating its interplay role between collagen production and activity of arginase enzyme. The basic fibroblast growth factor (FGF2) and VEGF promote the proliferation of endothelial cells, fibroblasts, and also enhance the goblet cell hyperplasia, angiogenesis and collagen deposition in the lung of asthmatics. The VEGF is an important mediator of vascular remodelling, extra-vascular remodelling and pulmonary inflammation that has been shown in the VEGF lung transgenic mice [37–39]. BSO significantly reduced lung remodelling by attenuating the mRNA expression of growth factors such as TGF-β, FGF2 and VEGF in the lung of asthmatic rats.
Conclusion
In summary, our study demonstrates that BSO can attenuate the allergen-induced pathological changes of lung remodelling by decreasing collagen deposition, arginase pathways, expression of TGF-β 1, FGF2 and VEGF and End1 as well as MMP3 in the lungs of asthmatic rats. These findings strongly suggest that BSO might be useful in the treatment of lung remodelling in asthmatic patients in future. Nevertheless, further study is required to explore the potential clinical usefulness of pure BSO for lung remodelling during asthma as well as to determine the potent compounds in the mixture of BSO that play an effective role in reducing the structural and functional alteration in allergic asthmatics.
Correspondence:
Dr. Shemin Lu
Department of Genetics and Molecular Biology
Xi’an Jiaotong University College of Medicine
Xi’an, Shaanxi 710061, P.R. China
lushemin@mail.xjtu.edu.cn or shemin.lu@gmail.com
References
1 Rennard SI. Repair mechanisms in asthma. J Allergy Clin Immunol. 1996;98(6 Pt 2):S278–86.
2 James AL, Wenzel S. Clinical relevance of airway remodelling in airway diseases. Eur Respir J. 2007;30(1):134–55.
3 Fattouh R, Jordana M. TGF-beta, eosinophils and IL-13 in allergic airway remodeling: a Critical appraisal with therapeutic considerations. Inflamm Allergy Drug Targets. 2008;7(4):224–36.
4 Walsh GM. Novel therapies for asthma: advances and problems. Curr Pharm Des. 20 05;11(23):3027–38.
5 Huntley A, Ernst E. Herbal medicines for asthma: a systematic review. Thorax. 2000;55(11):925–9.
6 Salem ML. Immunomodulatory and therapeutic properties of the Nigella sativa L. seed. Int Immunopharmacol. 2005;5(13-14):1749–70.
7 Khan M. 1976. A translation of Al-Bukhari. Collection of Authentic Prophet (PBUH) saying. Division 71 (the book of medicine). 2nd ed. Ankara (Turkey): Hilal Yayinlari Publishers.
8 Kalus U, Pruss A, Bystron J, Jurecka M, Smekalova A, Lichius JJ, et al. Effect of Nigella sativa (black seed) on subjective feeling in patients with allergic diseases. Phytother Res. 2003;17(10):1209–14.
9 Shahzad M, Yang X, Raza Asim MB. Qingzhu S, Yan H, Fujun Z, et al. Black seed oil ameliorates allergic airway inflammation by inhibiting T-cell prolife ration in rats. Pulm pharmacol ther. 2009;22(1):37–43.
10 Chakravarty N. Inhibition of histamine release from mast cells by nigellone. Ann Allergy. 1993;70(3):237–42.
11 Li LF, Liao SK, Huang CC, Hung MJ, Quinn DA. Serine/threonine kinase-protein kinase B and extracellular signal-regulated kinase regulate ventilator-induced pulmonary fibrosis after bleomycin-induced acute lung injury: a prospective, controlled animal experiment. Crit Care. 2008;12(4):R103.
12 Bates LS, Waldren RP. Teare ID. Rapid determination of free proline for water-stress studies. Plant and Soil. 1973;39(1):205–7.
13 Corraliza IM, Soler G, Eichmann K, Modolell M. Arginase induction by suppressors of nitric oxide synthesis (IL-4, IL-10 and PGE2) in murine bone-marrow-derived acrophages.Biochem Biophys Res Commun. 1995;206(2):667–73.
14 Ngo TT, Brillhart KL, Davis RH, Wong RC, Bovaird JH, Digangi JJ, et al. Spectrophotometric assay for ornithine decarboxylase. Anal Biochem. 1987;160(2):290–3.
15 Smith MA, Davies PJ. Separation and Quantitation of Polyamines in Plant Tissue by High Performance Liquid Chromatography of Their Dansyl Derivatives. Plant Physiol. 1985;78(1):89–91
16 Rydell-Tormanen K, Uller L, Erjefalt JS. Remodeling of extra-bronchial lung vasculature following allergic airway inflammation. Respir Res. 2008;9:18.
17 Zhou B, Comeau MR, De Smedt T, Liggitt HD, Dahl ME, Lewis DB, et al. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol. 2005;6(10):1047–53.
18 Pini L, Torregiani C, Martin JG, Hamid Q, Ludwig MS. Airway remodelling in allergen challenged Brown Norway rats: distribution of proteoglycans. Am J Physiol Lung Cell Mol Physiol. 2006;290(6):L1052–8.
19 Kershenobich D, Fierro FJ, Rojkind M. The relationship between the free pool of proline and collagen content in human liver cirrhosis. J Clin Invest. 1970;49(12):2246–49.
20 Wu G, Morris SM, Jr. Arginine metabolism: nitric oxide and beyond. Biochem J. 1998;336(Pt):1–17.
21 Kenyon NJ, Bratt JM, Linderholm AL, Last MS, Last JA. Arginases I and II in lungs of ovalbumin-sensitized mice exposed to ovalbumin: sources and consequences. Toxicol Appl Pharmacol. 2008:230(3):269–75.
22 Zimmermann N, King NE, Laporte J, Yang M, Mishra A, Pope SM, et al. Dissection of experimental asthma with DNA microarray analysis identifies arginase in asthma pathogenesis. J Clin Invest. 2003;111(12):1863–74.
23 Warnken M, Haag S, Matthiesen S, Juergens UR, Racké K. Species differences in expression pattern of arginase isoenzymes and differential effects of arginase inhibition on collagen synthesis in human and rat pulmonary fibroblasts. Naunyn Schmiedebergs Arch Pharmacol. 2010;381(4):297–304.
24 Hobbs CA, Gilmour SK. High levels of intracellular polyamines promote histone acetyltransferase activity resulting in chromatin hyperacetylation. J Cell Biochem. 2000;77(3): 345–60.
25 Chen B, Calvert AE, Cui H, Nelin LD. Hypoxia promotes human pulmonary artery smooth muscle cell proliferation through induction of arginase. Am J Physiol Lung Cell Mol Physiol. 2009;297(6):L1151-9.
26 Bergeron C, Boulet LP, Page N, Laviolette M, Zimmermann N, Rothenberg ME, et al. Influence of cigarette smoke on the arginine pathway in asthmatic airways: increased expression of arginase I. J Allergy Clin Immunol. 2007;119(2):391–7.
27 Deng K, He H, Qiu J, Lorber B, Bryson JB, Filbin MT. Increased synthesis of spermidine as a result of upregulation of arginase I promotes axonal regeneration in culture and in vivo. J Neurosci. 2009;29(30):9545–52.
28 Fagan KA, McMurtry IF, Rodman DM. Role of endothelin-1 in lung disease. Respir Res. 2001;2(2):90–101.
29 Patel KV, Sheth HG, Schrey MP. Stimulation or endothelin-1 secretion by human breast cancer cells through protein kinase A activation: a possible novel paracrine loop involving breast fibroblast-derived prostaglandin E2. Mol Cell Endocrinol. 1997;126(2):143–51.
30 Pe´gorier S, Arouche N, Dombret MC, Aubier M, Pretolani M. Augmented epithelial endothelin-1 expression in refractory asthma. J Allergy Clin Immun. 2007; 120(6):1301–7.
31 Furuya M. Analysis of matrix metalloproteinases and related tissue inhibitors in cystic fluids of ovarian tumors. Hokkaido Igaku Zasshi. 1999;74(2):145–55.
32 Warner RL, Beltran L, Younkin EM, Lewis CS, Weiss SJ, Varani J, et al. Role of stromelysin 1 and gelatinase B in experimental acute lung injury. Am J Respir Cell Mol Biol. 2001;24(5):537–44.
33 Dahlen B, Shute J, Howarth P. Immunohistochemical localisation of the matrix metalloproteinases MMP-3 and MMP-9 within the airways in asthma. Thorax. 1999;54(7):590–96.
34 Fingleton B. MMPs as therapeutic targets—Still a viable option. Semin Cell Dev Biol. 2008;19(1):61–8.
35 Phromnoi K, Yodkeeree S, Anuchapreeda S, Limtrakul P. Inhibition of MMP-3 activity and invasion of the MDA-MB-231 human invasive breast carcinoma cell line by bioflavonoids. Acta Pharmacol Sin. 2009;30(8):1169–76.
36 Homer RJ, Elias JA. Airway Remodeling in Asthma: Therapeutic Implications of Mechanisms Physiology. 2005;20(1):28–35.
37 Hostettler KE, Roth M, Burgess JK, Gencay MM, Gambazzi F, Black JL, et al. Airway epithelium-derived transforming growth factor-beta is a regulator of fibroblast proliferation in both fibrotic and normal subjects. Clin Exp Allergy. 2008;38(8):1309–17.
38 Kitowska K, Zakrzewicz D, Konigshoff M, Chrobak I, Grimminger F, Seeger W, et al. Functional role and species-specific contribution of arginases in pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2008;294(1):L34–45.
39 Broide DH. Immunologic and inflammatory mechanisms that drive asthma progression to remodeling. J Allergy Clin Immunol. 2008;121(3):560–70.





