Current clinical management of brainstem cavernomas
DOI: https://doi.org/10.4414/smw.2010.13120
O
Bozinov, T
Hatano, JK
Burkhardt, J
Sarnthein, H
Bertalanffy
Summary
Over the last two decades a favourable course for treated or nontreated brainstem cavernomas has become possible with enhanced diagnostic tools and clinical experience, as well as minimally invasive microsurgical improvements. Currently, brainstem cavernoma can be treated microsurgically with excellent results and an acceptable morbidity rate. The preferred surgical route has progressively shifted from a dorsal to a lateral approach, but this remains dependent on the location of the lesion in the brainstem. Surgical evaluation and management of all cases of this rare disease should be performed by experienced teams from the outset.
* Authors contributed equally
Introduction
In recent decades the incidence of cerebral cavernous malformations (CCM) has increased due to diagnostic advances with widespread use of magnetic resonance imaging (MRI) in clinical practice (prevalence 0.4–0.9%) [9, 24]. Brainstem cavernomas account for 8–22% of all intracranial cavernomas [13]. This subgroup of CCMs has a substantially higher propensity for bleeding (up to 30%) [21], is more likely to result in severe neurological deficits [7, 13, 21] and moreover has a higher incidence of recurrent haemorrhage than those in other locations [22, 30]. Altogether, our experience with brainstem cavernoma includes over 180 cases, most of them (130) being referred and treated microsurgically by the senior author (HB). The remaining lesions were managed conservatively and regularly monitored by MRI. Here we briefly summarise the literature and our experience in managing this disease.
Clinical symptoms
The annual risk of brainstem cavernoma haemorrhage accounts for 3.8–6% per person/year and shows a remarkable 30~60% rehaemorrhage rate per person/year [22, 30]. The extent of persistent neurological deficits correlates with the number of recurrent haemorrhages, and rebleeding episodes tend to occur at progressively shorter time intervals [22]. Haemorrhage from a brainstem cavernoma can be fatal in 20% of cases [7, 13, 22]. Neurological deficits depend greatly on the localisation of the lesion and vary significantly, including various degrees of internuclear ophthalmoplegia, worsening hemiparesis, facial or abducens paresis, gaze palsy, facial, truncal and extremity numbness, dysphagia, dysarthria, and gait ataxia, among others [2, 13, 22]. The clinical symptoms usually appear in a subacute manner over hours or days, and most cases are treated temporarily with dexamethasone to avoid malignant swelling of the brainstem and secondary problems. Acute incidents with loss of consciousness or breathing disability occur very rarely.
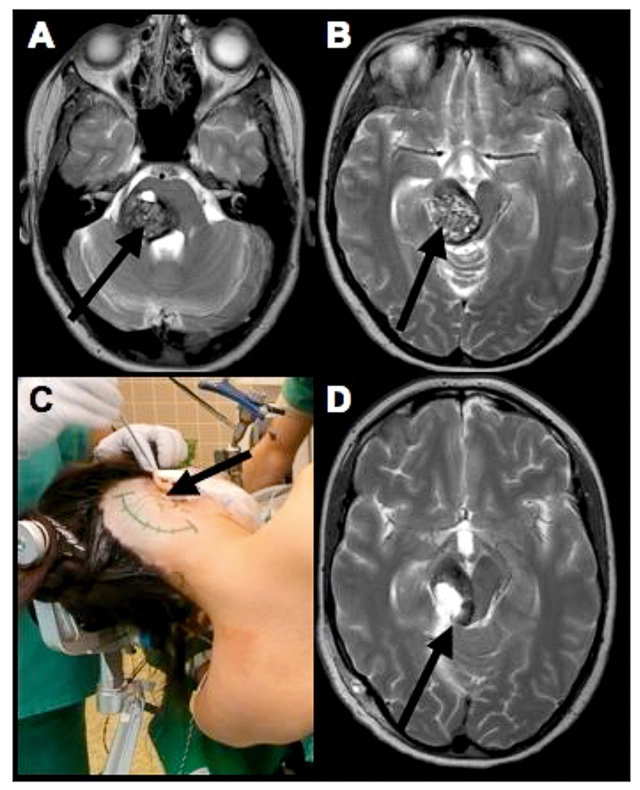
Figure 1
A 25 yo/f suffering from a right pontomesencephalic cavernomal haemorrhage (arrows) with subacute ataxia, hemiparesis and facial palsy (A and B). The surgery was performed on the left side via a combined supracerebellar and retrosigmoid approach (C). The arrows represent the approach to the cavernoma. Postoperative MRI image in T2 (D) revealed complete resection of the cavernoma with residual haemosiderin from the haemorrhages in the intact brainstem tissue (arrow). Postoperatively, only a temporary slight increase of ataxia was noted and further follow-up showed almost complete resolution of the preoperative symptoms.
Imaging
The gold standard for visualisation of the anatomical as well as pathological findings, such as the extent of the lesion and haemorrhage, is MRI. High field (1,5 or 3,0 Tesla) images with T1 (with and without contrast enhancement), T2 and gradient echo sequences in all three planes (axial, coronal, sagittal) are critical for guidance of all decisions. Additional tools such as T2-based imaging and fibre tracking have further improved the visualisation and understanding of these lesions [6].
Surgical treatment
Indications, goal and timing of surgery
Expert opinion varies regarding the indication and timing of surgery, but if haemorrhage appears associated with worsening of the neurological deficit surgical evacuation of the lesion and haematoma is recommended (fig. 3) [28]. Exceptionally, surgery for even asymptomatic patients is also proposed [27]. As a general rule clinical symptoms should be the main indication for surgery, and the patient option should preferably also be included in the decision process (fig. 3). The main goal of surgery is eliminating the risk of renewed haemorrhage and avoiding complications [1, 2, 22, 30]. Hence complete removal of the lesion is essential to prevent re-haemorrhage, which may occur in up to 43% of surgical cases [5]. However, in our brainstem cavernoma series we found a postoperative rebleeding rate of 4.4%. The risk of leaving residual portions of the lesion behind depends on surgeon experience. The larger the series, the lower the incidence of residuals [22, 30]. In the past two decades, waiting four to six weeks after a haemorrhagic event was recommended to stabilise the patient’s condition and waiting for the haematoma to become organised to achieve less active gliosis [10]. However, the incidence of rebleedings is highest within one month after surgery (21.8%) [22]. Prior to surgery, treatment with steroids for one or two weeks is recommended to resolve oedema and take advantage of haematoma cavity formation [30].
Surgical approaches to the brainstem and intraoperative monitoring
A great variety of surgical approaches, such as the suboccipital midline, retrosigmoid or subtemporal approaches exist in many instances of brainstem cavernoma [5, 8, 10, 16, 17, 22, 23, 30]. The choice of the proper approach depends on the relationship between the cavernoma and the pial or ependymal surface of the brainstem. As the floor of the fourth ventricle contains structures with important functions [3, 23], a lateral entry is preferred whenever possible. The supracerebellar infratentorial approach (fig. 1) is suitable for many lesions and has yielded favourable patient outcomes; it is one of our preferred access routes. Intraoperative electrophysiological monitoring of long tracks (MEP and SEP), AEP and cranial nerves is obligatory during brainstem surgery [2, 25].
Complications, morbidity and mortality
Postoperative morbidity may be due to manipulation or oedema of brainstem parenchyma, and permanent morbidity was reported earlier in the range of 12% ~21% [12, 22, 26, 30]. However, the morbidity rate is clearly related to surgical experience [1, 2].
Alternative treatments
Stereotactic radiosurgery
The use of radiosurgery for cavernomas has remained controversial, since the main goal of radiosurgery should be a significant reduction in bleeding risk. Some authors have insisted on the efficacy of radiosurgery for intracranial cavernomas, due to the reduced risk of haemorrhage after a latency period of 2 years [14, 15, 19]. However, the annual risk of haemorrhage during the latency period after radiosurgery is greater than 10% [15]. In our opinion stereotactic radiosurgery should not be considered as the first-step treatment for intracranial or brainstem cavernoma, since it fails to eliminate the risk of haemorrhage. If radiosurgery is adopted as the treatment modality of choice, accurate evaluation in a neurosurgical centre should be considered since not every so-called “surgically untreatable” lesion is surgically inaccessible [2, 29]. For instance, in their recently published serial Lunsford et al. presented a figure of a cerebellar cavernoma which is surgically accessible in experienced hands [20].

Figure 2
Left MRI images (A) show a typical mesencephalic cavernoma haemorrhage on the left side (arrows) in an axial T1 (no gadolinium-DTPA) and coronal T1 (with gadolinium-DTPA). The patient suffered from a slight headache and partial hemi-hypoaesthesia. Surgery in this case was not advised. The MRI images 2 months later (B) showed resorption of the acute haemorrhage with no sign of residual cavernoma (arrows). The MRI images after one year (C) confirmed the positive follow-up with complete resolution of all symptoms. The upper axial T1 (with gadolinium-DTPA) demonstrates again the old lesion with no sign of recurrence. The lower axial MRI image is a special sequence (gradient echo) to detect haemosiderin in the brain. It shows the old lesion and status after haemorrhage, which should not be mistaken for a new haemorrhage.
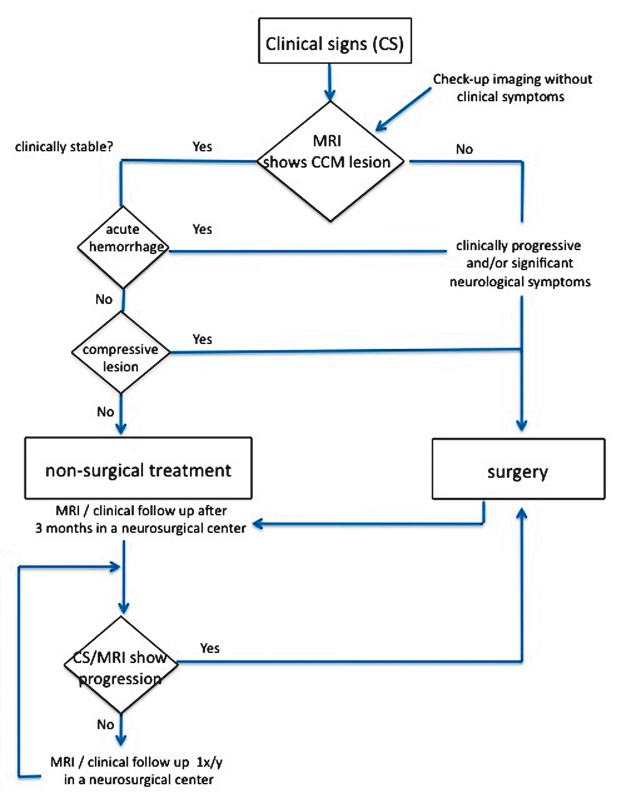
Figure 3
Flowchart to differentiate between nonsurgical versus surgical management in patients with brainstem cavernomas.
Conservative management
Long-term outcomes may be worse in a nonsurgical group (42% poorer outcome) than in surgically treated patients (9%) [22]. But conservative treatment plays an important role in patients with small lesions, rapid clinical improvement after bleeding episodes and a nonaggressive appearance of the lesion on MRI (fig. 2). In such cases it is important to inform the patient of the estimated individual bleeding risk and, additionally, all treatment options and possible morbidities should be discussed in detail. Nevertheless, mortality may occur whatever the decision [7, 13, 22]. We have followed conservatively more than 50 patients with either initially minor or nonhaemorrhaging (incidental findings) lesions. None of these patients has ever suffered life-threatening bleeding.
Follow-up and further management
We perform an initial MRI postoperatively or days after the first haemorrhage, and a follow-up MRI 2–3 months afterwards. Yearly MRI should be considered for all patients with or without surgery, and should be performed in a neurosurgically experienced centre.
Genetics and research
Some research groups have focused on the biological behaviour of cerebral cavernous malformations despite the rarity of this disease. Recent work has uncovered the association of three mutations CCM1 (KRIT1), CCM2 (MGC4607) and CCM3 (PDCD10) [11], PTEN promoter methylation [32] and disease modulating factors such as the HEG transmembrane receptor [18] and the RhoA GTPase [31] with this disease. Further research will aim to elucidate the pathogenesis of cavernomas in terms of cellular mechanisms of pathological angiogenesis [4] and de novo mutations, with a view to defining specific targets for future therapeutic interventions. Better genetic knowledge will also improve genetic counselling, which is recommended in familial cases [11].
Conclusion
Modern treatment options for brainstem cavernomas include a variety of diagnostic and surgical tools, experience and dedication. Altogether, favourable outcomes can be achieved and surgically nontreatable lesions are extremely rare. The most important factor is involvement of a surgically experienced clinic at the beginning of the diagnosis.
Correspondence:
Oliver Bozinov, MD
Department of Neurosurgery
University Hospital Zürich
Frauenklinikstrasse 10
CH-8091 Zürich
Switzerland
oliver.bozinov@usz.ch
References
1 Bertalanffy H, Gilsbach JM, Eggert HR, Seeger W. Microsurgery of deep-seated cavernous angiomas: report of 26 cases. Acta Neurochir. (Wien) 1991;108:91–9.
2 Bertalanffy H, Benes L, Miyazawa T, Alberti O, Siegel AM, Sure U. Cerebral cavernomas in the adult. Review of the literature and analysis of 72 surgically treated patients. Neurosurg Rev. 2002;25:1–53; discussion 54–55.
3 Bertalanffy H, Tissira N, Krayenbühl N, Bozinov O, Sarnthein J. (2010) Inter- and intra-patient variability of facial nerve response areas in the floor of the 4th ventricle. Neurosurgery, accepted for publication.
4 Burkhardt JK, Schmidt D, Schoenauer R, Brokopp C, Agarova I, Bozinov O, et al. Upregulation of transmembrane endothelial junction proteins in human cerebral cavernous malformation. Neurosurg Focus. 2010;29(3):E3.
5 Cenzato M, Stefini R, Ambrosi C, Giovanelli M. Post-operative remnants of brainstem cavernomas: incidence, risk factors and management. Acta Neurochir. (Wien) 2008;150:879–86; discussion 887.
6 Chavhan GB, Babyn PS, Thomas B, Shroff MM, Haacke EM. Principles, techniques, and applications of T2*-based MR imaging and its special applications. Radiographics. 2009;29:1433–49.
7 Ciurea AV, Nastase C, Tascu A, Brehar FM. Lethal recurrent hemorrhages of a brainstem cavernoma. Neurosurg Rev. 2007;30:259–62; discussion 262.
8 de Oliveira JG, Lekovic GP, Safavi-Abbasi S, Reis CV, Hanel RA, Porter RW, et al. Supracerebellar infratentorial approach to cavernous malformations of the brainstem: surgical variants and clinical experience with 45 patients. Neurosurgery. 2010;66:389–99.
9 Del Curling O Jr, Kelly DL Jr, Elster AD, Craven TE. An analysis of the natural history of cavernous angiomas. J Neurosurg. 1991;75:702–8.
10 Fahlbusch R, Strauss C, Huk W, Rockelein G, Kompf D, Ruprecht KW. Surgical removal of pontomesencephalic cavernous hemangiomas. Neurosurgery. 1990;26:449–56; discussion 456–47.
11 Felbor U, Sure U, Grimm T, Bertalanffy H. Genetics of cerebral cavernous angioma. Zentralbl Neurochir. 2006;67:110–6.
12 Ferroli P, Sinisi M, Franzini A, Giombini S, Solero CL, Broggi G. Brainstem cavernomas: long-term results of microsurgical resection in 52 patients. Neurosurgery. 2005;56:1203–12; discussion 1212–04.
13 Fritschi JA, Reulen HJ, Spetzler RF, Zabramski JM. Cavernous malformations of the brain stem. A review of 139 cases. Acta Neurochir. (Wien) 1994;130:35–46.
14 Gross BA, Batjer HH, Awad IA, Bendok BR. Brainstem cavernous malformations. Neurosurgery. 2009;64:E805-818; discussion E818.
15 Hasegawa T, McInerney J, Kondziolka D, Lee JY, Flickinger JC, Lunsford LD. Long-term results after stereotactic radiosurgery for patients with cavernous malformations. Neurosurgery. 2002;50:1190–7; discussion 1197–8.
16 Hauck EF, Barnett SL, White JA, Samson D. Symptomatic brainstem cavernomas. Neurosurgery. 2009;64:61–70; discussion 70–61.
17 Hebb MO, Spetzler RF. Lateral transpeduncular approach to intrinsic lesions of the rostral pons. Neurosurgery. 2010;66:26–9; discussion 29.
18 Kleaveland B, Zheng X, Liu JJ, Blum Y, Tung JJ, Zou Z, et al. Regulation of cardiovascular development and integrity by the heart of glass-cerebral cavernous malformation protein pathway. Nat Med. 2009;15:169–76.
19 Liu KD, Chung WY, Wu HM, Shiau CY, Wang LW, Guo WY, Pan DH. Gamma knife surgery for cavernous hemangiomas: an analysis of 125 patients. J Neurosurg. 2005;102(Suppl):81–6.
20 Lunsford LD, Khan AA, Niranjan A, Kano H, Flickinger JC, Kondziolka D. Stereotactic radiosurgery for symptomatic solitary cerebral cavernous malformations considered high risk for resection. J Neurosurg. 2010;113:23–9.
21 Porter PJ, Willinsky RA, Harper W, Wallace MC. Cerebral cavernous malformations: natural history and prognosis after clinical deterioration with or without hemorrhage. J Neurosurg. 1997;87:190–7.
22 Porter RW, Detwiler PW, Spetzler RF, Lawton MT, Baskin JJ, Derksen PT, et al. Cavernous malformations of the brainstem: experience with 100 patients. J Neurosurg. 1999;90:50–8.
23 Recalde RJ, Figueiredo EG, de Oliveira E. Microsurgical anatomy of the safe entry zones on the anterolateral brainstem related to surgical approaches to cavernous malformations. Neurosurgery. 2008;62:9–15; discussion 15–7.
24 Robinson JR, Awad IA, Little JR. Natural history of the cavernous angioma. J Neurosurg. 1991;75:709–14.
25 Sala F, Manganotti P, Tramontano V, Bricolo A, Gerosa M. Monitoring of motor pathways during brain stem surgery: what we have achieved and what we still miss? Neurophysiol Clin. 2007;37:399–406.
26 Sandalcioglu IE, Wiedemayer H, Secer S, Asgari S, Stolke D. Surgical removal of brain stem cavernous malformations: surgical indications, technical considerations, and results. J Neurol Neurosurg Psychiatry. 2002;72:351–5.
27 Sindou M, Yada J, Salord F. Functional results after microsurgical resection of brain stem cavernous malformations (retrospective study of a 12 patient series and review of the recent literature). Acta Neurochir. (Wien) 2000;142:843–52; discussion 852–43.
28 Steinberg GK, Chang SD, Gewirtz RJ, Lopez JR. Microsurgical resection of brainstem, thalamic, and basal ganglia angiographically occult vascular malformations. Neurosurgery. 2000;46:260–70; discussion 270–61.
29 Steiner L, Karlsson B, Yen CP, Torner JC, Lindquist C, Schlesinger D. Radiosurgery in cavernous malformations: anatomy of a controversy. J Neurosurg. 2010;113:16–21; discussion 21–12.
30 Wang CC, Liu A, Zhang JT, Sun B, Zhao YL. Surgical management of brain-stem cavernous malformations: report of 137 cases. Surg Neurol. 2003;59:444–54; discussion 454.
31 Whitehead KJ, Chan AC, Navankasattusas S, Koh W, London NR, Ling J, et al. The cerebral cavernous malformation signaling pathway promotes vascular integrity via Rho GTPases. Nat Med. 2009;15:177–84.
32 Zhu Y, Wloch A, Wu Q, Peters C, Pagenstecher A, Bertalanffy H, Sure U. Involvement of PTEN promoter methylation in cerebral cavernous malformations. Stroke. 2009;40:820–6.


