The impact of infections on critically ill acute heart failure patients: an observational study
DOI: https://doi.org/10.4414/smw.2010.13125
M
Streit, F
Businger, ER
Schmid, F
Follath
Summary
BACKGROUND: Hospitalised patients with acute heart failure (AHF) suffer from a high morbidity and mortality, which might, at least partly, be influenced by concomitant infections. The aim of this observational study was to investigate the impact of infections on the clinical course of critically ill patients with AHF, both present on intensive care unit (ICU) admission and acquired during the ICU stay.
METHODS: From 178 consecutive AHF patients, 76 were treated medically and 21 required emergency cardiac surgery. The remaining 81 patients, who underwent elective cardiac surgery, were excluded from the assessment of infections on ICU admission, but were included in the analysis of nosocomial infections during the ICU stay.
RESULTS: A total of 16% of patients (16/97) had infections on ICU admission. These patients had longer ICU (6 vs. 3 days, p = 0.04) and hospital (19 vs. 11 days, p = 0.04) stays than patients without infections. Although not statistically significant, there was a trend for increased mortality at 30 days (44% vs. 24%, p = 0.13) and 6 months (57% vs. 31%, p = 0.13) in AHF patients with infections on ICU admission. Infection complications during the ICU stay occurred in 17% (30/178) of AHF patients and significantly increased their mortality at 30 days (33% vs. 14%, p = 0.02) and 6 months (41% vs. 18%, p = 0.02).
CONCLUSIONS: In this observational study, infections present on ICU admission or occurring during the ICU stay had a negative impact on the morbidity and mortality of critically ill patients with AHF. Future studies are needed to gain a better understanding of the interactions between heart failure and infections, as a better knowledge of this field may have an important therapeutic potential.
Background
Hospitalised patients with acute heart failure (AHF) suffer from a high morbidity and mortality, which might, at least partly, be influenced by concomitant infections [1–5]. A Finnish multi-centre study investigating 620 hospitalised patients with AHF reported infections in 24% of all patients [3]. A French multi-centre study performed with 581 Intensive Care Unit (ICU) patients with AHF revealed an infection rate of 20% [5]. In the EuroHeart Failure Survey II, which described characteristics and outcome of 3580 AHF patients, infections were present in 18% of hospital admissions [1]. Despite this high frequency, the types of infections and their impact on the patients’ hospital course have not yet been investigated. In addition, some AHF patients will develop nosocomial infections during their hospital stay, which might complicate the hospital course and worsen the short and long-term outcomes. While it is generally recognised that nosocomial infections such as ventilator-associated pneumonia are common and associated with substantial morbidity, an increase in mortality and excess costs [6], no specific information is available regarding this issue in this particular population.
For the present study, consecutive AHF patients hospitalised in our medical and cardio-surgical ICUs were identified. In this population, we investigated the frequency and the types of infections, which were present on ICU admission. In addition, we studied nosocomial infections, which these AHF patients acquired during their ICU stay. The aim was to explore the impact of such infections on organ functions, hospital course and long-term mortality.
Methods
Study population
Between October 2004 and January 2005, a prospective observational study was conducted, using an identical evaluation protocol, at the 12-bed medical ICU and at the 10-bed cardio-surgical ICU of the University Hospital Zurich, Switzerland. Consecutive patients admitted to the two ICUs were screened for the diagnosis of AHF. Patients were included in the analysis only once, even when they were readmitted during the observation period. The patient characteristics have been presented earlier [7]. This study represents an additional analysis of this data set focusing on the impact of infections on AHF patients. The Institutional Review Board waived the need for written informed consent in this particular condition.
Diagnosis of acute heart failure
History, clinical findings, laboratory results and imaging were integrated by the clinicians in charge, which finally made the diagnosis of AHF. The patients were then classified in one of the following conditions described in the AHF guidelines published by the European Society of Cardiology [8]:
– Cardiogenic shock: Low cardiac output (<2.2 l/min/m2) with or without low blood pressure after correction of preload, with evidence of tissue hypoperfusion or organ dysfunction. Tissue hypoperfusion was diagnosed in the presence of cold, mottled skin, lactate levels >2.0 mmol/l, a central venous oxygen saturation < 65% or a mixed venous oxygen saturation <60%.
– Post-operative cardiac stunning: Transient and reversible impairment of contractility after cardiac surgery, resulting in low cardiac output after correction of preload, with need for inotropic support in order to prevent tissue hypoperfusion and organ dysfunction
– Pulmonary oedema: Heart failure induced severe respiratory distress, accompanied by crackles over both lungs and bilateral infiltrates in the chest X-ray
– Congestive heart failure: All other conditions with an underlying heart disease and AHF signs and symptoms, which do not fulfil the diagnostic criteria for the AHF syndromes described above. Clinical signs and symptoms included dyspnea NYHA III or IV, orthopnea, rales or elevated jugular venous pressure [2].
During the study period, six patients with sepsis-induced cardiac depression were identified, who suffered from a sepsis syndrome and therefore required inotropic support. However, these patients were not included in the present study. It is important to note that we did not diagnose AHF by elevated natriuretic peptide levels alone as it has been shown previously that these markers probably have a limited specificity in critically ill patients [9, 10]. AHF patients were divided into surgical and medical patients, regarding whether they had cardiac surgery preceding or during the ICU stay or not. Patients were further divided with regard to whether they were admitted electively to the ICU after planned cardiac surgery or whether they required emergency ICU admission.
Diagnosis of infections
Samples for microbiological analysis were collected if the clinicians suspected an infection. Microbiological specimens were analysed in the local microbiology department, and ambiguous cases were discussed with the infectious disease specialists. Infections were diagnosed in the presence of a positive culture from sterile body fluids or blood, or with evidence of a perforated bowel. As recommended in current guidelines, pneumonia was diagnosed in the presence of a typical infiltrate in the chest X-ray and purulent sputum [11, 12]. Only one patient, who presented with an infection on ICU admission, developed a nosocomial infection in the course of the ICU stay. Importantly, only the first episode of nosocomial infections was recorded.
Clinical and laboratory data
Renal dysfunction was defined as creatinine levels >177 µmol/l (>2 mg/dl) [1]. The upper detection limit for N- terminal pro-B-type natriuretic peptide (NTproBNP) was 70000 ng/l, and values higher than this were included in the analysis and labelled 70000 ng/l. The C-reactive protein (CRP) and procalcitonin normal values for healthy individuals at our institution are <5 mg/l and <0.1 µg/l, respectively. Clinical and laboratory data for the Simplified Acute Physiology Score II (SAPS II) were collected as the worst value within 24 hours after ICU-admission [13].
Statistics
Results are given as medians (range) or numbers (percentages). The chi-square test was used to compare categorical variables. We performed the group comparisons for continuous variables with the Mann Whitney U test, because a normal distribution of the clinical (e.g., ICU length of stay) and laboratory values (e.g., creatinine) was not assumed. Survival estimates for all cause mortality were calculated with the method of Kaplan and Meier (day 0 = ICU admission). The Log Rank test was used to compare the mortality of the subgroups. All testing was two-tailed, and a p-value <0.05 was considered significant. Analyses were performed with the use of SPSS 11.0.4 for Mac OS X.
Results
Patient population
During the 3-month observation period, 355 consecutive ICU-admissions were screened for the diagnosis of AHF. A total of 178 (50%) patients fulfilled the diagnostic criteria for AHF and were included in the study. Baseline characteristics of the AHF population are given in table 1. Left ventricular ejection fraction, which was assessed in 87 (49%) AHF patients during their ICU-stay, was 40 (10–75)%. Additional details of the population have been published previously [7].
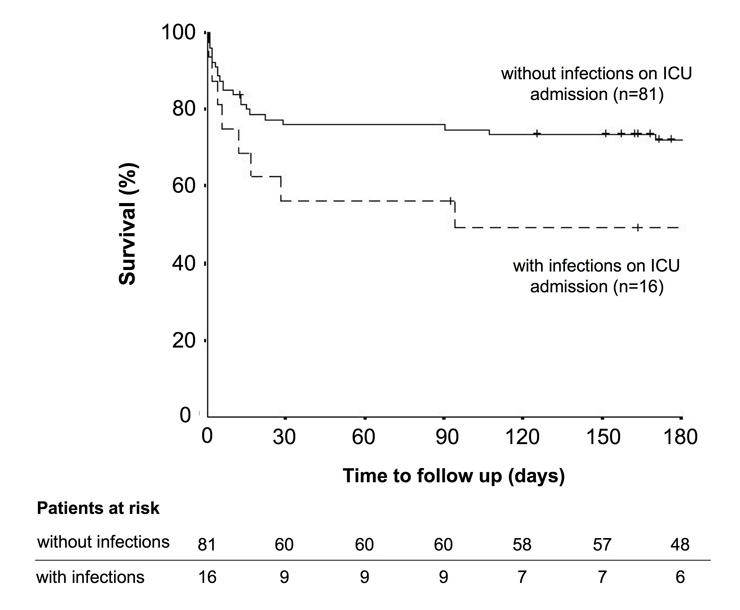
Figure 1
The curves show survival rates over time for acute heart failure patients with (dashed line, n = 16) and without (straight line, n = 81) infections on ICU admission (Log rank p = 0.08). The small vertical lines indicate the time points when a patient had his latest follow up. In addition, the numbers of patients at risk are tabulated for the main intervals of the rate of survival curve.
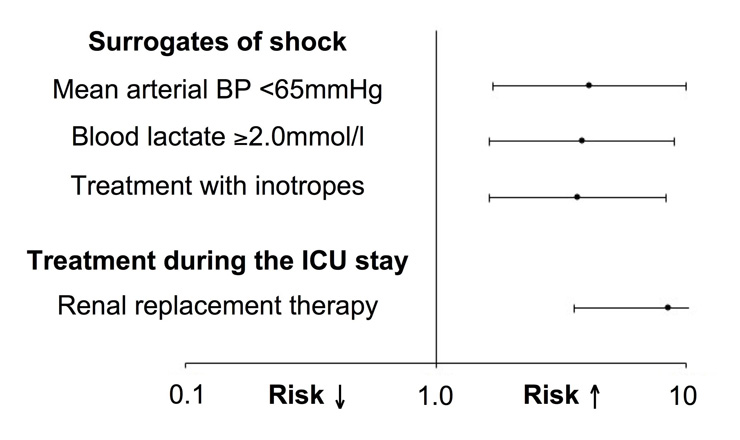
Figure 2
The figure shows risk factors associated with nosocomial infections during the ICU stay. Bars represent odd ratios with 95% confidence intervals. A value >1 indicates an increased risk for the occurrence of a nosocomial infection during the ICU stay. BP = blood pressure: ICU = intensive care unit.
Infections on ICU admission
As none of the 81 elective cardiac surgery patients showed any evidence of infection on ICU admission, this group was excluded from this particular analysis. Of the remaining 97 patients, infections on ICU admission were noted in 16 (17%) patients (16% in medical vs. 19% in emergency surgery patients; p = 0.74). Infections included pneumonia (n = 8), endocarditis (n = 4), cholecytitis (n = 1), diverticulitis (n = 1), urinary tract infection (n = 1) and skin infection (n = 1). Baseline characteristics on ICU admission are summarised in table 2. Age, gender and co-morbidities were not significantly different between patients with and without infections. Coronary artery disease (44% vs. 73%, p = 0.04) and myocardial infarction (19% vs. 56%, p = 0.01) were less common in infected patients compared to non-infected patients. SAPS II values were similar in AHF patients with and without infections on ICU admission (35 (15–84) vs. 24 (6–74), p = 0.46). Therapies and length of stay are summarised in table 3. Although not statistically significant, there was a trend for increased mortality at 30 days (44% vs. 24%, p = 0.13) and 6 months (57% vs. 31%, p = 0.13) in AHF patients with infections on ICU admission. Kaplan Meier survival curves are displayed in figure 1 and show a clear trend (p = 0.08) towards a worse outcome in AHF patients with infections on ICU-admission, compared to AHF patients without infections on ICU-admission.
Nosocomial infections during the ICU stay
Infectious complications occurred in 30 out of all 178 (17%) AHF patients (18% in medical, 24% in emergency surgery and 14% in elective surgery patients; p = 0.45) during their ICU stay. Nosocomial infections included pneumonias (n = 13), catheter related infections (n = 7), peritonitis secondary to bowel perforation (n = 4), urinary tract infections (n = 3), blood-stream infections of unknown origin (n = 2), and clostridium difficile colitis (n = 1). Patient characteristics for AHF patients with and without infectious complications are summarised in table 4. Age, gender and underlying heart diseases did not differ between patients with and without nosocomial infections. The frequencies of co-morbidities such as diabetes mellitus, chronic lung disease, malignancy, transplantation and alcoholism were not significantly different between the two groups either. However, SAPS II values were higher on ICU admission in patients who developed infectious complications during the ICU stay (33 (18–74) vs. 23 (6–67), p <0.01). Although the initial frequency of invasive ventilation was similar in both groups, patients with subsequent nosocomial infection were ventilated for longer than patients without nosocomial infections (40 (8–832) vs. 11 (1–119) hours, p <0.01, n = 77). The presence of cardiogenic shock on ICU admission and the need for renal replacement therapy increased the risk for the development of infections during the ICU stay (fig. 2). Left ventricular ejection fraction during the ICU stay was lower in patients who got infected, compared to patients who did not (35 (10–65) vs. 45 (15–75) %, p <0.01). Patients with nosocomial infections during their hospitalisation in ICU stayed longer in the ICU (12 (3–83) vs. 3 (1–38) days, p <0.01) and in hospital (16 (3–170) vs. 11 (2–78) days, p <0.01) than patients without infection complications. Nosocomial infections during the ICU stay significantly increased the mortality at 30 days (33% vs. 14%, p = 0.02) and 6 months (41% vs. 18%, p = 0.02). Survival curves are displayed in figure 3 and confirm the significantly worse outcome in AHF patients with nosocomial infections compared to AHF patients without infection complications.
|
Table 1: Baseline characteristics of acute heart failure patients on intensive care unit admission. |
| |
Emergency ICU
admissions
(n = 97) |
Elective ICU
admissions
(n = 81) |
p-value |
| Patient characteristics:
Age (years) – median (range)
Male gender |
66 (28-96)
69 (71%) |
67 (18-85)
51 (63%) |
0.99
0.27 |
| Type of patient:
Medical
Cardio-surgical |
76 (78%)
21 (22%) |
0 (0.0%)
81 (100%) |
<0.01
|
| Clinical presentation:
Cardiogenic shock
Pulmonary oedema
Congestive heart failure
Postoperative cardiac stunning |
32 (33%)
26 (27%)
38 (39%)
1 (1.0%) |
26 (32%)
1 (1.2%)
17 (21%)
37 (46%) |
1.0
<0.01
0.01
<0.01
|
| Underlying cardiac disease:
Coronary artery disease
Valve disease
Dilated cardiomyopathy |
66 (68%)
25 (26%)
9 (9.3%) |
52 (64%)
44 (54%)
8 (9.9%) |
0.64
<0.01
1.00 |
| Pre-existing diseases:
Diabetes mellitus
Chronic lung disease
Malignancy |
23 (24%)
10 (10%)
14 (14%) |
11 (14%)
17 (21%)
8 (9.9%) |
0.13
0.06
0.49 |
| Infections:
Present on ICU admission
Occurring during ICU stay |
16 (17%)
19 (20%) |
0 (0.0%)
11 (14%) |
<0.01
0.32 |
| Values are given as median (range) or number (%). ICU = intensive care unit. |
|
Table 2: Baseline characteristics of acute heart failure patients on intensive care unit admission. |
| |
AHF patients with infections
on ICU admission
(n = 16) |
AHF patients without infections
on ICU admission
(n = 81) |
p-value |
| Patient characteristics:
Age (years) – median (range)
Male gender |
61 (33–79)
12 (75%) |
66 (28–96)
57 (70%) |
0.50
1.00 |
| Type of patient:
Medical
Cardio-surgical |
12 (75%)
4 (25%) |
64 (79%)
17 (21%) |
0.74 |
| Clinical presentation:
Cardiogenic shock
Pulmonary oedema
Congestive heart failure
Postoperative cardiac stunning |
7 (44%)
5 (31%)
4 (25%)
0 (0.0%) |
25 (31%)
21 (26%)
34 (42%)
1 (1.2%) |
0.39
0.76
0.27
1.00 |
| Underlying cardiac disease:
Coronary artery disease
Valve disease
Dilated cardiomyopathy |
7 (44%)
8 (50%)
1 (6.3%) |
59 (73%)
17 (21%)
8 (9.9%) |
0.04
0.03
1.00 |
| Pre-existing diseases:
Diabetes mellitus
Chronic lung disease
Malignancy |
5 (31%)
3 (19%)
0 (0.0%) |
18 (22%)
7 (8.6%)
14 (17%) |
0.52
0.36
0.07 |
| Clinical findings:
Heart rate (1/min)
Mean arterial BP (mm Hg)
Temperature (°C) |
95 (72–170)
83 (42–120)
37.0 (36.1–39.6) |
85 (30–180)
80 (45–180)
36.8 (33.7–40.0) |
0.17
0.66
0.17 |
| Laboratory parameters:
Leukocyte count (1/µl)
Haemoglobin (g/l)
Troponin (µg/l) a
NT-proBNP (ng/l) b
C-reactive protein (mg/l) c
Procalcitonin (µg/l) d
Creatinine (µmol/l) |
10860 (4670–22060)
100 (75–197)
0.11 (0.01–3.5)
7476 (955–70000)
141 (21–365)
1.5 (0.07–22)
125 (61–386) |
10920 (3710–54790)
118 (55–175)
0.52 (0.01–27)
3454 (52–70000)
20 (1.0–316)
0.18 (0.06–7.67)
98 (57–2277) |
0.86
0.15
<0.01
0.04
<0.01
0.01
0.19 |
| Values are given as median (range). BP denotes blood pressure, NT-proBNP N-terminal pro-B-type natriuretic peptide. Values for calculations were available in a 91, b 80, c 90 and d 61 patients. AHF = acute heart failure; ICU = intensive care unit. |
|
Table 3: Parameters describing the hospital course of acute heart failure patients with and without infections on intensive care unit admission. |
| |
AHF patients with infections
on ICU admission
(n = 16) |
AHF patients without infections
on ICU admission
(n = 81) |
p-value |
| Therapies on ICU admission:
Vasopressors
Inotropes
Mechanical ventilation |
8 (50%)
4 (25%)
7 (44%) |
26 (32%)
23 (28%)
20 (25%) |
0.25
1.00
0.14 |
| Treatments during the
ICU stay:
Renal replacement therapy
Red blood cell transfusion
Administration of antibiotics |
4 (25%)
6 (38%)
16 (100%) |
18 (22%)
14 (17%)
30 (37%) |
0.75
0.09
<0.01
|
| Length of stay (days):
ICU
Hospital |
6 (2–38)
19 (2–54) |
3 (1–36)
11 (2–170) |
0.04
0.04
|
| Values are given as number (%) or median (range). AHF = acute heart failure; ICU = intensive care unit. |
|
Table 4: Patient characteristics of acute heart failure patients on ICU admission and during their ICU stay. |
| |
AHF patients with nosocomial infections during the ICU stay
(n = 30) |
AHF patients without nosocomial infections during the ICU stay
(n = 148) |
p-value |
| Patient characteristics:
Age (years) – median (range)
Male gender |
67 (31–85)
17 (57%) |
65 (18–96)
103 (70%) |
0.19
0.20 |
| Type of patient:
Medical
Emergency cardiac surgery
Elective cardiac surgery |
14 (47%)
5 (17%)
11 (37%) |
62 (42%)
16 (11%)
70 (47%) |
0.45 |
| Clinical presentation:
Cardiogenic shock
Pulmonary oedema
Congestive heart failure
Postoperative cardiac stunning |
20 (67%)
4 (13%)
3 (10%)
3 (10%) |
38 (26%)
23 (16%)
52 (35%)
35 (24%) |
<0.01
1.00
0.01
0.14 |
| Underlying cardiac disease:
Coronary artery disease
Valve disease
Dilated cardiomyopathy |
18 (60%)
10 (33%)
5 (17%) |
100 (68%)
59 (40%)
12 (8.1%) |
0.53
0.54
0.17 |
| Pre-existing diseases:
Diabetes mellitus
Chronic lung disease
Malignancy |
9 (30%)
2 (6.7%)
3 (10%) |
25 (17%)
25 (17%)
19 (13%) |
0.13
0.26
1.00 |
| Interventions during ICU:
Invasive ventilation
Renal replacement therapy |
19 (63%)
14 (47%) |
76 (52%)
13 (8.8%) |
0.32
<0.01
|
| Values are given as median (range). AHF = acute heart failure; ICU = intensive care unit. |
Discussion
In this study performed with consecutive medical and cardiac surgery patients with AHF, infections were diagnosed in 16% of all emergency ICU admissions. Infections present on admission prolonged the length of the ICU and hospital stay. Nosocomial infections acquired during the ICU stay occurred in 17% of all critically ill AHF-patients, prolonged the length of ICU and hospital stay, and had a negative impact on short and long-term mortality. Cardiogenic shock on admission, prolonged mechanical ventilation and renal replacement therapy were associated with the occurrence of nosocomial infections.
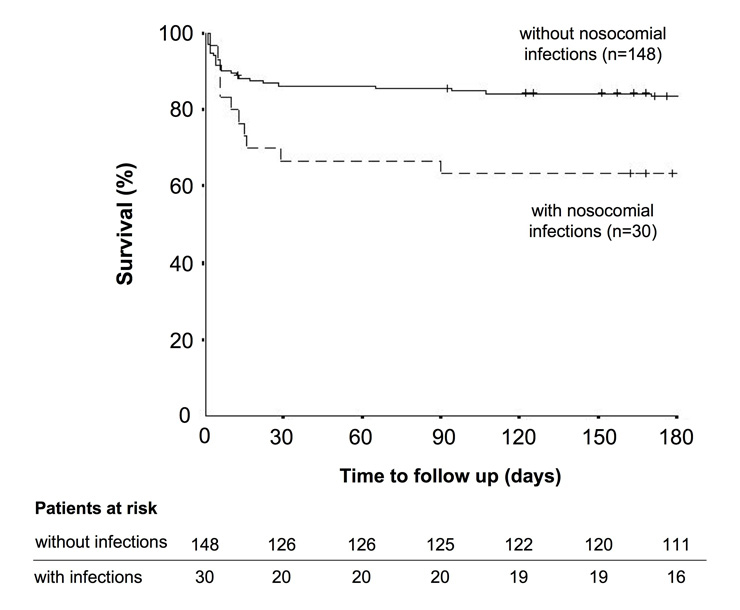
Figure 3
The curves show survival rates over time for acute heart failure patients with (dashed line, n = 30) and without (straight line, n = 148) infectious complications during their ICU stay (Log rank p = 0.02). The small vertical lines indicate the time points when a patient had his latest follow up. In addition, the numbers of patients at risk are tabulated for the main intervals of the rate of survival curve.
Infections on ICU admission
None of the AHF patients undergoing elective cardiac surgery were diagnosed with an infection on ICU admission. This is not surprising as elective surgery is usually postponed when infections are detected. Hence, elective surgery patients were excluded from this particular analysis, and infections on ICU admission were assessed in medical and emergency cardiac surgery patients only. The infection rate in this AHF population was 16%. This frequency is slightly lower as previously reported in epidemiological AHF studies [1, 3, 5]. Infections and pathogens identified in our study resembled the ones detected in a mixed cohort of hypotensive critically ill patients [14]. In our AHF patients with infections, there was a trend for higher SAPS II, more renal dysfunction and anaemia. These patient characteristics translated into a significantly longer ICU and hospital stay and a trend for a higher mortality.
Nosocomial infection during the ICU stay
In our AHF population, nosocomial infections occurred in 17% of all patients. Systolic LV function during the ICU stay was significantly lower in patients with infection complications, which can be explained by various mechanisms leading to sepsis-induced myocardial depression [15, 16]. In the present study, the occurrence of infection complications during the ICU stay was associated with a prolonged ICU and hospital stay. In addition, patients with nosocomial infections had a significantly higher short and long-term mortality.
Low arterial blood pressure, elevated blood lactate levels and the need for inotropes, all markers of shock, were associated with the development of nosocomial infections. A suggested mechanism is temporary mesenteric hypoperfusion with consecutive bacterial translocation from the bowel into the circulation [17]. A study, which looked at endotoxin levels in 17 patients with AHF, revealed higher levels in hepatic veins compared with the left ventricle [18]. Mesenteric ischemia can also lead directly to bowel necrosis and consecutive perforation. In the current study, 4 patients were diagnosed with this complication. Earlier, patient studies [14] and animal models [19] showed that the duration of arterial hypotension prior to antibiotic administration is a critical determinant of outcome.
Prolonged mechanical ventilation and renal replacement therapy were also associated with the presence of nosocomial infections. However, our data do not allow for any conclusion on whether nosocomial infections led to organ failure, or whether mechanical organ support promoted infection complications. Infections diagnosed and pathogens isolated in our study were comparable to other studies with critically ill patients [20]. The SHOCK trial included 297 patients with myocardial infarction complicated by cardiogenic shock, and 40 (13%) of them were diagnosed with a blood culture positive infection [21]. Like in our study, infection complications were associated with a longer duration of mechanical ventilation and a prolonged length of hospital stay. It is encouraging that the prevention of such acquired ICU infections has the potential for improving morbidity and mortality of critically ill AHF patients. Guidelines and recommendations have been published that address the problems of ventilator-associated pneumonia [11, 22] and catheter related blood stream infections [23, 24]. Whether selective decontamination of the digestive tract is a beneficial option for AHF patients must be studied in future trials [25–27].
Limitations of the study
The study population is inhomogeneous in terms of underlying heart diseases, clinical presentations and treatment modalities, because we investigated consecutive patients with AHF hospitalised in the medical or cardio-surgical ICU. Larger studies are needed to assess particular sub-groups. A problem is the differentiation between inflammation and infection, as the inflammatory cascade can be activated by non-infectious stimuli (e.g., ischemia-reperfusion injury) in heart failure patients [28–30]. We tried to keep this confounding factor to a minimum by applying stringent criteria for the diagnosis of infection. Apart from daily clinical investigations (including body temperature) and laboratory measurements (leukocyte count, CRP), any other screening measures were not applied for the detection of infections, which might be useful in future studies. Due to the design of our study, we cannot distinguish between cause and effect of our findings. For example, nosocomial infections were associated with a lower systolic function of the left ventricle. While there is strong evidence from animal and clinical studies suggesting that sepsis depresses cardiac function [15], it could also be argued that patients with low ejection fraction were more prone to infection complications. Another example is the relationship between nosocomial infections and the length of ICU stay. On the one hand, infection complications are likely to prolong the ICU course. However on the other hand, the risk of nosocomial infections will rise with prolonged organ dysfunctions requiring intensive care. More studies are needed to investigate these interactions, as they may have important therapeutic potential for the future.
Conclusions
In this observational study, infections were common in AHF patients admitted to the ICU. They contributed to organ dysfunction and prolonged the length of ICU and hospital stay. Nosocomial infections occurring during the ICU stay had a negative impact on short and long-term mortality of patients with AHF. Future studies are needed to gain a better understanding of the interactions between heart failure and infections as improved knowledge in this field may have important therapeutic consequences.
Authors’ contributions
AR designed the study, collected and analysed the data, and drafted the manuscript. FB and MS collected and analysed the data. ES, FF and MM designed the study, analysed the data and drafted the manuscript. All authors read and approved the final version of the manuscript.
Correspondence:
Dr. A. Rudiger
Medical Intensive Care Unit
University Hospital
Raemistrasse 100
CH-8091 Zurich
Switzerland
alain.rudiger@usz.ch
References
1 Nieminen MS, Brutsaert D, Dickstein K, Drexler H, Follath F, Harjola V-P, et al. EuroHeart Failure Survey II (EHFS II): a survey on hospitalized acute heart failure patients: description of population. Eur Heart J. 2006;27:2725–36.
2 Rudiger A, Harjola V-P, Müller A, Mattila E, Säila P, Nieminen M, et al. Acute heart failure: clinical presentation, one-year mortality and prognostic factors. Eur J Heart Fail. 2005;7:662–70.
3 Siirila-Waris K, Lassus J, Melin J, Peuhkurinen K, Nieminen MS, Harjola VP. Characteristics, outcomes, and predictors of 1-year mortality in patients hospitalized for acute heart failure. Eur Heart J. 2006;27:3011–7.
4 Tavazzi L, Maggioni A, Lucci D, Cacciatore G, Ansalone G, Olivia F, et al. Nationwide survey on acute heart failure in cardiology ward services in Italy. Eur Heart J. 2006;27:1207–15.
5 Zannad F, Mebazaa A, Juillière Y, Cohen-Solal A, Guize L, Alla F, et al. Clinical profile, contemporary management and one-year mortality in patients with severe acute heart failure syndromes: The EFICA study. Eur J Heart Fail. 2006;8:697–705.
6 Safdar N, Dezfulian C, Collard HR, Saint S. Clinical and economic consequences of ventilator-associated pneumonia: a systematic review. Crit Care Med. 2005;33:2184–93.
7 Rudiger A, Businger F, Streit M, Schmid ER, Maggiorini M, Follath F. Presentation and outcome of critically ill medical and cardiac-surgery patients with acute heart failure. Swiss Med Wkly. 2009;139:110–6.
8 Nieminen MS, Bohm M, Cowie MR, Drexler H, Filippatos GS, Jondeau G, et al. Executive summary of the guidelines on the diagnosis and treatment of acute heart failure. The Task Force on Acute Heart Failure of the European Society of Cardiology. Eur Heart J. 2005;26:384–416.
9 Rudiger A, Fischler M, Harpes P, Gasser S, Hornemann T, von Eckardstein A, et al. In critically ill patients, B-type natriuretic peptide (BNP) and N-terminal pro-BNP levels correlate with C-reactive protein values and leukocyte counts. Int J Cardiol. 2008;126:28–31.
10 Rudiger A, Gasser S, Fischler M, Hornemann T, Von Eckardstein A, Maggiorini M. Comparable increase of B-type natriuretic peptide and amino-terminal pro-B-type natriuretic peptide levels in patients with severe sepsis, septic shock, and acute heart failure. Crit Care Med. 2006;34:2140–4.
11 The American Thoracic Society and the Infecious Disease Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416.
12 Masterton RG, Galloway A, French G, Street M, Armstrong J, Brown E, et al. Guidelines for the management of hospital-acquired pneumonia in the UK: report of the working party on hospital-acquired pneumonia of the British Society for Antimicrobial Chemotherapy. J Antimicrob Chemother. 2008;62:5–34.
13 Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270:2957–63.
14 Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34:1589–96.
15 Rudiger A, Singer M. Mechanisms of sepsis-induced cardiac dysfunction. Crit Care Med. 2007;35:1599–608.
16 Chen D, Assad-Kottner C, Orrega C, Torre-Amione G. Cytokines and acute heart failure. Crit Care Med. 2008;36:S9–16.
17 Paulus WJ. How are cytokines activated in heart failure? Eur J Heart Fail. 1999;1:309–12.
18 Peschel T, Schoenauer M, Thiele H, Anker SD, Schuler G, Niebauer J. Invasive assessment of bacterial endotoxin and inflammatory cytokines in patients with acute heart failure. Eur J Heart Fail. 2003;5:609–14.
19 Kumar A, Haery C, Paladugu B, Kumar A, Symeoneides S, Taiberg L, et al. The duration of hypotension before the initiation of antibiotic treatment is a critical determinant of survival in a murine model of Escherichia coli septic shock: association with serum lactate and inflammatory cytokine levels. J Infect Dis. 2006;193:251–8.
20 Eggimann P, Pittet D. Infection control in the ICU. Chest. 2001;120:2059–93.
21 Kohsaka S, Menon V, Lowe AM, Lange M, Dzavik V, Sleeper LA, et al. Systemic inflammatory response syndrome after acute myocardial infarction complicated by cardiogenic shock. Arch Int Med. 2005;165:1643–50.
22 Zilberberg MD, Shorr AF, Kollef MH. Implementing quality improvements in the intensive care unit: ventilator bundle as an example. Crit Care Med. 2009;37:305–9.
23 O’Grady NP, Alexander M, Dellinger EP, Gerberding JL, Heard SO, Maki DG, et al. Guidelines for the prevention of intravascular catheter-related infections. Infect Control Hosp Epidemiol. 2002;23:759–69.
24 Pronovost P, Needham D, Berenholtz S, Sinopoli D, Chu H, Cosgrove S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355:2725–32.
25 de Smet AM, Kluytmans JA, Cooper BS, Mascini EM, Benus RF, van der Werf TS, et al. Decontamination of the digestive tract and oropharynx in ICU patients. N Engl J Med. 2009;360:20–31.
26 de Jonge E, Schultz MJ, Spanjaard L, Bossuyt PM, Vroom MB, Dankert J, et al. Effects of selective decontamination of digestive tract on mortality and acquisition of resistant bacteria in intensive care: a randomised controlled trial. Lancet. 2003;362:1011–6.
27 Pugin J, Auckenthaler R, Lew DP, Suter PM. Oropharyngeal decontamination decreases incidence of ventilator-associated pneumonia. A randomized, placebo-controlled, double-blind clinical trial. JAMA. 1991;265:2704–10.
28 Felker GM, Cotter G. Unraveling the pathophysiology of acute heart failure: an inflammatory proposal. Am Heart J. 2006;151:765–7.
29 Mann DL. Inflammatory mediators and the failing heart: past, present, and the foreseeable future. Circ Res. 2002;91:988–98.
30 Hasper D, Hummel M, Kleber FX, Reindl I, Volk HD. Systemic inflammation in patients with heart failure. Eur Heart J. 1998;19:761–5.


