
Figure 1
Endobronchial valve (Zephyr®).
DOI: https://doi.org/10.4414/smw.2010.13140
Abbreviations
COPD: Chronic obstructive pulmonary disease
VATS: Video-assisted thoracoscopy
EGFR: Epidermal growth factor receptor
FEV1: Forced expiratory volume in 1 second
SPECT: Single photon emission tomography
mPAP Mean pulmonary arterial pressure
MMRC: Modified Medical Research Council Dyspnoea Scale
IVC: Inspiratory vital capacity
RV: Residual volume
TLC: Total lung capacity
VO2 max: Maximal oxygen uptake
SF 36: Short Form (36) Health Survey
SGQ: Saint George’s Respiratory Questionnaire
CAT: COPD Assessment Test
The epidemic spread of smoking all over the world since World War II has resulted in an enormous rise in the number of patients with COPD. According to the World Health Organization (WHO), COPD will be the third leading cause of death by the year 2020. Lung emphysema is the pathoanatomical correlate of severe COPD associated with homogeneous or heterogeneous destruction of lung tissue and marked hyperinflation considerably limiting patient activity. Lung transplantation is a therapeutic option for only a selected group of younger patients with advanced COPD and no relevant comorbidities [1]. Lung volume reduction surgery (LVRS) was introduced more than fifteen years ago as an alternative to lung transplantation [2]. Initially the procedure was performed via sternotomy followed by bilateral video-assisted thoracoscopy (VATS) [3]. Despite improvements in quality of life, lung function parameters, 6-minute walk distance and medium-term survival [4], the early enthusiasm with this procedure has markedly subsided due to high perioperative mortality in selected groups of COPD patients [5]. As a result, new bronchoscopic technologies have been introduced to perform lung volume reduction without the risk associated with surgery. The idea is to reduce lung hyperinflation by bronchoscopic implantation of implantable devices such as biodegradable material, insertion of endobronchial valves or implantation of bronchopulmonary stents. Due to their advantages compared with surgical therapy, bronchoscopic lung volume reduction procedures have won mounting recognition in the pulmonary literature. Accordingly, a comprehensive update of lung volume reduction therapies for advanced emphysema has recently been published [6]. Most thus far published clinical trials on bronchoscopic lung volume reduction have also included patients suitable for surgical lung volume reduction, i.e. those suffering from heterogeneous upper lobe emphysema with marked hyperinflation and no contraindication for surgical lung volume reduction (table 1).
What do we expect as potential results when a new medication or technology is introduced? Unfortunately we cannot cure the majority of disorders. Expectations largely depend on current options for the treatment and/or control of a disease. Therefore a new drug or procedure must fulfil different criteria if it is to find its way into clinical practice. Table 2 depicts various options for the successful introduction of a new drug/procedure. The following examples may perhaps clarify this notion: in cases where no medical treatment is available, e.g. idiopathic lung fibrosis, a new drug improving a minor area of a disease is easily introduced. Another example is advanced lung cancer: except for the minority of patients with EGFR positive adenocarcinoma, current chemotherapeutic agents are associated with only a marginal improvement in survival. A drug prolonging survival for a median of 4 months would, therefore, have no problem gaining approval for routine use. Conversely, in cases where the available treatment is effective, a new drug or device must display considerable superiority. This was the case when combined inhaled corticosteroids and long-acting β2-agonists became available for the treatment of moderate asthma. Alternatively, a new drug or procedure may be equally effective but associated with fewer side effects. An example of this is sleeve resection, a localised resection of locally advanced lung cancer performed by thoracic surgeons to avoid the morbidity associated with pneumectomy. It has been shown that the cure rate associated with sleeve resection is similar to that achieved by pneumectomy, though perioperative mortality is significantly lower in patients undergoing sleeve resection. Finally, a drug or procedure may also be successfully deployed if, though not inferior in regard to efficacy and side effects, it is much less costly than current treatment. Regular inhalation of hypertonic saline in patients with cystic fibrosis has proved equally effective but its cost is one tenth that of recombinant DNase. All the above criteria should be taken into account in judging the clinical potential of bronchoscopic lung volume reduction. Specific goals of therapy in COPD, as shown on table 3, should be also considered. These endpoints are regarded as relevant and have usually been chosen for verification of efficacy in COPD trials. Similar endpoints must be addressed for bronchoscopic lung volume reduction, but it should be noted that candidates for surgical or bronchoscopic lung volume reduction are most often in an advanced stage of disease.

Figure 1
Endobronchial valve (Zephyr®).
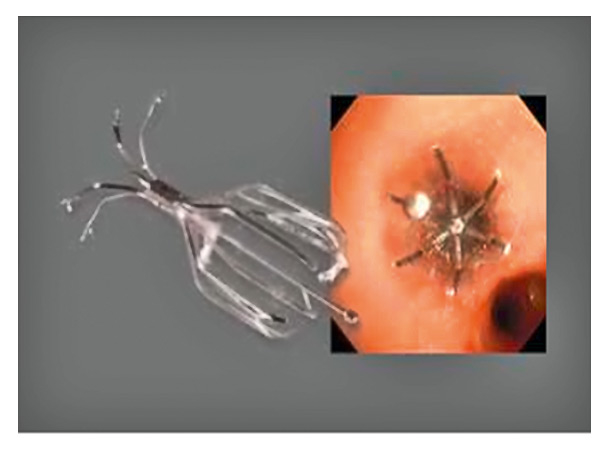
Figure 2
Endobronchial valve of 6 mm diameter (Spiration®).
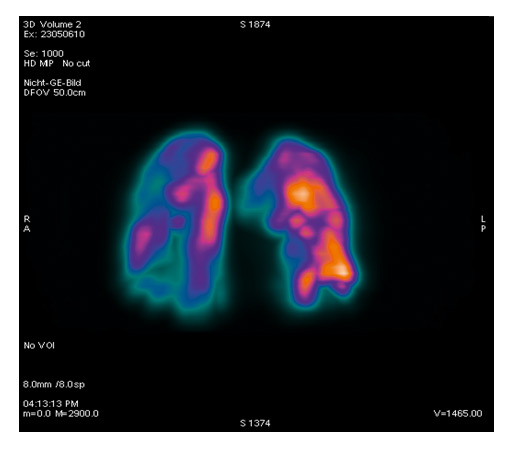
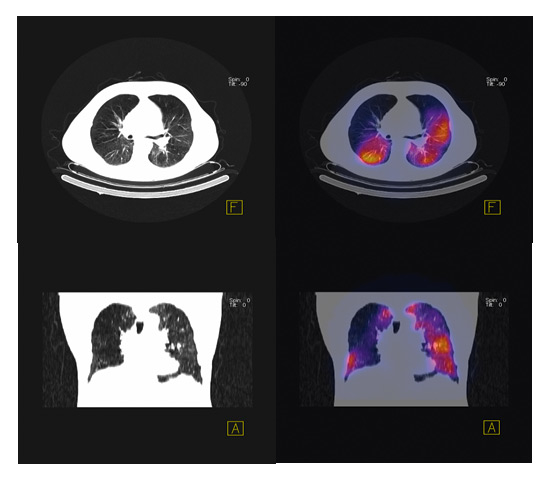
Figure 3
SPECT scan showing heterogeneous emphysema.
The results of a large randomised trial comparing endobronchial valves with conventional therapy were published in September 2010 [7]. A total of 321 patients with heterogeneous emphysema were randomly assigned to receive either endobronchial valves or standard medical care. At six months there was a significant difference in FEV1 of 6.8% comparing the two groups. In contrast, there was no significant difference in 6-minute walk distance, the other primary endpoint of the study. Patients with implanted valves suffered from exacerbations of COPD more often than controls. Preliminary results of two other large randomised clinical trials were presented at the European Respiratory Society Conference at Barcelona in September 2010. Using a similar randomised approach the EuroVENT trial produced comparable results [8]. As presented in table 4, the authors should be commended for a well-designed randomised study including a large number of patients with heterogeneous upper lobe dominant emphysema. Procedures within the EuroVENT trial aimed for unilateral lobar occlusion using the Zephyr® valve (fig. 1). Although the primary endpoints were well chosen, the results showed only a small improvement in lung function with no increase in 6-minute walk distance. Side effects were slightly more frequent in patients receiving valves as compared to controls. The EASE trial evaluated another approach to achievement of lung volume reduction [9]. After localising peribronchial vessels via endobronchial ultrasound, transbronchial puncture was performed followed by dilatation and stent implantation between bronchi and adjacent homogeneous, hyperinflated lung parenchyma. This approach was expected to lead to lung deflation immediately after the procedure. As depicted in Table 5, the study was very well designed, including randomisation of a large number of patients to the active or sham bronchoscopic procedure. Interestingly, despite the somewhat courageous intervention close to large vessels there were no major complications. There was an impressive decrease in residual volume within one week of the procedure. However, the effect on deflation was lost within six months, chiefly due to stent occlusion.
Taking into consideration the above-mentioned aspects, we now return to our initial question as to why a new procedure may become a successful therapeutic option and what aims may be achieved in COPD (table 6–7). Surgical lung volume reduction is currently more effective than bronchoscopic lung volume reduction both in regard to changes in lung volumes and exercise capacity. However, morbidity and mortality are much lower in bronchoscopic lung volume reduction than with the surgical procedure. We have recently shown that implantation of a new generation of intrabronchial valves –an umbrella-shaped, self-expanding device (fig. 2) can be performed safely via flexible bronchoscopy under conscious sedation with propofol, even in patients with major contraindications for surgical volume reduction [10]. Figure 2 shows an example of an endobronchial valve of 6 mm diameter (Spiration®). The duration of the whole procedure involving placing of 3 to 10 valves is 35 to 60 minutes, and patients can be discharged from hospital within 24 hours. A recently published multicentre study of 91 patients with severe obstruction suggests a highly significant improvement in health-related quality of life but no significant change in FEV1, exercise tests and total lung volumes [11]. Importantly, in contrast to the Euro-VENT trial, the aim of the Spiration® approach is bilateral valve placement. Flexible bronchoscopy is much less costly than the surgical procedure, although the current cost of endobronchial valves may partly counteract its advantages. Health related quality of life is improved following valve implantation but exercise capacity seems not to be significantly influenced. There is no evidence of a decreased exacerbation rate or even a trend to slightly more exacerbations. At the current stage no beneficial effect on medium-term mortality can be expected.
| Table 1: Contraindications for surgical lung volume reduction. |
| Persistent smoking |
| Not fit for pulmonary rehabilitation |
| Pulmonary arterial hypertension (mPAP >35 mm Hg) |
| Very severe obstruction (FEV1 <20% predicted) |
| Severe impairment of gas exchange |
| Diffusion capacity <20% predicted |
| Severe hypoxaemia |
| Hypercapnia |
| Major comorbidities |
| Lung infection / bronchiectasis |
| Table 2: Criteria for successful introduction of new therapeutic approaches. |
| No effective therapy available |
| Poor prognosis despite available therapy |
| Superiority over available therapy |
| Not inferior but fewer side effects than available therapy |
| Not inferior, similar side effects but much less costly than available therapy |
| Table 3: Specific aims of COPD therapy. |
| Reduction of symptoms (MMRC) |
| Improvement in lung function (FEV1, IVC,RV, TLC) |
| Improvement in exercise capacity (VO2 max, endurance, 6-min walk distance) |
| Improvement in general health (SF 36, SGQ, CAT…) |
| Reduction of COPD exacerbations |
| Reduction of mortality |
What approaches could improve the efficacy of bronchoscopic lung volume reduction? A major advance will be achieved if endobronchial stents used for homogeneous emphysema can be modified to remain open over a longer period of time, so that the initial marked benefit on hyperinflation can be maintained. This could lead to a lasting decrease in hyperinflation and potentially to an improvement in exercise capacity. With respect to valve implantation, the indication for the procedure needs further refining. In a small randomised trial Eberhard et al. showed that complete unilateral lobar exclusion provides better results than bilateral segmental occlusion [12]. Valve placement should primarily target the areas of the lung which are poorly perfused. Thus the optimal placement of valves might not be efficaciously judged by morphology on CT scan only. Figure 3 shows a SPECT (single photon emission tomography) scan which superimposes the morphology on CT scan with the perfusion pattern in a patient with heterogeneous emphysema (courtesy F. Forrer, University Hospital Basel). Seven valves were implanted into the areas of lowest perfusion with the aim of reducing lung volume without loss of diffusion capacity. Valves can therefore be placed not only to the upper but also to the lower lobes [10]. Persistence of collateral ventilation seems to be associated with poorer results of valve implantation as assessed in the US and EuroVENT trials [8]. This problem can be partly overcome by assessing CT scans for total fissures between the different lobes or by evaluating collateral ventilation during bronchoscopy with a specific catheter [13]. An alternative approach to prevention of collateral ventilation is to occlude segments or lobes completely by instilling a polymeric sealant [14, 15] or steam [16]. However, the latter procedure appears to be associated with a marked inflammatory response, thus questioning its clinical value in a patient population already suffering from persistent respiratory symptoms.
In summary, bronchoscopic lung volume reduction is a safe procedure, but one whose efficacy needs to be improved by further technical refinement.
| Table 4: EuroVENT trial. | ||
| Trial design | Randomised 2:1 treatment/control | +++ |
| Therapeutic procedure | Unilateral lobar treatment | +++ |
| Number of patients included | 171 heterogeneous emphysema | +++ |
| Primary endpoint | FEV1 and 6-min walk distance | +++ |
| Results efficacy | 6.6% FEV1 | + |
| 8.6% 6-min walk, 7.5% control | ||
| Responders, high responders, total | ||
| Results serious adverse events | Combined endpoint 17.3% versus 8.3% | ++ |
| Table 5: EASE trial. | ||
| Randomised design | 2:1 treatment/control | +++ |
| Therapeutic procedure | Bilateral treatment; sham control procedure | +++ |
| Number of patients | 315 also homogeneous emphysema | +++ |
| Primary endpoint | +++ | |
| Safety as a composite endpoint | ++ | |
| Results efficacy | Improvement at day one | + |
| No effect at 6 months | ||
| Table 6: Criteria for new therapeutic approaches: bronchoscopic lung volume reduction. | |
| No therapy available | no |
| Poor prognosis despite available therapy | yes |
| Superiority over available therapy | no |
| Not inferior but fewer side effects than available therapy | yes / no |
| Not inferior, similar side effects but much less costly han available therapy | ? |
| Table 7: Specific aims of COPD therapy: goals achieved by bronchoscopic lung volume reduction. | |
| Reduction of symptoms | maybe |
| Improvement in lung function | moderate |
| Improvement in exercise capacity | no |
| Improvement in general health | yes |
| Reduction of COPD exacerbations | no |
| Reduction of mortality | unlikely |
1 Lahzami S, Bridevaux PO, Soccal PM, Wellinger J, Robert JH, Ris HB, Aubert JD. Survival impact of lung transplantation for COPD. Eur Respir J. 2009:36(1);74-80.
2 Cooper JD, Trulock EP, Triantafillou AN, Patterson GA, Pohl MS, Deloney PA, Sundaresan RS, Roper CL. Bilateral pneumectomy (volume reduction) for chronic obstructive pulmonary disease. J Thorac Cardiovasc Surg. 1995;109(1):106-16; discussion 116-9.
3 Weder W, Schmid RA, Russi EW. Thoracoscopic lung volume reduction surgery for emphysema. Int Surg. 1996:81(3);229-34.
4 Hamacher J, Bloch KE, Stammberger U, Schmid RA, Laube I, Russi EW, Weder W. Two years’ outcome of lung volume reduction surgery in different morphologic emphysema types. Ann Thorac Surg. 1999:68(5);1792-98.
5 National, Emphysema, Treatment, Trial, Research, Group. Patients at high risk of death after lung-volume-reduction surgery. N Engl J Med. 2001:345(15);1075-83.
6 Berger RL, Decamp MM, Criner GJ, Celli BR. Lung volume reduction therapies for advanced emphysema: an update. Chest Epub. 2010:138(2);407-17.
7 Sciurba FC, Ernst A, Herth FJ, Strange C, Criner GJ, Marquette CH, Kovitz KL, Chiacchierini RP, Goldin J, McLennan G. A randomized study of endobronchial valves for advanced emphysema. N Engl J Med Epub. 2010:363(13);1233-44.
8 Herth FJP, Marquette C, Ernst A, Gasparini S, Valipour A, Criner G, Eberhardt R. Endobronchial valves for emphysema palliation trial – The EURO VENT trial. Eur Respiratory J. 2010:36(54).
9 Sybrecht GW, Shah P, Slebos D, Cardoso PFG, Levine B, Voelker K, Russell M, Abtin F, Goldin J, Cooper JD. First release of EASE (Exhale Airway Stents for Emphysema) randomized trial of airway bypass in homogeneous emphysema. Eur Respiratory J. 2010:36(54).
10 Tamm M, Ortmann M, Ferke B, Forrer F, Stolz D. Bronchial valve implantation by flexible bronchoscopy in high risk patients with advanced COPD. Eur Respiratory J. 2010:36(54).
11 Sterman DH, Mehta AC, Wood DE, Mathur PN, McKenna RJ, Jr., Ost DE, Truwit JD, Diaz P, Wahidi MM, Cerfolio R, Maxfield R, Musani AI, Gildea T, Sheski F, Machuzak M, Haas AR, Gonzalez HX, Springmeyer SC. A multicenter pilot study of a bronchial valve for the treatment of severe emphysema. Respiration Epub. 2010:79(3);222-33.
12 Eberhardt R, Gompelmann D, Schuhmann M, Heussel CP, Herth FJF. Unilateral vs. bilateral endoscopic lung volume reduction in patients with severe heterogeneous emphysema: A comparative randomised case study. Eur Respiratory J. 2010:36(54).
13 Judge E, Tuohy M, Cornell T, Lawrie I, Egan J. Direct assessment of collateral ventilation to optimize patient selection for endobronchial valve treatment. Eur Respiratory J. 2010:36(54).
14 Eberhardt R, Herth F, Stanzel F, Bonett R, Behr J, Marquette C, Valipour A, Kramer M. Polymeric lung volume reduction (PLVR) therapy for advanced homogeneous emphysema. Eur Respiratory J. 2010:36(54).
15 Herth F, Eberhardt R, Stanzel F, Bonett R, Behr J, Schmidt B, Magnussen H. Responses to polymeric lung volume reduction (PLVR) therapy at 6 months in patients with GOLD stage III emphysema. Eur Respiratory J. 2010:36(54).
16 Herth F. Glueing and steaming. Treatment beyond inhalers: endoscopic lung volume reduction European Respiratory Society Conference – Personal communication 2010.
No funding. No conflict of interest.