Postoperative serum pro-calcitonin and C-reactive protein levels in patients with orthopaedic infections
DOI: https://doi.org/10.4414/smw.2010.13124
C
Garzoni, T
Ferry, S
Harbarth, R
Stern, M
Assal, P
Hoffmeyer, D
Lew, L
Bernard
Summary
QUESTIONS UNDER STUDY / PRINCIPLES: The value of postoperative pro-calcitonin (PCT) in the follow-up of patients with localised infections in the orthopaedic domain is unknown.
METHODS: We conducted a retrospective study comparing postoperative ultra-sensitive serum PCT (upper normal level 0.25 µg/l) and C-reactive protein (CRP; upper normal level 10 mg/l) levels in adult patients with localised non-bacteremic orthopaedic infections.
RESULTS: A total of 165 paired PCT and CRP samples were retrieved from 60 infected patients. PCT levels exceeded normal in only half of the patients and practically only on the first postoperative day, despite a clinically active infection in all cases. PCT values did not differ between patients with or without surgical re-interventions (36 patients; median 0.09 mg/l vs. 24 patients; median 0.08 µg/l; Wilcoxon-rank sum-test, p= 0.34). CRP was elevated in 54 patients (90%) with a maximum at day 2, and normalised by the tenth day. Both markers correlated poorly with each other (Kendall-tau-test 0.47). The cost for one analysis (phlebotomy by nurses, transport and laboratory) was US$70 for PCT and $20 for CRP.
CONCLUSIONS: Postoperative serum PCT levels in orthopaedic infections were rarely elevated, even if patients continued to be infected. They quickly fell to within a normal range at day 2. PCT does not seem to be better than the less expensive CRP in the follow-up of these patients.
Introduction
Serum inflammatory markers like C-reactive protein (CRP) are widely used in the follow-up of patients with localised infections in the orthopaedic domain, but trauma or surgery may also result in their transient elevation [1, 2]. In 1993, pro-calcitonin (PCT) was introduced as a more accurate marker for general bacterial infections than CRP, erythrocyte sedimentation rate or leukocyte counts [3]. Today, the value of PCT is uncontested in sepsis and bacteremia [4], and many reports suggest it is a benefit even for localised infections [5–7]. For the diagnosis of osteomyelitis, PCT has a reported sensitivity ranging from 25% [8] and 33% [9] to 55% [10] and 58% [11], while sensitivity for septic arthritis is mediocreat best [8, 12]. In total opposition to previous reports yielding an unacceptable low sensitivity for the diagnosis of osteo-articular infections, a recent analysis from Switzerland identified PCT as a helpful diagnostic marker for the differentiation of infectious from non-infectious causes of fever after orthopaedic surgery [13]. However, the authors did not distinguish patients with localised osteo-articular infections from others with nosocomial infections for which PCT is already validated, for example bacteremia [4] or respiratory tract infections [5]. The latter group made up the largest patient population in their study [13]. While the value of PCT is debated for the diagnosis of purely localised orthopaedic infections, the performance of PCT for the follow-up of proven infected patients has not been evaluated.
Ideally, these questions can only be answered by a prospective, randomised (maybe even double-blinded) trial with a long duration since infections in orthopaedics are scare. Before organising such a time-consuming and costly trial, preliminary pilot evaluations about the feasibility and pertinence of the study question are clearly warranted. In this “pilot study”, postoperative ultra-sensitive serum PCT levels were compared to classical serum CRP in patients with proven localised orthopaedic infections. It is of interest to investigate if PCT falls to negative levels even if infection persists. If not, a randomised trial would be warranted to investigate the use of PCT for the diagnosis of osteo-articular infections, and/or its performance of predilection of recurrent / persistent infection.
Methods
The Geneva University Hospital is a 2’200-bed teaching hospital. The Orthopaedic Service has 132 acute care beds and performs more than 5’000 surgical procedures annually. Ultra-sensitive serum PCT has been used within the service since July 2007.
Data collection and definitions
Data were retrospectively retrieved from electronic databases for the period from July 2007 to July 2008. We included all operated adult patients (older than 16 years) with a localised orthopaedic infection, for whom paired postoperative PCT and CRP data were available. Informed consent was not required. To avoid confusion with other inflammatory processes, patients with the following were excluded: inflammation other than orthopaedic infection, nosocomial infections other than at orthopaedic site, bacteremia [4] and uninfected haematoma [6]. Localised orthopaedic infections included orthopaedic implant material, arthritis, bursitis, infected haematomas, bone or deep soft tissue abscesses. Diagnosis of infection was based upon the presence of pus and of pathogens obtained from intraoperative microbiological cultures.
Serum sampling
Postoperative serum samplings of PCT and CRP were performed during the same phlebotomy, in order to match the postsurgical sampling time. Several samples per patient were permitted as long as they were obtained on different days. Otherwise, only the morning sample was accepted for analysis. Samples obtained after cessation of antibiotic treatment were excluded, because the patients were no longer considered to be infected. Blood samples were collected using vacuum tubes (BD vacutainer SST II Plus tubes, Becton Dickinson Diagnostic Systems, Allschwil, Switzerland). PCT levels were quantitatively measured with the time-resolved amplified cryptate emission assay (Kyrptor PCT, Brahms AG, Henningdorf, Germany) with an assay sensitivity of 0.06 µg/l, four-fold above mean levels [14]. CRP levels were analysed per automate (DXC 800, Beckman Coulter). Cut off-values were 0.25 µg/l for PCT and 10 mg/l for CRP. Values above were considered as elevated. PCT and CRP levels were determined immediately after sampling; the serum was not stored. The cost for one analysis (phlebotomy by nurses, transport and laboratory) was US$70 for PCT and $20 for CRP. Pathogens were identified according to the Clinical Laboratory and Standards Institute’s recommendations [15].
Statistical analyses
The primary study question was the evolution of postoperative PCT levels in infected patients. Postoperative days were counted from the last surgical intervention. The Pearson χ2-test, the Fisher exact-test or the Wilcoxon-ranksum-test, used as appropriate, compared between the groups of patients with one or multiple surgeries. Correlations of the paired PCT and CRP values were computed with the Kendall-tau test. p values ≤0.05 (two-tailed) were considered significant. STATA software (9.0, STATA Corp, College Station, USA) was used.
Results
Study population and treatment
A total of 60 patients (median age, 58 years; 17 females) with localised orthopaedic infections were included (table 1) with a median follow-up of 26 months. No patient had a recurrence of infection before 30th June 2010. The five most frequent pathogens were: Staphylococcus aureus (n = 81), coagulase-negative staphylococci (n = 22), streptococci (n = 18), Pseudomonassp (n = 14), and enterococci (n = 7). All patients were treated with antibiotics and underwent surgery to cure the infection. The median time delay between infection onset and surgery was 7.5 days. The mean and the median number of surgical interventions was 1.7 and 1 (range, 1 to 8), respectively. A total of 24 of the 60 patients (40%) had multiple interventions (Table 1). Re-interventions occurred within a median of 3 days.
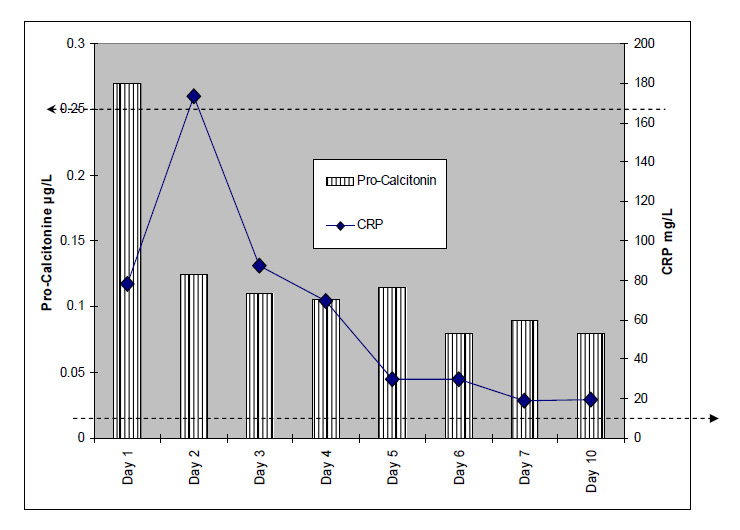
Figure 1
Median postoperative PCT and CRP levels in patients with orthopaedic infections.
The horizontal axis represents postoperative days. The thick intermitted dotted lines represent the upper limit of normal range: Left arrow for PCT (0.25 µg/l); right arrow for CRP (10 mg/l).
The line above indicates the number of paired PCT and CRP samples per postoperative day.
A total of 165 paired postoperative serum PCT and CRP samples were retrieved. The samplings occurred between day 1 and day 89 postoperative (median, 7 days; mean 10.8 days). A total of 20 patients had 1 pair sampled; 17 had 2 pairs; 7 had 3 pairs; 5 had 6 pairs; 5 had 4 pairs; 3 had 6 pairs; 2 had 7 pairs; and 1 patient had 8 pairs sampled.
Pro-calcitonin
Overall, 15 infected patients (15/60, 25%) had elevated PCT values. Median PCT levels were elevated only during the first day and only in half of the patients of that day (9/18, 50%). PCT levels fell to a negative range by day 2 despite persistent infection (fig. 1). In general, PCT levels were not different between patients undergoing one vs. multiple interventions (table 1). PCT levels were equally similar when the comparison of both patient groups was stratified for each postoperative day (1-5) individually (Wilcoxon-ranksum-tests; corresponding p values of 0.33, 0.52, 0.63, 0.91, and 0.12, respectively). Formally, sensitivity, specificity, positive and negative predictive values of elevated postoperative PCT levels for identification of persistent infections requiring re-intervention were 0.21, 0.83, 0.48 and 0.59, respectively.
C-reactive protein
Overall, 54 patients (90% of the study population) had elevated postoperative CRP levels. This proportion was significantly higher than for PCT (54/60 vs. 15/60 patients, Pearson χ2-test: p <0.001). Median CRP levels reached a maximum on day 2 and remained elevated during the first 10 days, more closely corresponding to the clinical evolution (fig. 1). CRP levels were not different between patients undergoing one vs. multiple interventions (table 1).
Correlation between pro-calcitonin and CRP
Overall, the correlation between CRP and PCT was 0.47. Stratified into postoperative periods, correlation results were 0.49 for the first three days (39 pairs), 0.39 for days 4–10 (67 pairs), 0.45 for days 11-30 (42 pairs), and 0.35 for more than one month (10 pairs).
Subgroup analyses
Due to the heterogeneity of the patient population and infecting pathogens, several subgroup analyses were performed.
– First surgery vs. subsequent surgery for infection
We analysed postoperative PCT and CRP levels only after the first surgical intervention and censored them for subsequent operations. Here, the median postoperative PCT level was 0.1 µg/l and the median CRP level was 64 mg/l. This result was similar to the analysis with postoperative PCT including subsequent surgeries for the same patient (χ2-test; p = 0.51), highlighting that inclusion of subsequent surgeries does not significantly change the immediate postoperative follow-up.
– Results according to the virulence of pathogens
We investigated whether the presumed virulence of the dominant pathogen would alter the general results. S. aureus was considered as virulent and the group of coagulase-negative staphylococci as less virulent pathogens. The individual number of other pathogens was too small to draw conclusive results. Overall, the 81 episodes due to S. aureusrevealed 9 (12%) elevated postoperative PCT values, of which three occurred during the first day. The corresponding results for the 22 episodes of coagulase-negative staphylococci were 3 (13%) and 1, respectively. These differences were not significant (Fisher-exact-tests; p = 0.73 and p = 1.0, respectively), highlighting that the presumed virulence of the underlying pathogen does not change immediate PCT evolution.
– Soft-tissue vs. osteo-articular infections
PCT levels were elevated in 13 of 85 soft-tissue infections and 13 of 80 osteo-articular infections; the difference being insignificant (χ2-test; p = 1.0). This was also the case regarding only day 1 of both subgroups (Fisher-exact-test; p = 0.64).
|
Table 1: Patients’ characteristics with one vs. multiple surgeries for orthopaedic infections. |
| |
One intervention
n = 36 |
Multiple interventions
n = 24 |
Pvalues* |
| PATIENTS |
|
|
|
| Female gender |
9 (25%) |
8 (33%) |
0.48 |
| Median age (years) |
57, range 18–92 |
60, range 19–84 |
0.94 |
| Median Charlson Score (points) |
0, range 0–4 |
1, range 0–13 |
0.24 |
| Immunosuppressed patients |
19+ (53%) |
7+ (29%) |
0.07 |
| – Diabetes |
14 (39%) |
7 (29%) |
0.44 |
| – Chronic renal insufficiency±
|
2 (6%) |
1 (4%) |
|
| – AIDS |
0 (0%) |
1 (4%) |
|
| – Immunsuppressive treatment
due to autoimmune disease |
3 (8%) |
1 (4%) |
|
| – Chronic alcoholism |
4 (11%) |
None (0%) |
|
| INFECTIONS |
|
|
|
| Implant-associated infections |
6 (17%) |
6 (25%) |
0.43 |
| – Total joint arthroplasties |
3 |
3 |
|
| – Osteosynthesis material |
3 |
2 |
|
| Skin/soft tissue infections |
22 (61%) |
16 (67%) |
0.66 |
| Abscess /infected haematomas |
9 |
6 |
|
| Diabetic foot infections |
6 |
5 |
|
| Bursitis |
3 |
5 |
|
| Amputation stump infections |
4 |
|
0.64 |
| Infectious Arthritis |
6 |
2 |
0.46 |
| Osteomyelitis |
2 |
|
|
| Polymicrobial infection |
16 (44%) |
18 (75%) |
0.02 |
| – S. aureus
|
17 (47%) |
12 (50%) |
0.83 |
| – coagulase-negative staphylococci |
14 (39%) |
8 (33%) |
0.66 |
| TREATMENT |
|
|
|
| Prior antibiotics before surgery |
15 (42%) |
10 (42%) |
1.00 |
| Type of interventions |
36 |
69+
|
|
| – Debridement and irrigation |
25 |
62 |
|
| – Implant removal |
1 |
2 |
|
| – Amputation |
6 |
1 |
|
| – Bursectomy |
3 |
5 |
|
| – Biopsy and lavage |
1 |
|
|
| LABORATORY |
|
|
|
| Total number of blood samples |
95 |
70 |
|
| Median overall postoperative PCT |
0.09 µg/l, range 0.02–11.89 µg/l |
0.08 µg/l, range 0.02–38.83 µg/l |
0.34 |
| Median PCT: Day 1 |
0.06 µg/l, range 0.02–0.96 µg/l |
0.34 µg/l, range 0.02–38.83 µg/l |
0.33 |
| Median overall postoperative CRP |
34 mg/l, range 1–385 mg/l |
34.5 mg/l, range 3–315 mg/l |
0.58 |
| Median CRP: Day 1 |
186 mg/l, range 11–341 mg/l |
75.5 mg/l, range 13–315 mg/l |
0.44 |
| PCT = Pro-Calcitonin; CRP = C-reactive protein; ± Creatinine clearance ≤30 ml/min
* Pearson χ2-, Fisher exact or Wilcoxon-ranksum tests, as appropriate+
One patient may have more than one type of immune suppression disease |
Discussion
Postoperative serum PCT levels from infected adult orthopaedic patients were elevated on the first postoperative day and only in half of the patients of that day, despite ongoing infection during the following days. Moreover, while PCT testing was at least three times more expensive than CRP sampling, correlations of PCT with paired CRP results were inconclusive and not in favour of PCT. The follow-up of CRP corresponded more closely to the clinical course of ongoing infection and was less costly to obtain.
It may be concluded that PCT had no additional value in the follow-up of infected patients than the much cheaper CRP. It is very important to note that this conclusion is based on retrospective data. This study does not directly address the question of the use of PCT in the primary diagnosis of localised orthopaedic infections. The current study is a preliminary evaluation of possible prospective trials, which may investigate the use of PCT in the predilection of recurrent osteo-articular infections and maybe the role of PCT in the diagnosis of localised infections. Only a well-designed prospective, randomised (and double-blinded) study would attribute the best clarity to these issues.
Literature concerning postsurgical PCT is sparse. While Rothenburger et al. failed to show any PCT elevation in wound infections in cardiovascular patients [16],others attributed some diagnostic value for surgical site infections [13] and other complications in previously uninfected neurosurgical [17],traumatic [18], cardiovascular [2, 16, 19, 20] and abdominal surgery patients [7]. On the other hand, many authors deny the utility of PCT sampling for the diagnosis of osteo-articular infections [8–12]. Importantly, all these studies investigated previously uninfected patients. Similar to the present study, only one study assessed the utility of PCT in the follow-up of orthopaedic patients with presumed infections, where serum PCT levels correlated with wound dehiscence of the extremities [21].
In uninfected patients, PCT usually reaches peak levels on the first and secondpostsurgical day and falls rapidly thereafter to negative values. The physiological half-life of PCT is 18 to 24 hours. This could be a possible explanation for the decrease in PCT levels by day 2 [2, 19, 22, 23]. It rarely exceeds the level of 2 µg/l [2, 6, 23]. Postoperative CRP levels reaches its peak on the second or third day [22, 23], and becomes negative slowly in a range encompassing 5 to 21 days [23]. Interestingly, we found a similar pattern of both inflammatory markers in infected patients, suggesting that the infection itself does not change the natural evolution of general inflammation.
Despite multiple trials about its use to the contrary, little is so far known about the cells of production or the biological activity of PCT. In the case of sepsis, a hepato-spanchnical production [24] is assumed via the calcitonin family of receptors. PCT could eventually be a mediator in host-own responses to sepsis [25]. Currently, studies also ignore if different bacterial species affect PCT levels differently. One study evaluated the accuracy of PCT to distinguish blood contamination from true bloodstream infection due to coagulase-negative staphylococci [26]. However, the study revealed once again the possibility of PCT to detect clinical bacteremia, and does not report differences between different clinical infecting isolates. Of note, in our retrospective analysis, the pattern of postoperative PCT was not different between infections due to S. aureus and coagulase-negative staphylococci.
Our study has limitations. i) It is a retrospective, single-centre, non-randomised study with a small sample size, large heterogeneity of the study population and of pathogens, large differences in the extent of surgical procedures, and blood samples at variable time points, therefore limiting the ability to generalise the findings. ii) It involved only adult and operated patients. iii) It had no comparator arm, since we excluded patients without infection. iv) We have, in some cases, used multiple samples from the same patient as long as they were not from the same day. This may not be relevant for PCT, since PCT with its short half-life time is only elevated on the first operative day, and re-operation is normally not performed the day following surgery. However, it may be relevant for CRP values that have a maximum at day 2 and which decline only slowly thereafter. iv) Other reports used higher cut-off values (0.5 or 1 µg/l) [2, 6, 8, 9, 11, 18, 22] than we did (0.25 µg/l). Lower cut-off values would theoretically raise the number of “positive” results, but we would not know how to interpret them when they are still within a negative range as validated by the manufacturer and our laboratory. Of note, we used a modern ultra-sensitive PCT test, excluding inherent insufficiencies of former tests [6, 14].
We thank to all colleagues of the Orthopedic Service, the Laboratory of Chemistry and the Laboratory of Bacteriology for their help. We appreciate the external advice of Dr P. Schriber.
Correspondence:
Ilker Uçkay MD
Orthopedic Surgery Service
Geneva University Hospitals
4, Rue Gabrielle Perret-Gentil
CH-1211 Geneva 14
Switzerland
ilker.uckay@hcuge.ch
References
1 Mimoz O, Benoist JF, Edouard AR, Assicot M, Bohuon C, Samii K. Procalcitonin and C-reactive protein during the early posttraumatic systemic inflammatory response syndrome. Intensive Care Med. 1998;24:185–8.
2 Aouifi A, Piriou V, Bastien O, Blanc P, Bouvier H, Evans R, et al. Usefulness of procalcitonin for diagnosis of infection in cardiac surgical patients. Crit Care Med. 2000;28:3171–6.
3 Assicot M, Gendrel D, Carsin H, Raymond J, Guilbaud J, Bohuon C. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet. 1993;341:515–8.
4 Nobre V, Harbarth S, Graf JD, Rohner P, Pugin J. Use of procalcitonin to shorten antibiotic treatment duration in septic patients: a randomized trial. Am J Respir Crit Care Med. 2008;177:498–505.
5 Christ-Crain M, Jaccard-Stolz D, Bingisser R, Gencay MM, Huber PR, Tamm M, et al. Effect of procalcitonin-guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster-randomised, single-blinded intervention trial. Lancet. 2007;363:600–7.
6 Becker KL, Snider R, Nylen ES. Procalcitonin assay in systemic inflammation, infection, and sepsis: clinical utility and limitations. Crit Care Med. 2008;36:941–52.
7 Rau B, Kruger CM, Schilling MK. Procalcitonin: improved biochemical severity stratification and postoperative monitoring in severe abdominal inflammation and sepsis. Langenbecks Arch Surg. 2004;389:134–44.
8 Faesch S, Cojocaru B, Hennequin C, Pannier S, Glorion C, Lacour B, et al. Can procalcitonin measurement help the diagnosis of osteomyelitis and septic arthritis? A prospective trial. Ital J Pediatr. 2009;35:33–9.
9 Bottner F, Wegner A, Winkelmann W, Becker K, Erren M, Götze C. Interleukin-6, procalcitonin and TNF-alpha: markers of peri-prosthetic infection following total joint replacement. J Bone Joint Surg Br. 2007;89:94–9.
10 Martinot M, Sordet C, Soubrier M, Puéchal X, Saraux A, Lioté F, et al. Diagnostic value of serum and synovial procalcitonin in acute arthritis: a prospective study of 42 patients. Clin Exp Rheumatol. 2005;23:303–10.
11 Butbul-Aviel Y, Koren A, Halevy R, Sakran W. Procalcitonin as a diagnostic aid in osteomyelitis and septic arthritis. Pediatr Emerg Care. 2005;21:828–32.
12 Fottner A, Birkenmaier C, von Schulze Pellengahr C, Wegener B, Jansson V. Can serum procalcitonin help to differentiate between septic and nonseptic arthritis? Arthroscopy. 2008;24:229–33.
13 Hunziker S, Hügle T, Schuchardt K, Groeschl I, Schütz P, Müller B, et al. The value of serum procalcitonin level for differentiation of infectious from noninfectious causes of fever after orthopedic surgery. J Bone Joint Surg Am. 2010;92:138–48.
14 Nylen E, Müller B, Becker KL, Snider R. The future diagnostic role of procalcitonin levels: the need for improved sensitivity. Clin Infect Dis. 2003;36:823–4.
15 Wayne PA. Clinical and Laboratory Standards Institute: Performance standards for antimicrobial susceptibility testing 17th informational supplement. Standard 100–17; 2007.
16 Rothenburger M, Markewitz A, Lenz T, Kaulbach HG, Marohl K, Kuhlmann WD, et al. Detection of acute phase response and infection. The role of procalcitonin and C-reactive protein. Clin Chem Lab Med. 1999;37:275–9.
17 Laifer G, Wasner M, Sendi P, Graber P, Gratzl O, Huber P, et al. Dynamics of serum procalcitonin in patients after major neurosurgery. Clin Microbiol Infect. 2005;11:679–81.
18 Yasmin D, Bulut G, Yıldız M. Can procalcitonin be used for the diagnosis and follow-up of postoperative complications after fracture surgery? Acta Orthop Traumatol Turc. 2006;40:15–21.
19 Sponholz C, Sakr Y, Reinhart K, Brunkhorst F. Diagnostic value and prognostic implications of serum procalcitonin after cardiac surgery: a systematic review of the literature. Crit Care. 2006;10:145–56.
20 Meisner M, Rauschmayer C, Schmidt J, Feyrer R, Cesnjevar R, Bredle D, et al. Early increase of procalcitonin after cardiovascular surgery in patients with postoperative complications. Intensive Care Med. 2002;28:1094–102.
21 Forsberg JA, Elster EA, Andersen RC, Nylen E, Brown TS, Rose MW, et al. Correlation of procalcitonin and cytokine expression with dehiscence of wartime extremity wounds. J Bone Joint Surg Am. 2008;90:580–8.
22 Molter GP, Soltesz S, Kottke R, Wilhelm W, Biedler A, Silomon M. Procalcitonin plasma concentrations and systemic inflammatory response following different types of surgery. Anaesthesist. 2003;52:210–7.
23 Arkader R, Troster EJ, Abellan DM, Lopes MR, Júnior RR, Carcillo JA, et al. Procalcitonin and C-reactive protein kinetics in postoperative pediatric cardiac surgical patients. J Cardiothorac Vasc Anesth. 2004;18:160–5.
24 Silomon M, Bach F, Ecker D, Graeter T, Grundmann U, Larsen R. Procalcitonin after extracorporeal circulation. Synthesis in the hepatosplanchnic region. Anaesthesist. 1999;48:395–8.
25 Sexton PM, Christopoulos G, Christopoulos A, Nylen ES, Snider RH Jr, Becker KL. Procalcitonin has bioactivity at calcitonin receptor family complexes: potential mediator implications in sepsis. Crit Care Med. 2008;36:1637–40.
26 Schütz P, Müller B, Trampuz A. Serum procalcitonin for discrimination of blood contamination from bloodstream infection due to coagulase-negative staphylococci. Infection. 2007;35:352–5.
