Three decades of endothelium research
DOI: https://doi.org/10.4414/smw.2010.13122
Andreas J.
Flammer c, Thomas F.
Lüscher ab
aCardiovascular Centre, Cardiology University Hospital Zurich, Switzerland
bInstitute of Physiology, Cardiovascular Research, University Zürich-Irchel, Zurich, Switzerland
cDivision of Cardiovascular Diseases, Department of Internal Medicine, Mayo Clinic and College of Medicine, Rochester, USA
Summary
The endothelium is more than just a passive interface between the blood and the vessel wall. Since the pioneering discovery of nitric oxide as an important endothelium-derived vasorelaxing molecule three decades ago, vascular research has developed exponentially and remains fascinating for the entire research community. Endothelial dysfunction is a pathological condition characterized by an imbalance between vasodilatating and vasoconstricting substances. Most, if not all, cardiovascular risk factors have been attributed with endothelial dysfunction and their therapeutic modification with an improvement in vascular function. This overview aims to provide a glimpse into this fascinating research field with the emphasis on vasoactive substances and the assessment of endothelial function.
Introduction
In the past the endothelium was believed to represent a simple semipermeable membrane covering the endoluminal part of all blood vessels. However, in recent years, abundant research on the endothelium and its function has brought to light its impressive and indeed indispensable physiological functions, especially in maintaining the homeostasis of vascular tone and structure. Loss of function of the endothelium not only makes the vessel prone to vasoconstriction, but also leads to atherothrombotic changes such as proliferation of vascular smooth muscle, expression of proinflammatory molecules and thrombosis.
Moreover, in humans endothelial dysfunction is one of the first detectable vascular alteration in the evolution of atherosclerosis, and its presence also correlates well with future cardiovascular events.
It is the aim of the present article to provide an overview of the physiological and pathophysiological function of the endothelium, its main vasoactive substances, and the possibilities of measuring and therapeutically influencing vascular function. The article is based upon the recently published overview article in a special issue on endothelium-dependent vasodilatation in honour of Robert Furchgott [1].
The vascular endothelium and its vasoactive substances
The endothelium represents the inner layer of the vessel wall. It is a continuous and smooth monolayer of cells providing a nonthrombogenic surface with highly selective permeability properties. In
total, it represents a surface area of about 4000 to 7000 m2. The endothelium controls vascular permeability and actively regulates the exchange of molecules in response to environmental and molecular signals (fig. 1) [2]. Moreover, healthy endothelial cells are crucial in the prevention of thrombotic events. A feature of note is that endothelial cells express antiplatelet and anticoagulant molecules [3], whereas dysfunctional cells make the vessel prone to thrombotic events with tissue factor playing an important role [4].
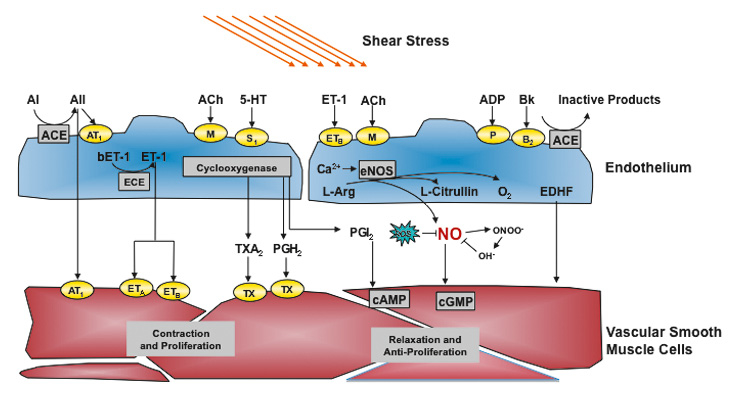
Figure 1
Endothelium-derived vasoactive substances.
Endothelial nitric oxide synthase is induced by shear stress and a variety of receptors and leads to a release of nitric oxide (NO), which exerts relaxation of vascular smooth muscle cells and other important effects such as antiproliferation and inhibition of thrombocyte aggregation and leukocyte adhesion. Other endothelium-derived relaxing factors including endothelium-derived hyperpolarisating factor (EDHF) and prostacyclin (PGI2) are also shown. ACE denotes angiotensin-converting enzyme, Ach, acetylcholine; AI, angiotensin I, AII, angiotensin II, AT1, angiotensin 1 receptor; Bk, bradykinin; COX, cyclooxygenase; ECE, ET-converting enzyme; EDHF, endothelium-derived hyperpolarizing factor; ETA and ETB, endothelin A and B receptors; ET-1, endothelin-1, L-Arg, L-arginine; PGH2, prostaglandin H2; ROS, reactive oxygen species; S1, serotoninergic receptor; TH, thromboxane receptor; Thr, Thrombin; TXA2, thromboxane; 5-HT, serotonin.
However, the endothelium is able to do much more; indeed, it is known to be a highly complex organ able to respond to a broad variety of endogenous and exogenous stimuli which also synthesizes and releases a vast amount of vasoactive substances.
Many endothelium-derived relaxing factors (EDRF) have been characterised chemically in recent years; most of them are released in response to an increase in intracellular calcium. The most studied EDRF molecules are nitric oxide (NO), prostacyclin (PGI2) and endothelial-derived hyperpolarisation factors (EDHF).
Furthermore, there are also important endothelial-derived constricting factors (EDCF), endothelin-1 (ET-1) representing the most potent molecule.
Given these important physiological (inter)actions of endothelial mediators, prompt repair of damaged or apoptotic cells by endothelial progenitor cells is essential. Thus, these cells are not only important for angiogenesis, but also prompt repair of defects in the endothelial lining of the vessel wall (for review see [5]).
Nitric oxide (NO)
Thirty years ago, Furchgott and Zawadzki demonstrated that endothelial cells produce a then unknown signaling molecule, which was at the time named endothelium-derived relaxing factor (EDRF) [6]. This molecule was shown to be able to relax vascular smooth muscles. Later on, Ignarro and Furchgott demonstrated that EDRF was indeed nitric oxide [7]. Since these discoveries a wealth of basic and clinical research has been triggered. For this seminal discovery Robert Furchgott and Louis Ignarro, together with Ferid Murad, were co-awarded the Nobel Prize in 1998.
In the course of further research, NO has not only been shown to have vasodilatory properties. Indeed, it also prevents platelet adhesion and aggregation, as well as leukocyte adhesion and migration into the arterial wall and inhibits smooth muscle cell proliferation, all key events in the development of atherosclerosis [8–13]. NO is a highly diffusible small molecule and is synthesised by NO synthase (NOS) from L-arginine. It is released by endothelial cells mainly in response to shear stress, but also by many other molecules such as acetylcholine, bradykinin, thrombin, and ADP among others, leading to a relaxation of vascular smooth muscle cells [6, 14–21].
Prostacyclin
Prostacyclin (PGI2) is another endothelium-derived relaxing factor which is released partly in response to shear stress [14, 22–24]. PGI2 is synthesised by cyclooxygenase-1 (COX-1) from arachidonic acid [25] and increases cAMP in smooth muscle cells as well as in platelets. In contrast to NO, PGI2 does not contribute to the maintenance of large conduit arteries’ basal vascular tone [20]. Importantly, however, it has potent platelet inhibitory effects. In contrast to NO, which is released continuously by agonists [26], PGI2 is released only in a transient manner [27]. PGI2 facilitates the release of NO by endothelial cells [28] and vice versa, the action of PGI2 in the vascular smooth muscle is also potentiated by NO and the half-life of the second messenger of prostacyclin is prolonged [29].
Endothelium-derived hyperpolarising factor(s)
Endothelium-derived hyperpolarising factors (EDHF) are molecules causing hyperpolarisation of smooth muscle cells. Their involvement in regulating vascular reactivity is defined as the endothelium-dependent response that persists in the presence of combined inhibition of NO and PGI2. They might represent a compensatory mechanism for endothelium-dependent vasodilatation in the presence of reduced NO availability [30].
Studies have identified several molecules or mediators that might act as EDHF in different tissues and species [31]: among them K+ [32], cytochrome P450 metabolites [33–35], lipoxygenase products [36], NO itself [37], reactive oxygen species (H2O2) [38], cyclic adenosine monophosphate [39], C-type natriuretic peptide [40], and electrical coupling through myoendothelial gap junctions [41, 42].
Endothelin
Some years after the detection of NO, the vasoconstrictor peptide endothelin (ET), which is also synthesised by vascular endothelial cells, was discovered [43, 44]. ET acts as a natural counterpart of NO [45]. Three isoformes of the peptide (ET-1, ET-2 and ET-3) exist, which are converted by the endothelin converting enzyme (ECE) from their precursors big endothelin originating from pre-proendothelin peptides cleaved by endopeptidases [46–50]. Similar to the expression of NO, there are also several factors modulating ET-1 production and release, among them shear stress, angiotensin II, thrombin, adrenaline, oxidised low-density lipoproteins and inflammatory cytokines [8, 51–62].
In humans, ET raises blood pressure [63, 64] and induces vascular and myocardial hypertrophy [65–67], both risk factors for cardiovascular morbidity and mortality [68–70].
Endothelial dysfunction
The term endothelial dysfunction is widely used to describe any form of abnormal activity of the endothelium. An imbalance of the above-mentioned vasoactive substances due to endothelium dysfunction affects vascular function negatively. Most commonly, endothelium dysfunction is characterised by an impaired NO bioavailability due to reduced production of NO by NOS or increased breakdown by reactive oxygen species [71]. In the early stages, endothelial function may be partly maintained by compensatory upregulation of prostacyclin and/or EDHF.
Endothelial dysfunction has been documented in almost every condition associated with atherosclerosis and cardiovascular disease. In humans, endothelial dysfunction has been observed in patients with hypertension [72, 73], in normotensive subjects with a family history of hypertension [74], in smokers [75, 76] and passive smokers [77], in dyslipidaemia [78, 79], in aging [73], diabetes mellitus [80–84], in obesity [84] in hyperhomocysteinemia [85, 86] and in patients with inflammatory or infectious diseases [87–89]. Many of these conditions also are characterised by overproduction of reactive oxygen species (ROS) and in turn increased oxidative stress [90]. ROS might interact with NO and reduce its bioavailability, and might directly damage cellular structures via the production of peroxynitrate. Hence oxidative stress is probably one of the major mechanisms in the development of endothelial dysfunction, if not its major contributor.
However, other factors also contribute to endothelial dysfunction, e.g. local factors such as chronic increases in shear stress, pressure and pulsatility as well as genetic predispositions and other so far unknown factors. Endothelial function therefore represents an integrated index of both the overall cardiovascular risk factor burden and the sum of all vasculoprotective factors in a given individual [91].
Given its role in the atherosclerotic process, it is not surprising that many studies demonstrate a prognostic role for endothelial function measurements in the coronary as well as in the peripheral and central circulation. First evidence came from patients with non-obstructive coronary artery disease, where two independent groups demonstrated a higher incidence of cardiovascular [92, 93] and cerebrovascular events in those with impaired coronary vascular function [94]. Coronary endothelial dysfunction predicts the incidence of further cardiovascular events even in patients without coronary artery disease [95, 96] and in heart transplant recipients [97]. Later on several other studies demonstrated incremental prognostic impact of peripheral endothelial dysfunction in patients with risk factors for coronary artery disease. Flow-mediated vasodilation was predictive for cardiovascular events beyond traditional risk factors in a large cohort of elderly patients [98], in patients with peripheral vascular disease [99], after elective vascular surgery [100], in postmenopausal women [101], in patients with chest pain [102], or in patients with coronary artery disease [103]. Similarly, venous occlusion plethysmography predicted CV events in patients with coronary artery disease [104] and in patients after acute coronary syndromes [105]. A recent study in patients with risk factors demonstrated that non-invasive peripheral arterial tonometry is able to predict late cardiovascular events [106]. However, whether peripheral endothelial dysfunction adds incremental information beyond classical risk factors in healthy humans is still debated. In a recent study in 3500 healthy subjects FMD was unable to predict the incidence of hypertension [107], whereas another study demonstrated the predictive value for incident CVD with FMD in 3026 healthy people [108].
Importantly, however, therapeutic interventions, which positively influence the cardiovascular risk profile of individuals, typically impact beneficially on endothelial function. In hypertension, for example, most classes of antihypertensive drugs improve endothelium-dependent vasodilatation in animals [109–116]. Depending on the antihypertensive drug and its pharmacological profile, improvements in endothelium-dependent vasodilatation can also be achieved in humans [117–136]. Indeed, calcium antagonists, ACE inhibitors and angiotensin-receptor antagonists, but not beta-blockers (with the exception of the NO-containing molecule nebivolol) improve endothelial function in hypertensives. Similar interventions with drugs or lifestyle changes have been studied with other risk factors and have shown similar results. The perhaps nowadays most important drugs in the prevention and treatment of atherosclerosis, the statins, have consistently been shown to improve endothelial dysfunction not only due to their lipid lowering properties but also due to their pleiotropic effects [137, 138]. Furthermore, direct infusion of reconstituted HDL is able to improve endothelial function significantly in hyperlipidemics [79].
Interestingly, not only drugs improve endothelial function in patients with cardiovascular risk factors, but also lifestyle modifications such as regular exercise [139, 140] or dietary interventions with foods rich in polyphenols, especially fruit, tea and cocoa [141–143].
Methods for assessing human endothelial dysfunction in vivo
Different techniques for measurement of endothelial dysfunction have been developed recently. The first demonstration of endothelial dysfunction in atherosclerotic coronary arteries using intracoronary infusion of acetylcholine was published in 1986 [144]. However, soon afterwards other less- and also non-invasive techniques have been developed to assess endothelial dysfunction mainly in the forearm circulation [145]. All the different techniques have their advantages and disadvantages and, importantly, different vascular beds are examined. The principle, however, is simple: large conduit arteries such as the brachial or radial artery dilate
in response to reactive hyperaemia (flow-mediated
vasodilatation) or upon intraarterial infusion of substances such as acetylcholine (Ach), bradykinin or serotonin in the presence of a functionally intact endothelium, capable of releasing NO or other vasodilator substances (see above).
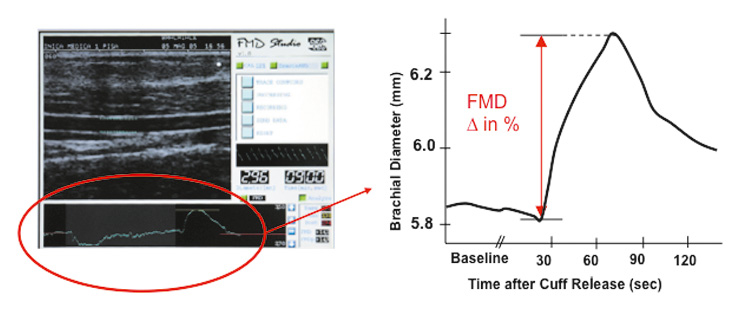
Figure 2
Flow-mediated vasodilatation.
Example of a typical ultrasound image of the brachial artery is demonstrated. Arterial diameter is shown schematically at baseline and after reactive hyperaemia induced flow-mediated vasodilatation. Blood pressure cuffs can be placed on the upper or the lower side of the transducer in the antecubital fossa, although the latter is the preferred method. On the right hand side, the time course of an FMD measurement is shown. See text for further explanation.
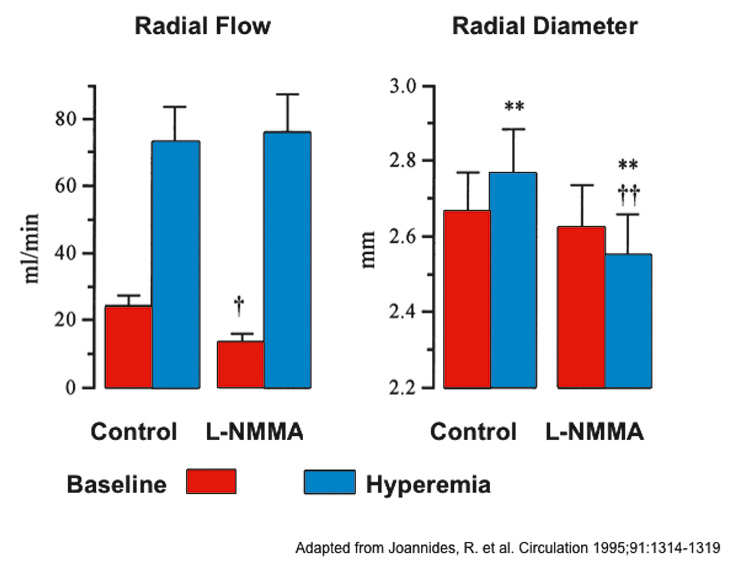
Figure 3
NO in flow-mediated vasodilatation.
Radial artery flow (mL/min) and radial artery diameter (mm) measured at baseline and during reactive hyperaemia before and after infusion of NG-monomethyl-L-arginine (L-NMMA). **P <.01 vs Base; P <.05 and P <.01 vs corresponding control value. Modified from Johannides et al. [20].
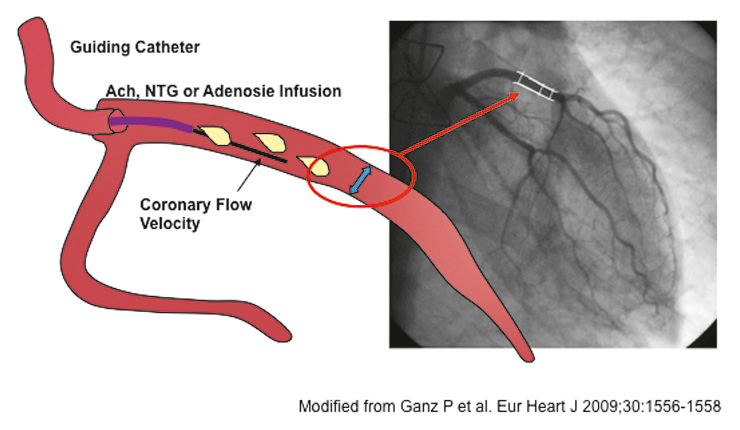
Figure 4
Measurement of coronary vascular reactivity.
Small catheters are positioned in proximal coronary arteries. Acetylcholine or nitroglycerin are infused to test conduit vessel endothelium-dependent and
-independent vasodilatation respectively. Changes in vascular diameter are measured by quantitative coronary angiography. Doppler flow-velocity measurements are used to assess small vessel vasoreactivity to acetylcholine and adenosine respectively. Modified from Ganz [161].
Flow-mediated vasodilatation as measured by ultrasound of the brachial artery
Due to its non-invasive properties flow-mediated vasodilatation of the forearm arteries has become the most important mode of measuring endothelial dysfunction. Many groups take advantage of this technique, which relies on the fact that endothelial cells release NO and other endothelium-derived relaxing factors in response to reactive hyperaemia (after a short occlusion of the brachial artery with a blood pressure cuff). Celermajer and his colleagues were the first to measure this response in vivo, and developed an elegant non-invasive technique to measure flow-mediated vasodilatation (FMD) of the conduit brachial or radial artery (fig. 2) [146]. We then demonstrated that indeed this response was nitric oxide dependent [20, 21] (fig. 3). The change in brachial or radial artery diameter in response to the increased shear stress induced by reactive hyperemia is measured by ultrasound technique. A feature of note is that peripheral endothelial function as assessed by FMD correlates with coronary artery endothelial function [147]. However, although the principle of this technique is simple, it is technically very challenging and therefore requires extensive training and standardization. Several attempts have been made to standardize the different protocols [148, 149].
Venous occlusion plethysmography
Although this technique is limited by its semi-invasive nature, requiring cannulation of the brachial artery, forearm venous plethysmography has the advantage that molecules, hormones or drugs can be infused intra-arterially, for instance acetylcholine (Ach) or nitroglycerine, to quantify endothelial-dependent and endothelial-independent vasodilatation respectively [72, 150]. It is of course also possible to administer other agonist and antagonists and even novel substances in a very low, systemically not effective dose into the brachial artery, with the contralateral limb serving as an internal control. Changes in forearm blood flow are measured by plethysmography in both forearms and results are expressed as the ratio of the changes in flow measured in both arms. Although the microcirculation in the forearm (which is assessed essentially by this technique) is not a target organ for atherosclerosis, it seems that the response to Ach nevertheless has an independent predictive value for future cardiovascular events [149].
Coronary endothelial function measurements
Coronary endothelial function can be measured in the catheterisation laboratory, but it is always limited by its invasive nature. Nevertheless, if applied appropriately, it provides very valuable information on the coronary vascular bed itself. Most protocols work with an intracoronary infusion of Ach to measure endothelial-dependent and nitroglycerine to measure endothelial-independent function respectively (fig. 4). As expected, coronary arteries with an intact endothelium will respond to intra-coronary Ach infusion with epicardial and microvascular dilatation, resulting in an increase in coronary blood flow. However, if the endothelial layer is dysfunctional or even interrupted, Ach produces a paradoxical vasoconstriction and a decrease in
144]. Similar to the other techniques, the response to intracoronary Ach gives important prognostic information [92].
Finger plethysmography
Because all present accepted methods of measuring endothelial function were either invasive or suffered from high inter- and intraobserver variability, other techniques in assessing vascular reactivity have been under investigation. Recently, a finger plethysmographic device which detects pulsatile arterial volume changes has been introduced [151, 152]. A decrease in arterial blood volume in the fingertip causes a decrease in pulsatile arterial column changes, thus decreasing the measured signal and vice versa. Similarly to the assessment of endothelial function via the FMD technique by ultrasound of the brachial artery, a pressure cuff is placed on one upper arm while the other arm serves as a control. After measuring baseline blood volume changes, the blood pressure cuff is inflated above systolic pressure and is deflated after 5 minutes to induce reactive hyperaemia on one arm. The calculated index between the arm with reactive hyperaemia and the control represents a measure for endothelial function. Similar volume changes after nitroglycerin can be measured. However, augmentation of the pulse amplitude after reactive hyperaemia is a complex response to ischaemia. It may reflect changes in flow, as well as in digital microvessel dilatation, and is only partly dependent on nitric oxide [153]. Studies demonstrated that impairment of peripheral finger endothelial function is correlated with coronary microvascular function in patients with early atherosclerosis [154]. In a cross-sectional study in 1957 patients in the Framingham cohort, digital vascular dysfunction was associated with traditional and metabolic cardiovascular risk factors [155].
Pulse wave analysis
The principle of this non-invasive technique is measurement of the pulse wave and velocity profile of the propagation of the arterial wave form and its reflected wave. The central aortic wave form can be calculated as the augmentation index [156]. Although not the only contributor, endothelial function plays an important role in arterial stiffness and thus affects the results of this methodology as well. It therefore has been used to determine effects of endothelial mediators on arterial stiffness
(for review see [157]). Importantly, aortic stiffness
expressed as aortic pulse wave velocity is a strong predictor for future cardiovascular events and mortality, especially in those patients with a higher baseline risk, as demonstrated recently in a meta-analysis in over 15 000 subjects [158].
Other methods of assessing endothelial function
There are other imaging tools capable of assessing vascular function, including magnetic resonance technique, but these will not be described in greater detail as their importance has still to be determined.
Other possible ways of evaluating endothelial function include direct measurement of biochemical and circulating endothelium markers. One possibility is to measure the plasma levels of endothelium-derived substances directly involved in vasoconstriction and vasorelaxation (e.g. endothelin, endothelial-derived NO compounds or prostacyclin metabolites). Because atherosclerosis is believed to be, at least in part, a chronic inflammatory disease and the expression of pro-inflammatory
cytokines and adhesion molecules may play an important role in the development of endothelial dysfunction, serum levels of (pro-)inflammatory markers such as C-reactive protein, interleukins and other cytokines, phospholipases and others have been measured. Due to their pro-atherogenic properties and their involvement in endothelial dysfunction, markers of oxidative stress (e.g. isoprostanes, oxLDL) or pro-angiogenic factors (e.g. VEGF) may provide further insights into early vascular changes. These markers certainly hold the
potential to provide mechanistic insights, though significant confounding with other diseases and conditions has to be taken into account.
Recently circulating endothelial progenitor cells (EPC) have emerged as a powerful marker of endothelial dysfunction. Indeed, endothelial dysfunction may reflect an imbalance between vascular injury and EPC-based repair [5, 159] and might indicate pre-clinical endothelial damage and a target for vascular protection [149]. Furthermore, genetics may provide more insights into the molecular mechanisms of endothelial function. In particular, single nucleotide polymorphisms of endothelial genes such as endothelin-1, eNOS among others, have been studied [149]. In the future, gene polymorphisms might help to further refine individual risk assessment for endothelial dysfunction and future events. For instance, coronary endothelial dysfunction in patients with coronary artery disease correlates with cytochrome P450 polymorphisms [160].
The future of endothelial dysfunction
In the last 30 years, an enormous number of studies have been published on endothelium function in experimental animals and humans. Currently, a search in PubMed results in 175 454 displayed studies, more than 10 000 in the year 2009 alone. This underscores the persistent interest and importance of endothelial research. Future areas of interest are the relation of endothelial dysfunction to endothelial progenitor cell number and function and their therapeutic modulation, as well as genetic factors influencing endothelial function. Finally, the assessment of endothelial function remains an important tool for assessment of the vascular effects of novel therapeutic agents in their clinical development.
Correspondence to:
Thomas F. Lüscher MD FRCP
Professor and Head of Cardiology and Cardiovascular Physiology
University Hospital and University-Irchel Zurich
Rämistrasse 100
CH-8091 Zurich
Switzerland
E-Mail: cardiotfl@gmx.ch
References
1 Flammer AJ, Luscher TF. Human endothelial dysfunction: EDRFs. Pflugers Arch. 2010;459:1005–13.
2 van Hinsbergh WM. Endothelial permeability for macromolecules. Mechanistic aspects of pathophysiological modulation. Arterioscler Thromb Vascular Biol. 1997;17:1018–23.
3 de Agostini AI, Watkins SC, Slayter HS, Youssoufian H, Rosenberg RD. Localization of anticoagulantly active heparan sulfate proteoglycans in vascular endothelium: antithrombin binding on cultured endothelial cells and perfused rat aorta. J Cell Biol. 1990;111:1293–304.
4 Steffel J, Luscher TF, Tanner FC. Tissue factor in cardiovascular diseases: molecular mechanisms and clinical implications. Circulation. 2006;113:722–31.
5 Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95:343–53.
6 Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–6.
7 Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci USA. 1987;84:9265–9.
8 Boulanger C, Lüscher TF. Release of endothelin from the porcine aorta. Inhibition of endothelium-derived nitric oxide. J Clin Invest. 1990;85:587–90.
9 Ross R. Atherosclerosis – an inflammatory disease. N Engl J Med. 1999;340:115–26.
10 Bhagat K, Moss R, Collier J, Vallance P. Endothelial “stunning” following a brief exposure to endotoxin: a mechanism to link infection and infarction? Cardiovasc Res. 1996;32:822–9.
11 Bhagat K, Vallance P. Inflammatory cytokines impair endothelium-dependent dilatation in human veins in vivo. Circulation. 1997;96:3042–7.
12 Hingorani AD, Cross J, Kharbanda RK, Mullen MJ, Bhagat K, Taylor M, et al. Acute systemic inflammation impairs endothelium-dependent dilatation in humans. Circulation. 2000;102:994–9.
13 Fichtlscherer S, Rosenberger G, Walter G, Breuer S, Dimmeler S, Zeiher AM. Elevated C-reactive protein levels and impaired endothelial vasoreactivity in patients with coronary artery disease. Circulation. 2000;102:1000–6.
14 Rubanyi GM, Romero JC, Vanhoutte PM. Flow-induced release of endothelium-derived relaxing factor. Am J Physiol. 1986;250:H1145–9.
15 Anderson EA, Mark AL. Flow-mediated and reflex changes in large peripheral artery tone in humans. Circulation. 1989;79:93–100.
16 Palmer RM, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;333:664–6.
17 Palmer RMJ, Rees DD, Ashton DS, Moncada S. L-arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. Biochem Biophys Res Commun. 1988;153:1251–6.
18 Vallance P, Collier J, Moncada S. Effects of endothelium-derived nitric oxide on peripheral arteriolar tone in man. Lancet. 1989;2:997–1000.
19 Stamler JS, Loh E, Roddy MA, Currie KE, Creager MA. Nitric oxide regulates basal systemic and pulmonary vascular resistance in healthy humans. Circulation. 1994;89:2035–40.
20 Joannides R, Haefeli WE, Linder L, Richard V, Bakkali EH, Thuillez C, et al. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circulation. 1995;91:1314–9.
21 Joannides R, Richard V, Haefeli WE, Linder L, Lüscher TF, Thuillez C. Role of basal and stimulated release of nitric oxide in the regulation of radial artery caliber in humans. Hypertension. 1995;26:327–31.
22 Pohl U, Holtz J, Busse R, Bassenge E. Crucial role of endothelium in the vasodilator response to increased flow in vivo. Hypertension. 1986;8:37–44.
23 Koller A, Kaley G. Prostaglandins mediate arteriolar dilation to increased blood flow velocity in skeletal muscle microcirculation. Circ Res. 1990;67:529–34.
24 Okahara K, Sun B, Kambayashi J. Upregulation of prostacyclin synthesis-related gene expression by shear stress in vascular endothelial cells. Arterioscler Thromb Vasc Biol. 1998;18:1922–6.
25 Moncada S, Gryglewski R, Bunting S, Vane JR. An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation. Nature. 1976;263:663–5.
26 Harrington LS, Carrier MJ, Gallagher N, Gilroy D, Garland CJ, Mitchell JA. Elucidation of the temporal relationship between endothelial-derived NO and EDHF in mesenteric vessels. Am J Physiol. 2007;293:H1682–8.
27 Mitchell JA, de Nucci G, Warner TD, Vane JR. Different patterns of release of endothelium-derived relaxing factor and prostacyclin. Br J Pharmacol. 1992;105:485–9.
28 Shimokawa H, Flavahan NA, Lorenz RR, Vanhoutte PM. Prostacyclin releases endothelium-derived relaxing factor and potentiates its action in coronary arteries of the pig. Br J Pharmacol. 1988;95:1197–203.
29 Delpy E, Coste H, Gouville AC. Effects of cyclic GMP elevation on isoprenaline-induced increase in cyclic AMP and relaxation in rat aortic smooth muscle: role of phosphodiesterase 3. Br J Pharmacol. 1996;119:471–8.
30 Taddei S, Ghiadoni L, Virdis A, Buralli S, Salvetti A. Vasodilation to bradykinin is mediated by an ouabain-sensitive pathway as a compensatory mechanism for impaired nitric oxide availability in essential hypertensive patients. Circulation. 1999;100:1400–5.
31 Duvall WL. Endothelial dysfunction and antioxidants. Mt Sinai J Med. 2005;72:71–80.
32 Edwards G, Dora KA, Gardener MJ, Garland CJ, Weston AH. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature. 1998;396:269–72.
33 Campbell WB, Falck JR. Arachidonic acid metabolites as endothelium-derived hyperpolarizing factors. Hypertension. 2007;49:590–6.
34 Larsen BT, Gutterman DD, Sato A, Toyama K, Campbell WB, Zeldin DC, et al. Hydrogen peroxide inhibits cytochrome p450 epoxygenases: interaction between two endothelium-derived hyperpolarizing factors. Circ Res. 2008;102:59–67.
35 Taddei S, Versari D, Cipriano A, Ghiadoni L, Galetta F, Franzoni F, et al. Identification of a cytochrome P450 2C9-derived endothelium-derived hyperpolarizing factor in essential hypertensive patients. J Am Coll Cardiol. 2006;48:508–15.
36 Faraci FM, Sobey CG, Chrissobolis S, Lund DD, Heistad DD, Weintraub NL. Arachidonate dilates basilar artery by lipoxygenase-dependent mechanism and activation of K(+) channels. Am J Physiol Regul Integr Comp Physiol. 2001;281:R246–53.
37 Bolotina VM, Najibi S, Palacino JJ, Pagano PJ, Cohen RA. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature. 1994;368:850–3.
38 Ellis A, Triggle CR. Endothelium-derived reactive oxygen species: their relationship to endothelium-dependent hyperpolarization and vascular tone. Can J Physiol Pharmacol. 2003;81:1013–28.
39 Popp R, Brandes RP, Ott G, Busse R, Fleming I. Dynamic modulation of interendothelial gap junctional communication by 11,12-epoxyeicosatrienoic acid. Circ Res. 2002;90:800–6.
40 Wei CM, Hu S, Miller VM, Burnett JC, Jr. Vascular actions of C-type natriuretic peptide in isolated porcine coronary arteries and coronary vascular smooth muscle cells. Biochem Biophys Res Commun. 1994;205:765–71.
41 Griffith TM. Endothelium-dependent smooth muscle hyperpolarization: do gap junctions provide a unifying hypothesis? Br J Pharmacol. 2004;141:881–903.
42 Griffith TM, Chaytor AT, Edwards DH. The obligatory link: role of gap junctional communication in endothelium-dependent smooth muscle hyperpolarization. Pharmacol Res. 2004;49:551–64.
43 Hickey KA, Rubanyi G, Paul RJ, Highsmith RF. Characterization of a coronary vasoconstrictor produced by cultured endothelial cells. Am J Physiol. 1985;248:C550–6.
44 Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–5.
45 Lüscher TF, Yang Z, Tschudi M, Von SL, Stulz P, Boulanger C, et al. Interaction between endothelin-1 and endothelium-derived relaxing factor in human arteries and veins. Circ Res. 1990;66:1088–94.
46 Ikegawa R, Matsumura Y, Tsukahara Y, Takaoka M, Morimoto S. Phosphoramidon, a metalloproteinase inhibitor, suppresses the secretion of endothelin-1 from cultured endothelial cells by inhibiting a big endothelin-1 converting enzyme. Biochem Biophys Res Commun. 1990;171:669–75.
47 Takahashi M, Matsushita Y, Iijima Y, Tanzawa K. Purification and characterization of endothelin-converting enzyme from rat lung. J Biol Chem. 1993;268:21394–8.
48 Ohnaka K, Takayanagi R, Nishikawa M, Haji M, Nawata H. Purification and characterization of a phosphoramidon-sensitive endothelin-converting enzyme in porcine aortic endothelium. J Biol Chem. 1993;268:26759–66.
49 Shimada K, Takahashi M, Tanzawa K. Cloning and functional expression of endothelin-converting enzyme from rat endothelial cells. J Biol Chem. 1994;269:18275–8.
50 Rossi GP, Albertin G, Franchin E, Sacchetto A, Cesari M, Palu G, et al. Expression of the endothelin-converting enzyme gene in human tissues. Biochem Biophys Res Commun. 1995;211:249–53.
51 Boulanger CM, Tanner FC, Bea ML, Hahn AW, Werner A, Lüscher TF. Oxidized low density lipoproteins induce mRNA expression and release of endothelin from human and porcine endothelium. Circ Res. 1992;70:1191–7.
52 Yoshizumi M, Kurihara H, Sugiyama T, Takaku F, Yanagisawa M, Masaki T, et al. Hemodynamic shear stress stimulates endothelin production by cultured endothelial cells. Biochem Biophys Res Commun. 1989;161:859–64.
53 Kourembanas S, Marsden PA, McQuillan LP, Faller DV. Hypoxia induces endothelin gene expression and secretion in cultured human endothelium. J Clin Invest. 1991;88:1054–7.
54 Shirakami G, Nakao K, Saito Y, Magaribuchi T, Jougasaki M, Mukoyama M, et al. Acute pulmonary alveolar hypoxia increases lung and plasma endothelin-1 levels in conscious rats. Life Sci. 1991;48:969–76.
55 Hieda HS, Gomez-Sanchez CE. Hypoxia increases endothelin release in bovine endothelial cells in culture, but epinephrine, norepinephrine, serotonin, histamine and angiotensin II do not. Life Sci. 1990;47:247–51.
56 Kohno M, Murakawa K, Yokokawa K, Yasunari K, Horio T, Kurihara N, et al. Production of endothelin by cultured porcine endothelial cells: modulation by adrenaline. J Hypertens Suppl. 1989;7:S130–1.
57 Ohta K, Hirata Y, Imai T, Kanno K, Emori T, Shichiri M, et al. Cytokine-induced release of endothelin-1 from porcine renal epithelial cell line. Biochem Biophys Res Commun. 1990;169:578–84.
58 Kanse SM, Takahashi K, Lam HC, Rees A, Warren JB, Porta M, et al. Cytokine stimulated endothelin release from endothelial cells. Life Sci. 1991;48:1379–84.
59 Miyamori I, Takeda Y, Yoneda T, Iki K, Takeda R. Interleukin-2 enhances the release of endothelin-1 from the rat mesenteric artery. Life Sci. 1991;49:1295–300.
60 Woods M, Bishop-Bailey D, Pepper JR, Evans TW, Mitchell JA, Warner TD. Cytokine and lipopolysaccharide stimulation of endothelin-1 release from human internal mammary artery and saphenous vein smooth-muscle cells. J Cardiovasc Pharmacol. 1998;31(Suppl 1):S348–50.
61 Dohi Y, Hahn AW, Boulanger CM, Bühler FR, Lüscher TF. Endothelin stimulated by angiotensin II augments contractility of spontaneously hypertensive rat resistance arteries. Hypertension. 1992;19:131–7.
62 Barton M, Shaw S, d’Uscio LV, Moreau P, Lüscher TF. Angiotensin II increases vascular and renal endothelin-1 and functional endothelin converting enzyme activity in vivo: role of ETA receptors for endothelin regulation. Biochem Biophys Res Commun. 1997;238:861–5.
63 Vierhapper H, Wagner O, Nowotny P, Waldhausl W. Effect of endothelin-1 in man. Circulation. 1990;81:1415–8.
64 Kiely DG, Cargill RI, Struthers AD, Lipworth BJ. Cardiopulmonary effects of endothelin-1 in man. Cardiovasc Res. 1997;33:378–86.
65 Ito H, Hirata Y, Hiroe M, Tsujino M, Adachi S, Takamoto T, et al. ET-1 induces hypertrophy with enhanced expression of muscle specific genes in cultured neonatal rat cardiomyocytes. Circ Res. 1991;69:209–15.
66 Barton M, d’Uscio LV, Shaw S, Meyer P, Moreau P, Luscher TF. ET(A) receptor blockade prevents increased tissue endothelin-1, vascular hypertrophy, and endothelial dysfunction in salt-sensitive hypertension. Hypertension. 1998;31:499–504.
67 Yang Z, Krasnici N, Lüscher TF. Endothelin-1 potentiates smooth muscle cell growth to PDGF: role of ETA and ETB receptor blockade. Circulation. 1999;100:5–8.
68 Kannel WB, Gordon T, Offutt D. Left ventricular hypertrophy by electrocardiogram. Prevalence, incidence, and mortality in the Framingham study. Ann Intern Med. 1969;71:89–105.
69 Bots ML, Hoes AW, Koudstaal PJ, Hofman A, Grobbee DE. Common carotid intima-media thickness and risk of stroke and myocardial infarction: the Rotterdam Study. Circulation. 1997;96:1432–7.
70 O’Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK, Jr. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med.1999;340:14–22.
71 Lüscher TF, Vanhoutte PM. (1990) The Endothelium: Modulator of Cardiovascular Function. CRC Press, Boca Raton.
72 Panza JA, Quyyumi AA, Brush JJ, Epstein SE. Abnormal endothelium-dependent vascular relaxation in patients with
essential hypertension. N Engl J Med. 1990;323:22–7.
73 Linder L, Kiowski W, Buhler FR, Luscher TF. Indirect evidence for release of endothelium-derived relaxing factor in human forearm circulation in vivo. Blunted response in essential hypertension. Circulation. 1990;81:1762–7.
74 Taddei S, Virdis A, Mattei P, Arzilli F, Salvetti A. Endothelium-dependent forearm vasodilation is reduced in normotensive subjects with familial history of hypertension. J Cardiovasc Pharmacol. 1992;20:S193–5.
75 Celermajer DS, Sorensen KE, Georgakopoulos D, Bull C, Thomas O, Robinson J, et al. Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults. Circulation. 1993;88:2149–55.
76 Zeiher AM, Schachinger V, Minners J. Long-term cigarette smoking impairs endothelium-dependent coronary arterial vasodilator function. Circulation. 1995;92:1094–100.
77 Celermajer DS, Adams MR, Clarkson P, Robinson J, McCredie R, Donald A, et al. Passive smoking and impaired endothelium-dependent arterial dilatation in healthy young adults. N Engl J Med. 1996;334:150–4.
78 Casino PR, Kilcoyne CM, Quyyumi AA, Hoeg JM, Panza JA. The role of nitric oxide in endothelium-dependent vasodilation of hypercholesterolemic patients. Circulation. 1993;88:2541–7.
79 Spieker LE, Sudano I, Hurlimann D, Lerch PG, Lang MG, Binggeli C, et al. High-density lipoprotein restores endothelial function in hypercholesterolemic men. Circulation. 2002;105:1399–402.
80 Makimattila S, Virkamaki A, Groop PH, Cockcroft J, Utriainen T, Fagerudd J, et al. Chronic hyperglycemia impairs endothelial function and insulin sensitivity via different mechanisms in insulin-dependent diabetes mellitus. Circulation. 1996;94:1276–82.
81 Calver A, Collier J, Vallance P. Inhibition and stimulation of nitric oxide synthesis in the human forearm arterial bed of patients with insulin-dependent diabetes. J Clin Invest. 1992;90:2548–54.
82 Cosentino F, Eto M, De Paolis P, van der Loo B, Bachschmid M, Ullrich V, et al. High glucose causes upregulation of cyclooxygenase-2 and alters prostanoid profile in human endothelial cells: role of protein kinase C and reactive oxygen species. Circulation. 2003;107:1017–23.
83 Smits P, Kapma JA, Jacobs MC, Lutterman J, Thien T. Endothelium-dependent vascular relaxation in patients with type I diabetes. Diabetes. 1993;42:148–53.
84 Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest. 1996;97:2601–10.
85 Virdis A, Ghiadoni L, Cardinal H, Favilla S, Duranti P, Birindelli R, et al. Mechanisms responsible for endothelial dysfunction induced by fasting hyperhomocystinemia in normotensive subjects and patients with essential hypertension. J Am Coll Cardiol. 2001;38:1106–15.
86 Tawakol A, Forgione MA, Stuehlinger M, Alpert NM, Cooke JP, Loscalzo J, et al. Homocysteine impairs coronary microvascular dilator function in humans. J Am Coll Cardiol. 2002;40:1051–8.
87 Hurlimann D, Forster A, Noll G, Enseleit F, Chenevard R, Distler O, et al. Anti-tumor necrosis factor-alpha treatment improves endothelial function in patients with rheumatoid arthritis. Circulation. 2002;106:2184–7.
88 Flammer AJ, Vo NT, Ledergerber B, Hermann F, Gamperli A, Huttner A, et al. Effect of atazanavir versus other protease inhibitor-containing antiretroviral therapy on endothelial function in HIV-infected persons: randomised controlled trial. Heart. 2009;95:385–90.
89 Flammer AJ, Sudano I, Hermann F, Gay S, Forster A, Neidhart M, et al. Angiotensin-converting enzyme inhibition improves vascular function in rheumatoid arthritis. Circulation. 2008;117:2262–9.
90 Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87:840–4.
91 Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23:168–75.
92 Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101:1899–906.
93 Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Jr., Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–54.
94 Targonski PV, Bonetti PO, Pumper GM, Higano ST, Holmes DR, Jr., Lerman A. Coronary endothelial dysfunction is associated with an increased risk of cerebrovascular events. Circulation. 2003;107:2805–9.
95 Halcox JP, Schenke WH, Zalos G, Mincemoyer R, Prasad A, Waclawiw MA, et al. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002;106:653–8.
96 Schindler TH, Hornig B, Buser PT, Olschewski M, Magosaki N, Pfisterer M, et al. Prognostic value of abnormal vasoreactivity of epicardial coronary arteries to sympathetic stimulation in patients with normal coronary angiograms. Arterioscler Thromb Vasc Biol. 2003;23:495–501.
97 Hollenberg SM, Klein LW, Parrillo JE, Scherer M, Burns D, Tamburro P, et al. Coronary endothelial dysfunction after heart transplantation predicts allograft vasculopathy and cardiac death. Circulation. 2001;104:3091–6.
98 Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation. 2007;115:2390–7.
99 Brevetti G, Silvestro A, Schiano V, Chiariello M. Endothelial dysfunction and cardiovascular risk prediction in peripheral arterial disease: additive value of flow-mediated dilation to ankle-brachial pressure index. Circulation. 2003;108:2093–8.
100 Gokce N, Keaney JF, Jr., Hunter LM, Watkins MT, Menzoian JO, Vita JA. Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelial function: a prospective study. Circulation. 2002;105:1567–72.
101 Rossi R, Chiurlia E, Nuzzo A, Cioni E, Origliani G, Modena MG. Flow-mediated vasodilation and the risk of developing hypertension in healthy postmenopausal women. J Am Coll Cardiol. 2004;44:1636–40.
102 Neunteufl T, Heher S, Katzenschlager R, Wolfl G, Kostner K, Maurer G, et al. Late prognostic value of flow-mediated dilation in the brachial artery of patients with chest pain. Am J Cardiol. 2000;86:207–10.
103 Chan SY, Mancini GB, Kuramoto L, Schulzer M, Frohlich J, Ignaszewski A. The prognostic importance of endothelial dysfunction and carotid atheroma burden in patients with coronary artery disease. J Am Coll Cardiol. 2003;42:1037–43.
104 Heitzer T, Schlinzig T, Krohn K, Meinertz T, Munzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104:2673–8.
105 Fichtlscherer S, Breuer S, Zeiher AM. Prognostic value of systemic endothelial dysfunction in patients with acute coronary syndromes: further evidence for the existence of the “vulnerable” patient. Circulation. 2004;110:1926–32.
106 Rubinshtein R, Kuvin JT, Soffler M, Lennon RJ, Lavi S, Nelson RE, et al. Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur Heart J. 2010;31:1142–8.
107 Shimbo D, Muntner P, Mann D, Viera AJ, Homma S, Polak JF, et al. Endothelial dysfunction and the risk of hypertension: the multi-ethnic study of atherosclerosis. Hypertension. 2010;55:1210–6.
108 Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, et al. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation. 2009;120:502–9.
109 Lüscher TF, Vanhoutte PM, Raij L. Antihypertensive treatment normalizes decreased endothelium-dependent relaxations in rats with salt-induced hypertension. Hypertension. 1987;9(Suppl III):193–7.
110 Tschudi MR, Criscione L, Novosel D, Pfeiffer K, Lüscher TF. Antihypertensive therapy augments endothelium-dependent relaxations in coronary arteries of spontaneously hypertensive rats. Circulation. 1994;89:2212–8.
111 Takase H, Moreau P, Kung CF, Nava E, Lüscher TF. Antihypertensive therapy prevents endothelial dysfunction in chronic nitric oxide deficiency. Effect of verapamil and trandolapril. Hypertension. 1996;27:25–31.
112 d’Uscio LV, Shaw S, Barton M, Lüscher TF. Losartan but not verapamil inhibits angiotensin II-induced tissue endothelin-1 increase: role of blood pressure and endothelial function. Hypertension. 1998;31:1305–10.
113 Maeso R, Rodrigo E, Munoz-Garcia R, Navarro-Cid J, Ruilope LM, Cachofeiro V, et al. Chronic treatment with losartan ameliorates vascular dysfunction induced by aging in spontaneously hypertensive rats. J Hypertens. 1998;16:665–72.
114 Rodrigo E, Maeso R, Munoz-Garcia R, Navarro-Cid J, Ruilope LM, Cachofeiro V, et al. Endothelial dysfunction in spontaneously hypertensive rats: consequences of chronic treatment with losartan or captopril. J Hypertens. 1997;15:613–8.
115 Boulanger CM, Desta B, Clozel JP, Vanhoutte PM. Chronic treatment with the CA2+ channel inhibitor RO 40-5967 potentiates endothelium-dependent relaxations in the aorta of the hypertensive salt sensitive Dahl rat. Blood Press. 1994;3:193–6.
116 Dohi Y, Criscione L, Pfeiffer K, Luscher TF. Angiotensin blockade or calcium antagonists improve endothelial dysfunction in hypertension: studies in perfused mesenteric resistance arteries. J Cardiovasc Pharmacol. 1994;24:372–9.
117 Spieker LE, Flammer AJ, Luscher TF The vascular endothelium in hypertension. Handb Exp Pharmacol. 2006;249–83.
118 Hirooka Y, Imaizumi T, Masaki H, Ando S, Harada S, Momohara M, et al. Captopril improves impaired endothelium-dependent vasodilation in hypertensive patients. Hypertension. 1992;20:175–80.
119 Creager MA, Roddy M-A, Coleman SM, Dzau VJ. The effect of ACE inhibition on endothelium-dependent vasodilation in hypertension. J Vasc Res. 1992;29:97.
120 Taddei S, Virdis A, Ghiadoni L, Mattei P, Salvetti A. Effects of angiotensin converting enzyme inhibition on endothelium- dependent vasodilatation in essential hypertensive patients. J Hypertens. 1998;16:447–56.
121 Lyons D, Webster J, Benjamin N. The effect of antihypertensive therapy on responsiveness to local intra-arterial NG-monomethyl-L-arginine in patients with essential hypertension. J Hypertens. 1994;12:1047–52.
122 Millgard J, Hagg A, Sarabi M, Lind L. Captopril, but not nifedipine, improves endothelium-dependent vasodilation in hypertensive patients. J Hum Hypertens. 1998;12:511–6.
123 Schiffrin EL, Deng LY, Larochelle P. Progressive improvement in the structure of resistance arteries of hypertensive patients after 2 years of treatment with an angiotensin I-converting enzyme inhibitor. Comparison with effects of a beta-blocker. Am J Hypertens. 1995;8:229–36.
124 Ghiadoni L, Virdis A, Magagna A, Taddei S, Salvetti A. Effect of the angiotensin II type 1 receptor blocker candesartan on endothelial function in patients with essential hypertension. Hypertension. 2000;35:501–6.
125 Schiffrin EL, Lariviere R, Li JS, Sventek P, Touyz RM. Deoxycorticosterone acetate plus salt induces overexpression of vascular endothelin-1 and severe vascular hypertrophy in spontaneously hypertensive rats. Hypertension. 1995;25:769–73.
126 Dawes M, Brett SE, Chowienczyk PJ, Mant TG, Ritter JM. The vasodilator action of nebivolol in forearm vasculature of subjects with essential hypertension. Br J Clin Pharmacol. 1999;48:460–3.
127 Millgard J, Lind L. Acute hypertension impairs endothelium-dependent vasodilation. Clin Sci (Lond). 1998;94:601–7.
128 Schiffrin EL, Deng LY. Structure and function of resistance arteries of hypertensive patients treated with a beta-blocker or a calcium channel antagonist. J Hypertens. 1996;14:1247–55.
129 Taddei S, Virdis A, Ghiadoni L, Uleri S, Magagna A, Salvetti A. Lacidipine restores endothelium-dependent vasodilation in essential hypertensive patients. Hypertension. 1997;30:1606–12.
130 Perticone F, Ceravolo R, Maio R, Ventura G, Iacopino S, Cuda G, et al. Calcium antagonist isradipine improves abnormal endothelium-dependent vasodilation in never treated hypertensive patients. Cardiovasc Res. 1999;41:299–306.
131 Panza JA, Quyyumi AA, Callahan TS, Epstein SE. Effect of antihypertensive treatment on endothelium-dependent vascular relaxation in patients with essential hypertension. J Am Coll Cardiol. 1993;21:1145–51.
132 Taddei S, Mattei P, Virdis A, Sudano I, Ghiadoni L, Salvetti A. Effect of potassium on vasodilation to acetylcholine in essential hypertension. Hypertension. 1994;23:485–90.
133 Yavuz D, Koc M, Toprak A, Akpinar I, Velioglu A, Deyneli O, et al. Effects of ACE inhibition and AT1-receptor antagonism on endothelial function and insulin sensitivity in essential hypertensive patients. J Renin Angiotensin Aldosterone Syst. 2003;4:197–203.
134 Ghiadoni L, Magagna A, Versari D, Kardasz I, Huang Y, Taddei S, et al. Different effect of antihypertensive drugs on conduit artery endothelial function. Hypertension. 2003;41:1281–6.
135 Flammer AJ, Hermann F, Wiesli P, Schwegler B, Chenevard R, Hurlimann D, et al. Effect of losartan, compared with atenolol, on endothelial function and oxidative stress in patients with type 2 diabetes and hypertension. J Hypertens. 2007;25:785–91.
136 Sudano I, Virdis A, Taddei S, Spieker L, Corti R, Noll G, et al. Chronic treatment with long-acting nifedipine reduces vasoconstriction to endothelin-1 in essential hypertension. Hypertension. 2007;49:285–90.
137 Lüscher TF, Tanner FC, Noll G. Lipids and endothelial function: effects of lipid-lowering and other therapeutic interventions. Curr Opin Lipidol. 1996;7:234–40.
138 Eto M, Luscher TF. The cardioprotective effects of statins. Coron Artery Dis. 2004;15:243–5.
139 Clarkson P, Montgomery HE, Mullen MJ, Donald AE, Powe AJ, Bull T, et al. Exercise training enhances endothelial function in young men. J Am Coll Cardiol. 1999;33:1379–85.
140 Hambrecht R, Fiehn E, Weigl C, Gielen S, Hamann C, Kaiser R, et al. Regular physical exercise corrects endothelial dysfunction and improves exercise capacity in patients with chronic heart failure. Circulation. 1998;98:2709–15.
141 Corti R, Flammer AJ, Hollenberg NK, Luscher TF. Cocoa and cardiovascular health. Circulation. 2009;119:1433–41.
142 Flammer AJ, Hermann F, Sudano I, Spieker L, Hermann M, Cooper KA, et al. Dark chocolate improves coronary vasomotion and reduces platelet reactivity. Circulation. 2007;116:2376–82.
143 Sudano I, Spieker LE, Hermann F, Flammer A, Corti R, Noll G, et al. Protection of endothelial function: targets for nutritional and pharmacological interventions. J Cardiovasc Pharmacol. 2006;47(Suppl 2):S136–50; discussion S72–6.
144 Ludmer PL, Selwyn AP, Shook TL, Wayne RR, Mudge GH, Alexander RW, et al. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med. 1986;315:1046–51.
145 Anderson TJ, Gerhard MD, Meredith IT, Charbonneau F, Delagrange D, Creager MA, et al. Systemic nature of endothelial dysfunction in atherosclerosis. Am J Cardiol. 1995;75:71B–4B.
146 Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–5.
147 Anderson TJ, Uehata A, Gerhard MD, Meredith IT, Knab S, Delagrage D, et al. Close relation of endothelial function in the human coronary and peripheral circulation. J Am Coll Cardiol. 1995;26:1235–41.
148 Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–65.
149 Deanfield J, Donald A, Ferri C, Giannattasio C, Halcox J, Halligan S, et al. Endothelial function and dysfunction. Part I: Methodological issues for assessment in the different vascular beds: a statement by the Working Group on Endothelin and Endothelial Factors of the European Society of Hypertension. J Hypertens. 2005;23:7–17.
150 Linder L, Kiowski W, Bühler FR, Lüscher TF. Indirect evidence for release of endothelium-derived relaxing factor in human forearm circulation in vivo. Blunted response in essential hypertension. Circulation. 1990;81:1762–7.
151 Kuvin JT, Patel AR, Sliney KA, Pandian NG, Sheffy J, Schnall RP, et al. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am Heart J. 2003;146:168–74.
152 Lavie P, Schnall RP, Sheffy J, Shlitner A. Peripheral vasoconstriction during REM sleep detected by a new plethysmographic method. Nat Med. 2000;6:606.
153 Nohria A, Gerhard-Herman M, Creager MA, Hurley S, Mitra D, Ganz P. Role of nitric oxide in the regulation of digital pulse volume amplitude in humans. J Appl Physiol. 2006;101:545–8.
154 Bonetti PO, Pumper GM, Higano ST, Holmes DR, Jr., Kuvin JT, Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol. 2004;44:2137–41.
155 Hamburg NM, Keyes MJ, Larson MG, Vasan RS, Schnabel R, Pryde MM, et al. Cross-sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart Study. Circulation. 2008;117:2467–74.
156 Wilkinson IB, Fuchs SA, Jansen IM, Spratt JC, Murray GD, Cockcroft JR, et al. Reproducibility of pulse wave velocity and augmentation index measured by pulse wave analysis. J Hypertens. 1998;16:2079–84.
157 Nelson MR, Stepanek J, Cevette M, Covalciuc M, Hurst RT, Tajik AJ. Noninvasive measurement of central vascular pressures with arterial tonometry: clinical revival of the pulse pressure waveform? Mayo Clinic Proceed. 2010;85:460–72.
158 Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1318–27.
159 Shantsila E, Watson T, Lip GY. Endothelial progenitor cells in cardiovascular disorders. J Am Coll Cardiol. 2007;49:741–52.
160 Hermann M, Hellermann JP, Quitzau K, Hoffmann MM, Gasser T, Meinertz T, et al. CYP4A11 polymorphism correlates with coronary endothelial dysfunction in patients with coronary artery disease – the ENCORE Trials. Atherosclerosis. 2009;207:476–9.
161 Ganz P, Ho JE, Hsue PY. Structural and functional manifestations of human atherosclerosis: do they run in parallel? Eur Heart J. 2009;30:1556–8.



