
Figure 1
The CoreValve® by Medtronic, Minneapolis, MN, for retrograde implantations only.
DOI: https://doi.org/10.4414/smw.2010.13127
State of the art techniques and future perspectives
Degenerative aortic valve stenosis (AS) occurs in the elderly and represents the most frequent acquired heart valve disease, with a prevalence of 4.6% in adults aged 75 years or more. Due to the ageing population in developed countries, the amount of elderly suffering from severe symptomatic AS is constantly increasing and cardiologists as well as cardiac surgeons worldwide are already facing a greater burden from this valvular disease [1–4].
Concerning the standard treatment for symptomatic AS, there is still a lack of pharmacological therapies to prevent or slowdown the progression of this potentially life-threatening disease and, unfortunately, percutaneous aortic valve ballooning (valvuloplasty) has revealed questionable short-term and mid-term results [5, 6]. Thus, this technique is no longer considered a valid therapy for AS but is still indicated to temporarily improve the patient’s haemodynamic (in case of AS followed by acute cardiac decompensation), or as a palliative treatment for patients not referred to open heart surgery. As a consequence, for the last fifty years the standard open heart surgery for aortic valve replacement (AVR) with use of cardiopulmonary bypass (CPB), sternotomy (or mini-sternotomy), aortic cross clamping and cardioplegic arrest represents the treatment of choice and the standard of care for patients carrying severe AS with symptoms, and the mid- and long-term results are very satisfactory [7–10]. Moreover, during the last twenty years it has been proven that the refinement of surgical techniques, anaesthesiological management and intensive care treatments have favourably influenced the outcome and the surgical results of patients aged 80 years or more, who underwent isolated AVR [11–16].
However, there is still a pool of patients affected by severe AS requiring AVR (estimated at 33% of patients with severe symptomatic AS) who do not undergo valve surgery because they are considered too old (nonagenarians, centenaries) for such an invasive procedure, or because they are affected by concomitant co-morbidities that noticeably increase the operative risk (too high risk for hospital mortality and/or onset of severe postoperative complications) [17]. The amount of people included in this subgroup will likely increase constantly during the upcoming decades because an ageing population will have more co-morbidities and, consequently, doctors and surgeons will deny high-risk AVRs.
In this scenario, transcatheter minimal invasive beating heart aortic valve therapies (transcatheter aortic valve implantation: TAVI) have been considered as attractive alternatives to the standard AVR for patients carrying an elevated predictable operative risk or in case of peculiar clinical situations that could compromise the patient’s outcome after standard open heart surgery.
In the 1990s, the visionary idea of implanting valved stents in the aortic annulus stimulated basic research, computer simulations, bench tests and animal models all over Europe, and, at the beginning of this century (April 2002), the first transcatheter aortic stent-valve implantation in human (using an antegrade trans-septal venous approach through a femoral vein) was performed by Dr. Cribier in Rouen, France [18–26].

Figure 1
The CoreValve® by Medtronic, Minneapolis, MN, for retrograde implantations only.
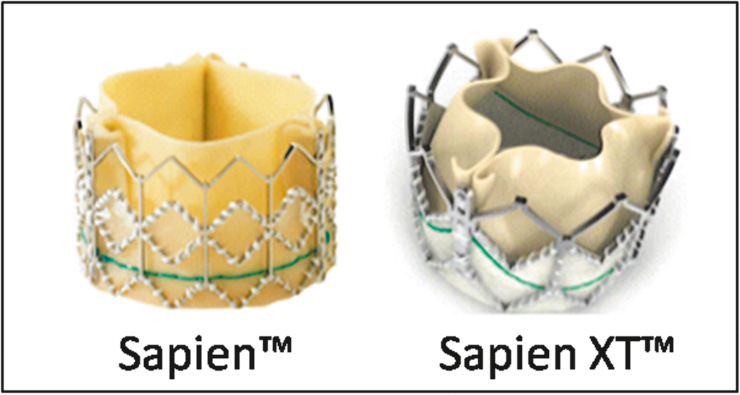
Figure 2
The SapienTM THV (left) and the new Sapien XTTM generation (right) by Edwards Lifesciences, Irvine, CA, for antegrade and retrograde implantations.
Since then, only two different sutureless transcatheter aortic stent-valves have been developed, tested and, subsequently, introduced into the current clinical practice. These two devices, which are routinely implanted in several highly specialised cardiac centres, are the CoreValve® system by Medtronic, Minneapolis, MN, (fig. 1), which is only available for a retrograde purpose, and the Edwards Sapien™ THV stent-valve system by Edwards Lifesciences INC, Irvine, CA, (fig. 2), which is available for both the antegrade and the retrograde applications. However, a few more devices, allowing for antegrade and/or retrograde implantations, have already passed the animal lab tests and are now under human clinical investigation (see the chapter: future perspectives).
The CoreValve® system (fig. 1) is a self-expandable nitinol stent with an inner porcine pericardial valve, which received the CE mark in 2007. The stent-valve is designed to sit into the aortic root and to anchor into the aortic annulus. However, the valve function is more supra-annular and a skirt of pericardium, bordering the lower portion of the stent-valve, prevents paravalvular leaks. The valve is available in two sizes, the 26 mm and the 29 mm, and the delivery system (only for retrograde applications) has an external diameter of 18F allowing for a transfemoral (TF) (recommended) or a trans-subclavian (off-label use) transcatheter stent-valve implantation.
The Edwards Sapien™ THV stent-valve (fig. 2) is a tube-like stainless steel balloon-expandable stent, of 14–16 mm in length, with an inner bovine pericardial valve treated with the ThermaFix™ anticalcification system, that received the CE mark approval in October and December 2007 for its transfemoral and transapical (TA) applications, respectively. The stent-valve is inserted and deployed within the aortic annulus and sits in a sub-coronary position. The Sapien™ stent-valve is available in two sizes, a 23 mm (14 mm length) and a 26 mm (16 mm length), and can be introduced via a transapical (antegrade, Edwards Ascendra™ system) or a transfemoral (retrograde, Edwards RetroFlex™ system) transcatheter approach that requires a 22F (for the 23 mm) or a 24F (for the 26 mm) sheath.
The new generation of Edwards Sapien™ THV, the XT generation (fig. 2), was officially launched in the second quarter of 2010. This new device, available for transapical and transfemoral applications, has important innovations: first of all, both the retrograde and the antegrade implantation will benefit from new smaller sheaths; an 18F sheath for transfemoral access (allowing for full percutaneous procedures) and a 22F or 24F sheath for the transapical access. The new transfemoral delivery system is called the NovaFlex™ system, whereas the new transapical delivery system is named Ascendra II™ and both are easier to use compared to the older versions. Other important innovations are the new stent (cobalt-chromium) that allows for a smaller crimped profile with a higher resistance to mechanical stress, and the re-designed valve leaflets that gain a “semi-closed” profile at rest (instead of an open profile) allowing for an easier closure under pressure once implanted. Moreover, a new 29 mm Sapien XT™ THV for transapical applications only, will be available in 2011.
With regards to the delivery systems and their introduction into the ascending aorta, two specific pathways have been explored so far: the antegrade way employing direct transapical access, and the retrograde way that uses either transfemoral or, alternatively, trans-subclavian access [27–30].
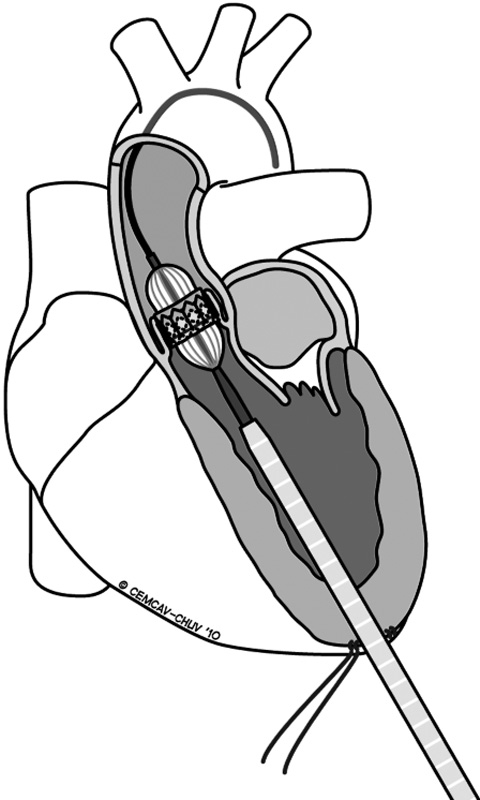
Figure 3
Transapical approach for implanting a transcatheter aortic stent-valve.
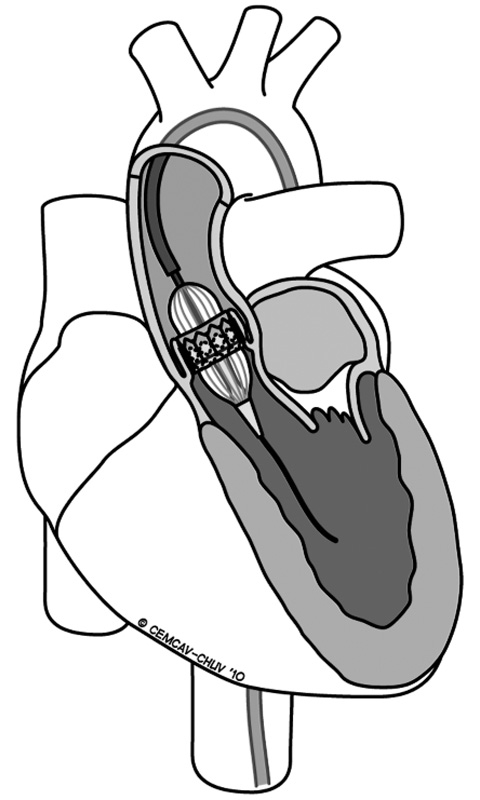
Figure 4
Transfemoral approach for implanting a transcatheter aortic stent-valve.
The main advantages of performing transapical procedures (fig. 3) are: i) the feasibility does not rely on the absence of a concomitant peripheral vascular disease or previous aortic surgery; ii) the delivery system seems to be more “steady” and the procedure itself more “straightforward”, and iii) this access potentially reduces the risk of calcium dislodgment due to the passage of a stiff transfemoral device into a diseased aortic arch. A transapical transcatheter aortic valve implantation (TA-TAVI) can be performed in the operating room, in a hybrid room, or in a catheterisation laboratory with patients under general anaesthesia (high epidural anaesthesia in a conscious patient has also been reported). Regardless to the place where the TA is performed, it is a prerequisite that high-quality fluoroscopic imaging must be guaranteed. Apical bleeding is very rare, mostly related to patients’ tissue fragility and/or to the team learning curve, and represents the most dangerous complication related to the transapical access itself. Once occurred, CPB resuscitation can be helpful in order to safely repair the tear [31]. In transapical TAVI, the cardiac apex is prepared through a small left anterolateral mini-thoracotomy using a purse-string or a crossing suture reinforced by pledgets and, after the procedure, a chest tube is routinely inserted into the left pleura with pain releasers injected in the intercostal tissue.
The transfemoral procedure (fig. 4) for transcatheter aortic valve implantation (TF-TAVI) is mostly performed in the cardiac catheterisation laboratory or in the hybrid room and one of the main advantages of this technique is that it allows fully percutaneous implantations in conscious patients, as long as the peripheral vessels have an adequate caliber (more than 6 mm diameter), there are no very tortuous vessels, and vascular closure devices are available. Alternatively, the standard technique requires surgical preparation of the common femoral artery (rarely, the iliac artery) under local or general anaesthesia. According to the latest international guidelines, the presence of peripheral vascular disease, small vessel diameters, tortuous vessels, aortic disease or previous aortic surgery contraindicates this approach. Major and minor postoperative vascular complications have been reported quite often in recent series (up to 40% of incidence) and some critical events (vessels dissections, ruptures or avulsions) might be catastrophic when not promptly and adequately treated.
Trans-subclavian access is an alternative retrograde pathway that has been recently explored. It requires a surgical exposure of the left subclavian artery and an adequate minimal vessel inner diameter for 18F delivery systems (6 mm diameter). There are some advantages in using this approach: first of all, the distance between the site of introduction and the aortic valve is short, compared to the transfemoral option, and it results in a steadier pathway. Secondly, as long as the subclavian artery is intact, the trans-subclavian procedure can be performed in case of a concomitant vascular disease involving the abdominal aorta or the legs and does not require a thoracotomy. Unfortunately, the presence of a patent internal mammary artery, such as a diseased subclavial artery, in redo coronary surgery contraindicates this approach. However, at the moment this interesting approach remains “off-label” and is not yet formally recommended by the industry.
In case of severe peripheral vascular disease and a concomitant contraindication to transapical procedures (i.e. an apical thrombus), an alternative, interesting, retrograde approach has been proposed: through an upper “J-shape” mini-sternotomy, the guidewires and the delivery system are inserted, retrogradely, into the ascending aorta and are secured with a double purse-string suture (4-0 polypropylene). Then, the TAVI is performed as a transfemoral procedure. The presence of “porcelain” aorta and the risk of postoperative massive bleeding limit this approach to selected cases.
First of all, we have to state that patients suitable for TAVI have to be selected by a multidisciplinary group of experts composed of cardiologists and cardiac surgeons, and the entire process requires a team approach involving an anaesthesiologist and doctors from the intensive care unit. The patients’ enrolment follows the latest international guidelines for TAVI [28]. Briefly, elderly patients with severe symptomatic AS and a very high predictable operative risk (defined as a Logistic Euroscore-above 20% or an STS score above 10%) are good candidates for a transcatheter therapeutic option. However, all cardiac centres performing TAVI have already experienced the screening of special candidates who carry a “clinically estimated” surgical risk too high for standard AVR, despite the Logistic Euroscore or the STS score lying below the above mentioned limits. In particular, a heavily calcified ascending aorta (“porcelain” aorta), the presence of a severe congenital thoracic distortion, a severe liver disease (i.e. a liver cirrhosis with a CHILD classification A or B), the presence of non-cardiac tumours with a good mid-term prognosis or a pre-dialysis renal insufficiency, have always been considered important risk factors contraindicating standard AVRs with CPB. Generally speaking, we can state that all contraindications to CPB use, sternotomy, cardioplegic cardiac arrest or aortic cross-clamping are good indications for a TAVI.
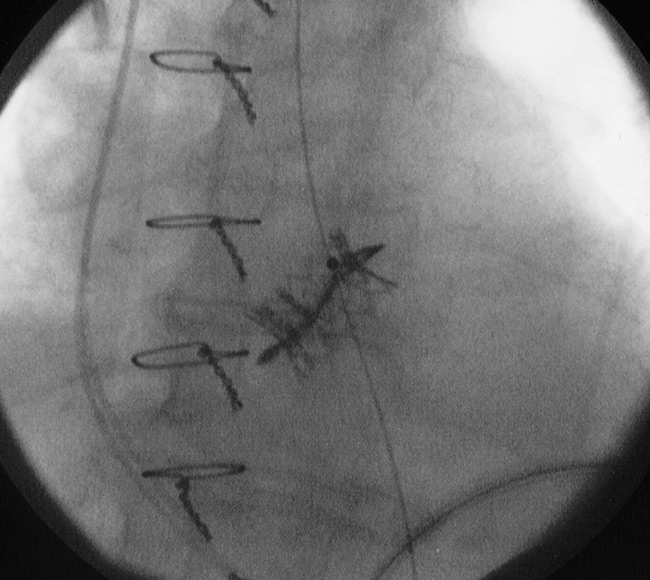
Figure 5
Image taken from the fluoroscopy system showing a transapical valve-in-valve procedure (a 23 mm SapienTM THV into a degenerated 23 mm Mitroflow bioprosthesis).
The native aortic annulus diameter also plays a key role: a valve annulus measuring 19 to 25 mm diameter is recommended for a 23 mm or a 26 mm Sapien™ THV, whereas an aortic annulus measuring 20 to 27 mm requires a CoreValve®. Smaller and bigger valve diameters are not suitable, at the moment, for transcatheter valve therapies despite the fact that, as soon as the 29 mm Sapien XT™ THV will be available, a wide 28 mm aortic annulus will also become treatable.
A recent myocardial infarction (less than 3 months), an extremely severe pulmonary dysfunction (avoiding thoracotomy and intubations), and the presence of an apical thrombus are considered formal contraindications for transapical TAVI, whereas a left ventricular ejection fraction (LVEF) below or equal to 20% contraindicates all TAVI procedures.
A congenitally bicuspid aortic valve is also included in the contraindication list for TAVI, however, a successful case of TAVI in a congenital aortic bicuspidy has recently been published [32].
All major peripheral vascular problems involving the aorta or the ileo-femoral arteries contraindicate transfemoral approaches for TAVI, but even a standard transapical TAVI normally requires, at least, one valid peripheral arterial access for the introduction of an arterial catheter (a “pigtail” catheter) to inject contrast medium into the ascending aorta. However, we were able to demonstrate that a transapical TAVI can also be successfully performed in the absence of any valid arterial access, when the intraoperative angiographies are avoided and the stent-valve is positioned solely under transeosophageal echo-guidance (see the chapter: echo-guided transapical TAVI without angiography) [33].
With regard to redo aortic valve surgery, in a few experienced cardiac centres it has recently become routine to perform “valve-in-valve” (VinV) procedures in case of degenerated aortic bio-prostheses in high-risk patients. In fact, the VinV strategy was developed to avoid high-risk redo cardiac surgery in old patients with degenerated valves, and is based on the implantation of a sized stent-valve into the stented or stentless diseased bio-prosthesis (the recommended minimal bio-prosthesis diameter for the transapical implantation of an Edwards Sapien™ stent-valve is 23 mm), and the latest postoperative clinical results are satisfactory [34, 35] (fig. 5). However the transcatheter VinV remains “off-label” and further clinical investigations are recommended.
Another new approach involving transcatheter procedures in case of a failed mitral valve repair is the “valve-in-a-ring” (VinR) procedure that has been very recently proposed (no clinical cases have been published yet and a case report is under review to the Editor of the European Journal of Cardio-Thoracic Surgery). The procedure is based on the implantation, retrogradely, via a transapical approach, of a stent-valve into a failed mitral repair with a ring. However this approach is under evaluation, is an “off-label” use and more studies are necessary to establish its feasibility with different rings (flexible, rigid, semi-rigid) and different sizes.
Patients enrolled for TAVI undergo preoperative clinical and radiological investigations in order to evaluate their general clinical state, the size of the aortic annulus, the presence of concomitant vascular disease and the presence of coronary disease [36].
The standard protocol for TAVI includes the following tests:
– thorax X-ray
– transthoracic echocardiogramme (first level of screening)
– peripheral vascular Doppler (first level of investigation)
– transoesophageal echocardiogramme (second level)
– heart catheterisation with coronary angiogramme, aortography and, eventually, peripheral injections.
– cardiac and vascular computed-tomography scan followed by 3-dimentional reconstructions of the heart, the aorta, the iliac, the femoral and, eventually, the subclavian artery to enable measurements to be done.
The transapical TAVI (TA-TAVI) (fig. 3) requires a left anterolateral mini-thoracotomy through the 5th or the 6th intercostal space. In this phase, a transthoracic echocardiogramme is useful to identify the cardiac apex, in order to place a mark on the skin. Once the pleura is opened, it is also useful to insert a finger through it in order to identify the apex position: if the apex is easily accessible, the procedure can be performed through this access, otherwise the upper or the lower intercostal space can be taken into consideration. After opening the pericardium, typically two concentric purse string sutures (2-0 or 3-0 polypropylene sutures with 1×0.5 cm large Teflon pledgets) have to be prepared on the left lateral wall of the cardiac apex. Care has to be taken in order to insert the needle through the muscle and not through the fat in order to avoid life threatening bleedings from ventricular tears. Once the guidewires are passed through the apex and across the aortic valve, it is important to advance them into the descending aorta using a standard pigtail catheter. Following this, the authors immediately insert the bigger sheet (30F) which is used to introduce the delivery system for the Edwards SapienTMTHV. Through this big sheet, we first perform the aortic valvuloplasty and then we perform the stent-valve implantation, both under rapid pacing (heart beat rate >180 bpm). In case of a VinV procedure, we do not perform the valvuloplasty in order to avoid the dislodgments of valve material.
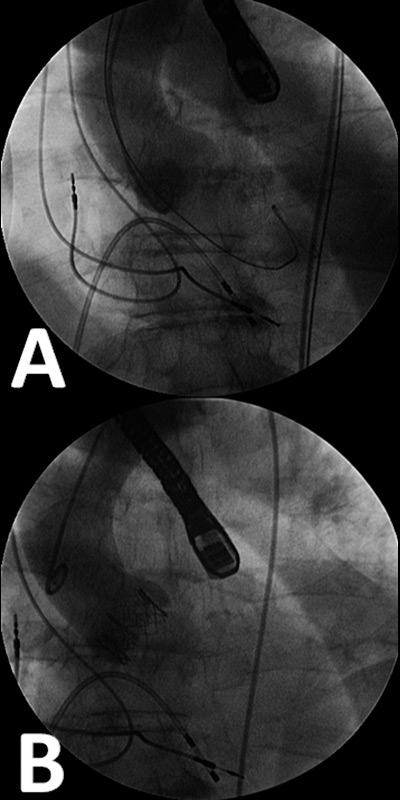
Figure 6
A) angiogrammes are performed before the valve positioning in order to assess the position of the fluoroscopic system which has to lie perpendicular to the aortic valve plane. B) an angiographic control performed after the stent-valve implantation.
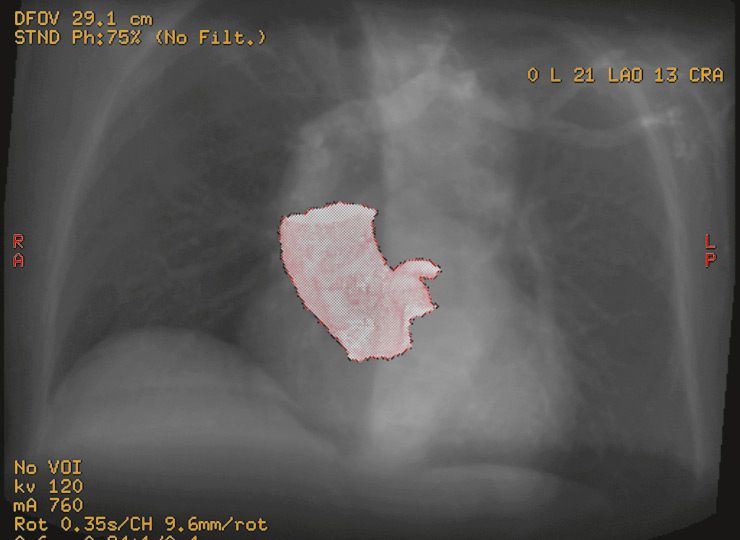
Figure 7
Preoperative CT-scan reconstruction of the aortic root. On the upper right side, two angles indicate the positioning of the aortic valve plane in the space and the fluoroscopy is orientated consequently (in this case, the angles are 21 degrees left-lateral and 16 degrees cranial).
In order to avoid parallax phenomena between the aorta and the stent-valve during the procedure, the fluoroscopy unit has to be precisely orientated: repeated angiographies are performed before the balloon valvuloplasty and the three leaflets of the aortic valve have to lie on one single line (fig. 6A). The stent-valve positioning requires angiographies to verify the placement of the crimped stent-valve is in the ideal landing zone (the stent-valve has to lie 40% on the aortic side and 60% on the ventricular side), and, at the end of the procedure when the valve is in place, a postoperative angiographic control is also routinely performed to verify the coronary patency and the absence of major paravalvular leaks (fig. 6B). The intraoperative TEE is used to confirm the valve positioning, the absence of major (>2+) paravalvular leaks as well as to verify the leaflets’ motion and integrity.
The transfemoral TAVI (TF-TAVI) (fig. 4) requires valid femoral access or, rarely, iliac access (where an 8 mm diameter vascular graft is anastomosed to the vessel). The TF procedure requires the surgical preparation of the femoral vessel or, since the advent of small 18F delivery systems, it can be performed fully percutaneously. Once inserted into the aorta, the guidewires are advanced and placed across the aortic arch and through the stenosed aortic valve. During this retrograde pathway it is important to prevent calcium dislodgment from a diseased aortic wall. The positioning of the fluoroscopy unit follows the same rules already described for the TA procedure and repeated angiographies are performed. Once the valvuloplasty is performed and the crimped stent-valve is inserted across the native valve, an aortography is performed to confirm the positioning. In the case of a conscious patient, the postoperative TEE control is replaced by TTE and angiography.
The two different stented valves which are available are deployed in two different ways: under rapid cardiac pacing (heart beat rate >180 bpm) the SapienTM THV is expanded using the rapid inflation of the inner catheter-balloon, whereas the CoreValve® auto-expands after the removal of the delivery system. Once the valve is in place, the delivery system is removed and a closure system is employed in case of a percutaneous access.
Despite disadvantages, intraoperative cardiac imaging is crucial for a successful stent-valve implantation. A major disadvantage, related to the use of high doses of contrast medium for the angiographies, is the quite relevant incidence of contrast-related postoperative acute renal failure after TAVI (up to 33%), which represents an important complication negatively affecting the patients’ outcome [37, 38].
Routine echo-guided transapical TAVI without angiography was proposed by our group in 2009 to prevent contrast-related acute postoperative renal failure [39–41], and is based on our wide experience (more than 500 procedures performed) on endovascular aorta repair (EVAR) without angiography [42].
The technique does not differ from standard transapical TAVI as far as the surgical details are concerned. However, preoperative and the intraoperative cardiac imaging are peculiar and technical details have already been described in a previous report [41]. The essential steps can be briefly summarised as follows: an injected (very low dose) cardiac computer tomography (CT-scan) is performed preoperatively to measure the annulus diameter and the distance with the coronary ostia, and to analyse the aortic root (3-D reconstructions). Two angles are identified, a left-lateral and a cranial one, indicating the orientation of the aortic valve plane in the space (fig. 7). Using these angles the fluoroscopy is orientated into its final position, and the use of repeated aortographies is no longer necessary (fig. 8). The valve positioning is the crucial phase and is based on high-quality TEE images acquired by experienced specialists [43] (fig. 9). The fluoroscopy without contrast still plays a key-role for the placement of guidewires and catheters, and for the valve ballooning and the stent-valve balloon inflation. At the end of the procedure, the haemodynamic control is assessed by TEE imaging and measurements and if the patient is stable the coronary angiogramme and the aortography are no longer performed.
Published results have shown satisfactory short-term outcomes after transapical and transfemoral TAVI but, to the best of our knowledge, there is not yet evidence that a superiority exists between the two techniques. Thus, it would be of great interest to develop a three-arm, randomised, prospective clinical trial that statistically compares the standard AVR with the transapical and the transfemoral TAVI.
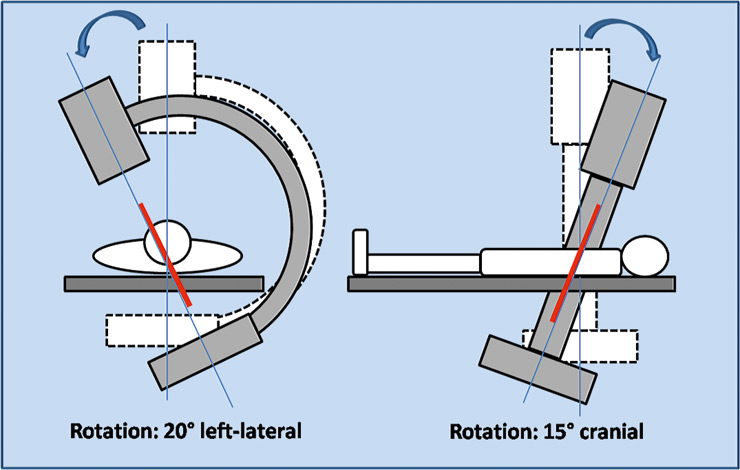
Figure 8
A schematic view of the fluoroscopy orientation in the operating room. The left-lateral and the cranial orientations are calculated preoperatively using CT-scan images.
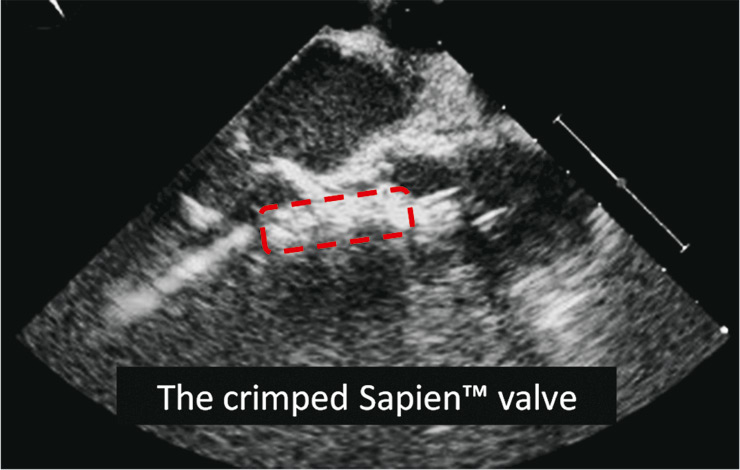
Figure 9
Image from the intraoperative transoesophageal echocardiogramme (TEE) showing the crimped Edwards SapienTM THV during the positioning in the ideal landing zone. Landmarks such as the hinge point of the aortic valve leaflets and the anterior mitral valve leaflet are identified.
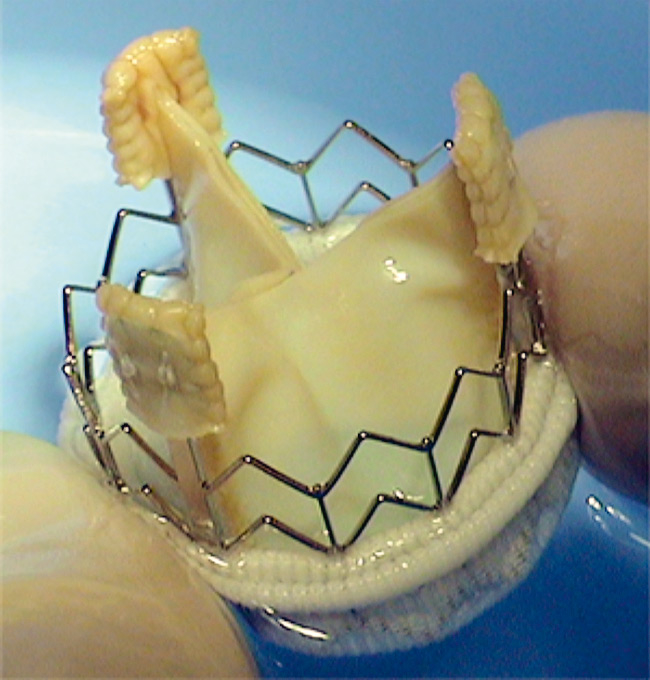
Figure 10
The ATS 3F® sutureless stent-valve.
This interesting trial has not yet been developed but, during the last four years, some articles reporting cohorts of TAVI patients treated in Europe and Canada have been published and the main contents are summarised in table 1 [44–52].
What is clear and evident is that the two populations of TF and TA patients are not comparable. In fact, the logistic Euroscore is usually 1/3 higher in the TA groups according to the fact that these groups suffer from a higher incidence of vascular disease. Concerning hospital mortality (30 days), the results show that the TA groups have a higher operative mortality compared to the TF groups, whereas the TF groups have more incidence of postoperative stroke. Another clear finding is that the TF procedures performed using the Core Valve® system have a higher incidence of pace maker implantation.
As an example of a transcatheter platform, we can share our clinical experience: we started in 2002 with animal experiments [19, 20, 22–24], and we continued in 2006 with the first transapical stented valve (ATS 3F) implanted into a human in Switzerland (fig. 10).
With regards to the Edwards SapienTM THV, we launched TA implants very early in 2008 and, during a period of 18 months, 40 transapical TAVI and 2 transfemoral cases (TF is still performed under proctor supervision) were performed. The mean age of the population was 81 ± 8 years, the mean logistic Euroscore was 35 ± 14.5% (range 14–75%), and there were 24 females (60%). Special indications included: previous thoracic radiotherapy (2 patients), porcelain aorta (3) and liver cirrhosis with oesophageal varices (CHILD B classification) in 2 patients. We also performed two VinV procedures: a 23 mm SapienTMin 2 degenerated 23 mm bio-prosthesis (a 7 year old Mitroflow and a 7 year old Edwards Perimount). Three patients were in a critical preoperative state, three had chronic renal failure with a pre-dialysis state (blood creatinine level chronically above 230, 250 and 300 µg/dl, respectively), 15 (37.5%) had chronic pulmonary disease (COPD), 30 (75%) had a peripheral vascular disease (6 patients underwent previous vascular surgery), 21 (52.5%) suffered from coronary disease (7 patients had previous surgery and 4 had coronary angioplasty/stenting), and 15 suffered from chronic renal insufficiency (the mean preoperative blood creatinine and urea levels were 108 ± 70 µg/dl and 9.2 ± 5.6 mmol/l, respectively). The mean ejection fraction was 53 ± 12%. The procedural success rate was 97.5% and we implanted a 26 mm SapienTMTHV 23 times and a 23 mm SapienTM THV 17 times. The 30-day mortality was 7.5% (3 patients died from severe right ventricular dysfunction, intra-procedural left-ventricular free wall rupture and late bilateral pneumonia) and we did not have patients with postoperative myocardial infarction, acute renal failure (the total mean postoperative blood creatinine and urea levels were 91.7 ± 60 µg/dl and 7.8 ± 3.6 mmol/l, respectively), and atrio-ventricular block. The post-procedural stroke rate was 0% but, unfortunately, a left hemiplegia occurred in an old lady at postoperative day 9, despite a therapy with aspirin (100 mg) and warfarin. The mean operative time was 108 ± 34 minutes and the postoperative recovery required a mean intensive care unit stay of 2.2 ± 3.5 days (median: 1 day) and a mean hospital stay of 15 ± 9 days (median: 12.5 days). A total of 34 patients were rapidly extubated and, among them, 8 patients were extubated in the operating theatre. The postoperative haemodynamic showed a mean trans-valvular gradient of 15 ± 9 (max) and 8.7 ± 5 mm Hg (mean), with 7 patients carrying a trivial (1+/4) paravalvular leak and 3 patients a mild (2+/4) paravalvular leak.
To summarise, our pioneering experience on TAVI is in line with the most-recent reports present in the literature (see the comparison in table 1). In particular, all centres who are familiar with this minimally invasive technique report a mean logistic Euroscore ranging between 27 and 35%, a procedural success rate mostly above 95% and a mean 30-day mortality rate between 7.5 and 17.5%. If the percutaneous transfemoral TAVI (with the two systems available at the moment) is taken into consideration, the published results show a lower mean logistic Euroscore ranging between 23 and 25.5%, a procedural success rate always above 90%, and a 30-day mortality rate around 7–10.8% [43–51].
It is remarkable that both the transapical and the transfemoral TAVI have a physiological learning curve and, in some recently published reports, the cohorts of patients are split into two groups: an earlier group (20–25 patients) that includes the learning curve phase, and a group of patients operated on more recently [46, 48]. In these papers, authors have concluded that the inclusion criteria did not change over the time and the mean logistic Euroscore remained unchanged or even increased in the most recent series, whereas the procedural successful rate, together with the 30-day mortality rate, ameliorated sensibly after the learning curve period. In our experience, we can confirm this downward trend for hospital mortality after the physiological learning curve phase, and we can also confirm that the surgical risk is higher in patients that were addressed most recently to transapical TAVI: in the first 20 cases performed in our centre, we had an observed 30-day mortality of 10% with a predicted operative mortality of 29% (by logistic Euroscore), whereas, within the second 20 cases performed, we had an amelioration in the observed 30-day mortality (5%) despite a worst predicted surgical risk of 40% (by log. Euroscore).
Nevertheless, more single- and multicentre reports with longer follow-ups are required to confirm the good trend of this promising transcatheter valve technology.
| Table 1Comparison between the most recently published series of transapical and transfemoral transcatheter aortic valve implantations using the Edwards Sapien™ THV and the Medtronic CoreValve®. | |||||||||
| Study/Author | Patients(n) | Meanage (Y) | logisticES (%) | STSscore (%) | Success(%) | 30-daymortality(%) | PMimplantation(%) | Strokerate (%) | Postop. renalfailure (%) |
| Edwards Sapien™: transapical | |||||||||
| Ferrari (Lausanne, Switzerland) | 40 | 81 | 35 | – | 97.5 | 7.5 | 2.5 | ||
| Ye (Vancouver, Canada) [44] | 71 | 80 | 34.5 | 12.1 | 95.8 | 16.9 | 8.5 | 1.4 | data not available |
| Rodés-Cabau (multicentric, Canada) [45] | 177 | 80 | – | 10.5 | 96.1 | 11.3 | 6.2 | 1.7 | haemodialysis: 3.4 |
| Walther (multicentric, Germany) [46] | 59 | 81 | 27 | – | 90 | 13.6 | data not available | 3.4 | haemodialysis: 13.5 |
| Wendt (Essen, Germany) [47] | 40 | 82 | 41.6 | 16.5 | 97.5 | 17.5 | data not available | 12.5 | |
| Himbert (Paris, France) [48] | 24 | 82 | 28 | 18 | 100 | 16 | 4 | data not available | |
| mean | 81.00 | 33.22 | 14.28 | 96.15 | 13.80 | 4.68 | 1.50 | ||
| Edwards Sapien™: transfemoral | |||||||||
| Rodés-Cabau (multicentric, Canada) [45] | 162 | 83 | − | 9 | 90.5 | 9.5 | 3.6 | 3 | haemodialysis: 1.8 |
| Himbert (Paris, France) [48] | 51 | 82 | 25 | 15 | 90 | 8 | 6 | 6 | data not available |
| Webb (Vancouver, Canada) [49] | 113 | 85 | 25 | 8.7 | 94 | 8 | 4.4 | 5 | 4.4 |
| mean | 83.33 | 25.00 | 10.90 | 91.50 | 8.50 | 4.67 | 4.67 | ||
| CoreValve® 18F: transfemoral | |||||||||
| Grube (Siegburg, Germany) [50] | 102 | 82 | 24.5 | 8.6 | 91.2 | 10.8 | 33.3 | 2.9 | data not available |
| Tamburino (Catania, Italy) [51] | 30 | 82 | 25.3 | − | 97 | 6.7 | 20 | 3 | |
| Piazza (multricentric) [52] | 646 | 81 | 23 | − | 97.2 | 8 | 9.3 | 1.9 | data not available |
| mean | 81.67 | 24.27 | 8.60 | 95.13 | 8.50 | 20.87 | 2.60 | ||
| Procedural success: at least one stent-valve in place, no need for full sternotomy and patient leaving alive the OR/hybrid room/cath. lab.Data not available: the variable does not appear in the manuscript, but it does not mean that the event did not happen.ES: Euroscore. | |||||||||
After the CE-mark approval, the transapical and the transfemoral TAVI performed with the Sapien™THV and the CoreValve® have become routine procedures in selected cardiac centres, allowing further hospitals to begin their own transcatheter valve programme soon. Therefore, we will probably face a constant increase in stent-valve implantations with great benefits in terms of wider clinical trials and new technical/research investments.

Figure 11
The stent-valve by Symetis SA, Lausanne, Switzerland.
Concerning the surgical indications, the high-risk elderly patient with severe and symptomatic AS will remain the ideal candidate for a while. However, as soon as the overall quality and longevity of these stent-valves has been proven by long-term follow-ups, patients carrying a lower predictable operative risk profile might be considered as candidates for TAVI. With regards to the technical developments, new futuristic toolsthat offer the opportunity to completely ablate, percutaneously, the diseased aortic valve leaflets before the stent-valve implantation are under development but, to the best of our knowledge, only few bench tests have been performed so far [53–56]. The stent-valve evolution will also provide new stent-valves for transapical and transfemoral applications, such as the Symetis stent-valve (Symetis SA, Lausanne, Switzerland) (fig. 11), and the Ventor Embracer™ stent-valve (Medtronic, Minneapolis, MN) for an exclusive transapical use, or the JenaClip™ stent-valve (JenaValve Technology, Munich, Germany) for both transapical and transfemoral applications [57, 58]. These new stented valves will bring innovations such as a greater variety of sizes, expected durability (due to the employed biological tissues and their treatment), simplicity in use (in particular for the valve positioning and implantation, and for the delivery system), and some interesting characteristics such as the self-orientation (the stent-valve leaflets will fit within the native aortic valve leaflets) and the self expandability without need for rapid cardiac pacing.
Another evolution plan is the required vessel caliber for peripheral applications. In fact, the minimal vessel diameter required for the delivery system insertion during transfemoral and trans-subclavian TAVI procedures will continue to decrease as long as smaller delivery systems and smaller crimped stent-valves are developed by the industry. However, the extreme downsizing of the crimped stent-valve can compromise the quality of the metallic stent (which is crimped under high compressive forces) and the integrity of the leaflets (the thickness of the tissue employed to create valve leaflets is artificially lowered to minimise the size of the crimped valve).
Regarding the transapical minimal invasiveness and the latest alternative access point, our group has already proposed, in an animal model, the use of “natural orifices”, such as the umbilicus, for transapical or transventricular stent-valve implantations, where the delivery system is introduced into the human body through a natural skin access point [59].
More realistically, the fully percutaneous transfemoral stent-valve implantation already exists thanks to the downsizing of the delivery system and the flexibility of the crimped stent-valve. However, a future perspective would be the transapical TAVI performed under thoracoscopy, and the development of new ventricular closure devices appears to be the most important step for a fully percutaneous transapical procedure [60].
Transapical and transfemoral TAVI are the most recent established techniques for the treatment of isolated high-risk AS in the elderly and the latest publications have shown satisfactory hospital outcomes and mid-term results (up to two years). As a consequence, it can be presumed that the indications for this disruptive procedure will expand towards younger and less risky candidates as soon as ongoing clinical trials provide supporting long-term results.
In the near future, the evolving technology will provide new stent-valves with ameliorated designs and performance, and new smaller delivery systems allowing for less invasive procedures and a lower risk of vascular and cardiac injuries.
It should not be forgetten that the cooperation between cardiac surgeons, cardiologists, anaesthesiologists, radiologists and technicians (the so-called teamwork or team approach) is one of the most important parts of the entire TAVI process and its focus is the patient’s physical and mental wellness. In conclusion, to continue to guarantee the extraordinary and, maybe, unexpected success of this new minimally invasive procedure, the team approach should remain a key-point for all teams who intend to create a new TAVI platform soon.
1 Lester SJ, Heilbron B, Gin K, Dodek A, Jue J. The natural history and rate of progression of aortic stenosis. Chest. 1998;113:1109–14.
2 Aronow WS, Kronzon I. Prevalence and severity of valvular aortic stenosis determined by Doppler echocardiography and its association with echocardiographic and electrocardiographic left ventricular hypertrophy and physical signs of aortic stenosis in elderly patients. Am J Cardiol. 1991;67:776–7.
3 Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005–11.
4 Ross J Jr, Braunwald E. Aortic stenosis. Circulation. 1968;38:61–7.
5 Otto CM, Mickel MC, Kennedy JW, Alderman EL, Bashore TM, Block PC, Brinker JA, Diver D, Ferguson J, Holmes DR Jr. Three-year outcome after balloon aortic valvuloplasty. Insights into prognosis of valvular aortic stenosis. Circulation. 1994;89:642–50.
6 Varadarajan P, Kapoor N, Bansal RC, Pai RG. Clinical profile and natural history of 453 nonsurgically managed patients with severe aortic stenosis. Ann Thorac Surg. 2006;82:2111–5.
7 Otto CM. Timing of aortic valve surgery. Heart. 2000;84:211–8.
8 Kvidal P, Bergström R, Hörte LG, Ståhle E. Observed and relative survival after aortic valve replacement. J Am Coll Cardiol. 2000;35:747–56.
9 Schwarz F, Baumann P, Manthey J, Hoffmann M, Schuler G, Mehmel HC, Schmitz W, Kübler W. The effect of aortic valve replacement on survival. Circulation. 1982;66:1105–10.
10 Bonow RO, Carabello BA, Kanu C, de Leon AC Jr, Faxon DP, Freed MD, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation. 2006;114:e84–231.
11 Ferrari E, Tozzi P, Hurni M, Ruchat P, Stumpe F, von Segesser LK. Primary isolated aortic valve surgery in octogenarians. Eur J Cardiothorac Surg. 2010. (paper in press) PMID: 20304666.
12 Asimakopoulos G, Edwards MB, Taylor KM. Aortic valve replacement in patients 80 years of age and older: survival and cause of death based on 1100 cases: collective results from the UK valve registry. Circulation. 1997;96:3403–8.
13 Sundt TM, Bailey MS, Moon MR, Mendeloff EN, Huddleston CB, Pasque MK, Barner HB, Gay WA Jr. Quality of life after aortic valve replacement at the age of >80 years. Circulation. 2000;102:III70–4.
14 Chukwuemeka A, Borger MA, Ivanov J, Armstrong S, Feindel CM, David TE. Valve surgery in octogenarians: a safe option with good medium-term results. J Heart Valve Dis. 2006;15:191–6.
15 Melby SJ, Zierer A, Kaiser SP, Guthrie TJ, Keune JD, Schuessler RB, Pasque MK, Lawton JS, Moazami N, Moon MR, Damiano RJ Jr. Aortic valve replacement in octogenarians: risk factors for early and late mortality. Ann Thorac Surg. 2007;83:1651–6.
16 Kolh P, Kerzmann A, Honore C, Comte L, Limet R. Aortic valve surgery in octogenarians: predictive factors for operative and long-term results. Eur J Cardiothorac Surg. 2007;31:600–6.
17 Iung B, Cachier A, Baron G, Messika-Zeitoun D, Delahaye F, Tornos P, Gohlke-Bärwolf C, Boersma E, Ravaud P, Vahanian A. Decision-making in elderly patients with severe aortic stenosis: why are so many denied surgery? Eur Heart J. 2005;26:2714–20.
18 Cribier A, Eltchaninoff H, Bash A, Borenstein N, Tron C, Bauer F, Derumeaux G, Anselme F, Laborde F, Leon MB. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation. 2002;106:3006–8.
19 Zhou JQ, Corno AF, Huber CH, Tozzi P, von Segesser LK. Self-expandable valved stent of large size: off-bypass implantation in pulmonary position. Eur J Cardiothorac Surg. 2003;24:212–6.
20 Ma L, Tozzi P, Huber CH, Taub S, Gerelle G, von Segesser LK. Double-crowned valved stents for off-pump mitral valve replacement. Eur J Cardiothorac Surg. 2005;28:194–8.
21 Andersen HR, Knudsen LL, Hasenkam JM. Transluminal implantation of artificial heart valves. Description of a new expandable aortic valve and initial results with implantation by catheter technique in closed chest pigs. Eur Heart J. 1992;13: 704–8.
22 Corno AF, Zhou J, Tozzi P, von Segesser LK. Off-bypass implantation of a self-expandable valved stent between inferior vena cava and right atrium. Interact Cardiovasc Thorac Surg. 2003;2:166–9.
23 Huber CH, Marty B, von Segesser LK. Acceptance and introduction of disruptive technologies – simple steps to build a fully functional pulmonary valved stent. Interact Cardiovasc Thorac Surg. 2007;6:430–2.
24 Huber CH, Tozzi P, Corno AF, Marty B, Ruchat P, Gersbach P, Nasratulla M, von Segesser LK. Do valved stents compromise coronary flow? Eur J Cardiothorac Surg. 2004:754–9.
25 Boudjemline Y, Bonhoeffer P. Steps toward percutaneous aortic valve replacement. Circulation. 2002;105:775–8.
26 Cribier A, Eltchaninoff H, Tron C, Bash A, Borenstein N, Bauer F, Derumeaux G, Pontier G, Laborde F, Leon MB. Percutaneous artificial heart valves: from animal experimentation to the first human implantation in a case of calcified aortic stenosis. Arch Mal Coeur Vaiss. 2003;96:645–52.
27 Walther T, Dewey T, Borger MA, Kempfert J, Linke A, Becht R, Falk V, Schuler G, Mohr FW, Mack M. Transapical aortic valve implantation: step by step. Ann Thorac Surg. 2009;87:276–83.
28 Vahanian A, Alfieri OR, Al-Attar N, Antunes MJ, Bax J, Cormier B, et al. Transcatheter valve implantation for patients with aortic stenosis: a position statement from the European Association of Cardio-Thoracic Surgery (EACTS) and the European Society of Cardiology (ESC), in collaboration with the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur J Cardiothorac Surg. 2008;34:1–8.
29 Cribier A, Litzler PY, Eltchaninoff H, Godin M, Tron C, Bauer F, Bessou JP. Technique of transcatheter aortic valve implantation with the Edwards-Sapien heart valve using the transfemoral approach. Herz. 2009;34:347–56.
30 Zierer A, Wimmer-Greinecker G, Martens S, Moritz A, Doss M. The transapical approach for aortic valve implantation. J Thorac Cardiovasc Surg. 2008;136:948–53.
31 Al-Attar N, Ghodbane W, Himbert D, Rau C, Raffoul R, Messika-Zeitoun D, Brochet E, Vahanian A, Nataf P. Unexpected complications of transapical aortic valve implantation. Ann Thorac Surg. 2009;88:90–4.
32 Ferrari E, Locca D, Sulzer C, Marcucci C, Rizzo E, Tozzi P, von Segesser LK. Successful transapical aortic valve implantation in a congenital bicuspid aortic valve. Ann Thorac Surg. 2010 (in press).
33 Ferrari E, Marcucci C, Sulzer C, Rizzo E, von Segesser LK. No arterial access: a “blind flight” for a transapical aortic valve implantation (TAVI). J Heart Valve Dis. 2010;19:266–8.
34 Walther T, Kempfert J, Borger MA, Fassl J, Falk V, Blumenstein J, Dehdashtian M, Schuler G, Mohr FW. Human minimally invasive off-pump valve-in-a-valve implantation. Ann Thorac Surg. 2008;85:1072–3.
35 Ferrari E, Marcucci C, Sulzer C, von Segesser LK. Which available transapical transcatheter valve fits into degenerated aortic bioprostheses? Interact Cardiovasc Thorac Surg. 2010 (in press).
36 Nietlispach F, Leipsic J, Al-Bugami S, Masson JB, Carere RG, Webb JG. CT of the ilio-femoral arteries using direct aortic contrast injection: proof of feasibility in patients screened towards percutaneous aortic valve replacement. Swiss Med Wkly. 2009;139:458–62.
37 Strauch JT, Scherner MP, Haldenwang PL, Pfister R, Kuhn EW, Madershahian N, et al. Minimally invasive transapical aortic valve implantation and the risk of acute kidney injury. Ann Thorac Surg. 2010;89:465–70.
38 Aregger F, Wenaweser P, Hellige GJ, Kadner A, Carrel T, Windecker S, Frey FJ. Risk of acute kidney injury in patients with severe aortic valve stenosis undergoing transcatheter valve replacement. Nephrol Dial Transplant. 2009;24:2175–9.
39 Ferrari E, Sulzer C, Rizzo E, von Segesser LK, A fully echo-guided trans-apical aortic valve implantation, Eur J Cardiothorac Surg. 2009;36:938–40.
40 Ferrari E, Marcucci C, Di Bernardo S, von Segesser LK. Feasibility of transapical aortic valve implantation guided by intracardiac ultrasound without angiography. J Thorac Cardiovasc Surg. 2010 (in press).
41 Ferrari E, Sulzer C, Marcucci C, Rizzo E, Tozzi P, von Segesser LK. Transapical aortic valve implantation without angiography – proof of concept. Ann Thorac Surg. 2010;89(6):1925–32.
42 von Segesser LK, Marty B, Ruchat P, Bogen M, Gallino A. Routine use of intravascular ultrasound for endovascular aneurysm repair: angiography is not necessary. Eur J Vasc Endovasc Surg. 2002;23:537–42.
43 Moss RR, Ivens E, Pasupati S, Humphries K, Thompson CR, Munt B, Sinhal A, Webb JG. Role of echocardiography in percutaneous aortic valve implantation. JACC Cardiovasc Imaging. 2008;1:15–24.
44 Ye J, Cheung A, Lichtenstein SV, Nietlispach F, Albugami S, Masson JB, Thompson CR, Munt B, Moss R, Carere RG, Jamieson WR, Webb JG. Transapical transcatheter aortic valve implantation: follow-up to 3 years. J Thorac Cardiovasc Surg. 2010;139:1107–13.
45 Rodés-Cabau J, Webb JG, Cheung A, Ye J, Dumont E, Feindel CM, et al. Transcatheter aortic valve implantation for the treatment of severe symptomatic aortic stenosis in patients at very high or prohibitive surgical risk: acute and late outcomes of the multicenter Canadian experience. J Am Coll Cardiol. 2010;55:1080–90.
46 Walther T, Simon P, Dewey T, Wimmer-Greinecker G, Falk V, Kasimir MT, et al. Transapical minimally invasive aortic valve implantation: multicenter experience. Circulation. 2007;116:I240–5.
47 Wendt D, Eggebrecht H, Kahlert P, Heine T, Kottenberg E, Massoudy P, et al. Experience and learning curve with transapical aortic valve implantation. Herz. 2009;34:388–97.
48 Himbert D, Descoutures F, Al-Attar N, Iung B, Ducrocq G, Détaint D, et al. Results of transfemoral or transapical aortic valve implantation following a uniform assessment in high-risk patients with aortic stenosis. J Am Coll Cardiol. 2009;54:303–11.
49 Webb JG, Altwegg L, Boone RH, Cheung A, Ye J, Lichtenstein S, et al. Transcatheter aortic valve implantation: impact on clinical and valve-related outcomes. Circulation. 2009;119:3009–16.
50 Grube E, Buellesfeld L, Mueller R, Sauren B, Zickmann B, Nair D, et al. Progress and current status of percutaneous aortic valve replacement: results of three device generations of the CoreValve Revalving system. Circ Cardiovasc Interv. 2008;1:167–75.
51 Tamburino C, Capodanno D, Mulè M, Scarabelli M, Cammalleri V, Barbanti M, Calafiore A, Ussia G. Procedural success and 30-day clinical outcomes after percutaneous aortic valve replacement using current third-generation self-expanding CoreValve prosthesis. J Invasive Cardiol. 2009;21:93–8.
52 Piazza N, Grube E, Gerckens U, den Heijer P, Linke A, Luha O, et al. Procedural and 30-day outcomes following transcatheter aortic valve implantation using the third generation (18 Fr) corevalve revalving system: results from the multicentre, expanded evaluation registry 1-year following CE mark approval. EuroIntervention. 2008;4:242–9.
53 Vandenberghe S, Salizzoni S, Bajona P, Zehr KJ, Speziali G. In vitro testing of a temporary catheter-based aortic “parachute” valve. ASAIO J. 2008;54:574–7.
54 Salizzoni S, Bajona P, Zehr KJ, Anderson WD, Vandenberghe S, Speziali G. Transapical off-pump removal of the native aortic valve: a proof-of-concept animal study. J Thorac Cardiovasc Surg. 2009;138:468–73.
55 Quaden R, Attmann T, Schünke M, Theisen-Kunde D, Cramer J, Lutter G. Percutaneous aortic valve replacement: endovascular resection of human aortic valve in situ. J Thorac Cardiovasc Surg. 2008;135:1081–6.
56 Quaden R, Attmann T, Boening A, Cremer J, Lutter G. Percutaneous aortic valve replacement: resection before implantation. Eur J Cardiothorac Surg. 2005;27:836–40.
57 Vergnat M, Henaine R, Kalejs M, Bommeli S, Ferrari E, Obadia JF, Von Segesser LK. A new self-expanding aortic stent valve with annular fixation: in vitro haemodynamic assessment. Eur J Cardiothorac Surg. 2009;35:970–6.
58 Lauten A, Ferrari M, Petri A, Ensminger SM, Gummert JF, Boudjemline Y, et al. Experimental evaluation of the jenaclip transcatheter aortic valve. Catheter Cardiovasc Interv. 2009;74:514–9.
59 Kalejs M, Ferrari E, von Segesser LK. Towards no-scar cardiac surgery – minimally invasive access through umbilicus for aortic valve replacement. Eur J Cardiothorac Surg. 2009;36:773–5.
60 Tozzi P, Pawelec-Wojtalic M, Bukowska D, Ferrari E, Siniscalchi G, von Segesser LK. Endoscopic access closure for direct implantation of valved stents. Swiss Med Wkly. 2007;137:182–4.
No funding; no competing interests.