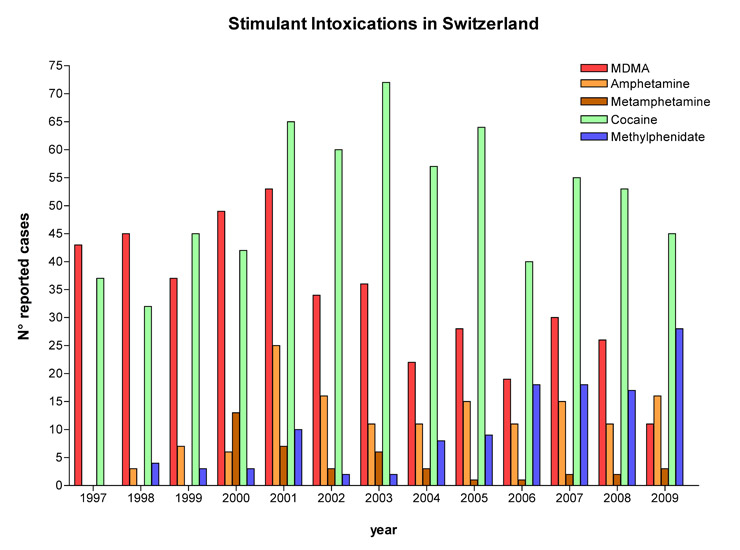
Figure 1
Annual numbers of exposures to stimulants reported to the Swiss Toxicological Information Centre from 1997 to 2009.
DOI: https://doi.org/10.4414/smw.2010.13115
Recreational drug use, i.e. the use of psychoactive drugs other than for approved medical purposes, is a major public health problem in many countries [1–7]. Although cannabis is by far the most widely used illicit drug of abuse, the psychostimulants cocaine, amphetamines (including 3,4‑methylenedioxymethamphetamine [MDMA, ecstasy] and related designer drugs) have a use prevalence of 2–5% in 17–18 year olds [8]. In 2007, an estimated 4.4% of the adolescent and young adult (15–39-year-old) population in Switzerland reported having used cocaine at least once [9]. The respective prevalences of use were 1% for amphetamine and 1.8% for MDMA in 2007 [9]. In recent years the unlicensed non-medical use of prescription psychostimulants such as methylphenidate for recreational purposes or as cognitive enhancers has also become a focus of concern. There is a perception of widespread misuse, particularly in the student population [1, 2, 10]. Among US college students, lifetime prevalence of non-medical prescription stimulant use (mainly methylphenidate and dextroamphetamine) was reported to be 6.9%, and non-prescribed use of stimulant medications is associated with consumption of recreational drugs [1]. No data on the prevalence of non-medical prescription stimulant use is available for Switzerland.
While recreational psychostimulant use is prevalent in Switzerland [9, 11], there are no data describing toxicity related to abuse of prescription stimulants in that country, and only one study has analysed acute medical problems with ecstasy [12]. In addition, only a few studies have described common amphetamine- and cocaine-related medical problems in other countries [13–17]. We therefore analysed stimulant drug exposure cases reported to the Swiss Toxicological Information Centre (STIC) between January 1997 and December 2009. The aim of the study was to describe case characteristics including toxicity related to stimulant exposure, frequency of reports to the STIC and temporal trends.
The study was approved by the Ethics Committee of the Canton of Zurich, Switzerland (Nr. 2010-0015/0). The STIC provides 24-hour nationwide free medical advice in cases of poisoning to health professionals and members of the general public. The STIC records demographic and clinical information on exposure cases at the time of the initial phone call, using an in-house computer-based and structured data-recording system (TOXI) [18, 19]. For reports by health professionals, the STIC also collects written clinical data (signs and symptoms at presentation, final diagnosis, laboratory results, treatment, outcome) using a questionnaire (follow-up) which is sent to the physician who treated the patient. A physician trained in toxicology then enters this follow-up data into the TOXI database to complement the case files. Severity of poisoning is assessed using the Poison Severity Score (PSS) developed by the European Association of Poison Centres and Clinical Toxicologists (EAPCCT), the International Programme on Chemical Safety, and the European Commission [20]. Medical outcome is classified as death, severe toxicity, moderate toxicity, minor toxicity or no effect.
We conducted a retrospective review of exposure/abuse cases with psychostimulants including cocaine, amphetamines (amphetamine, methamphetamine, MDMA), and methylphenidate reported to the STIC from January 1997 to December 2009. Abuse of methylphenidate was defined as intake without prescription, use of a dose higher than the one prescribed, or non-oral administration. Cases were included in the final analysis only if the physician in charge provided the STIC with clinical information on the follow-up questionnaire. Clinical information had to be sufficiently detailed to allow meaningful characterisation of the case (heart rate, blood pressure, Glasgow Coma Scale Scores, and main symptoms). Additional clinical information (discharge letter, outcome, ECG findings, laboratory values) was often also available to describe exposure cases in the study (tables 1 and 2). For all cases the information in the TOXI database was verified using the archived original follow-up questionnaire and supplemented with information from discharge letters when available. Cases were included if exposure was confirmed by a positive drug screening test in either blood or urine. Cases where laboratory confirmation of exposure was missing were also included if the exposure was considered “likely”, i.e. related in time to symptoms and signs of stimulant exposure (e.g. sympathomimetic or serotonergic toxicity, and hyponatraemia in the case of MDMA exposure) and in the absence of medical conditions or drug intake that might alternatively have explained the reported findings. In cases of co-ingestants, cases were included if either stimulant exposure was confirmed by a positive drug screening test or the stimulant was considered the main responsible agent for the clinical findings. Cases related to ingestion of stimulants with suicidal intention or related to a criminal context (i.e. body packer or body stuffer) were excluded.
Statistical analyses were performed with NCSS [21]. To assess potential differences in patient characteristics and medical problems between the amphetamine, MDMA, and cocaine groups we used χ2 analyses for all categorical outcomes. Logistic regression analyses were used to assess effects of age, gender, co-use (monointoxication vs. additional substances), and route of drug administration (oral vs. non-oral) on severity of poisoning (severe/fatal vs. non-severe). Wald probability levels, adjusted odds ratios (ORs) and 95% confidence intervals (CIs) are reported. The significance level was set at p <0.05. The group size for methylphenidate was too small to allow for meaningful statistical comparisons with the other groups.
| Table 1: Patient characteristics. | |||||
| MDMA | Amphetamine | Cocaine | Methylphenidate | ||
| Number of cases (%) | 150 (100) | 76 (100) | 264 (100) | 38 (100) | |
| Demographics | |||||
| male | 88 (58.6) | 45 (59.2) | 176 (66.7) | 20 (52.6) | |
| mean age [range] | 21.1 [11-62] | 22.7 [14-54] | 28.3 [14-57] | 24.9 [11-50] | |
| ≤25 | 100 (66.6)** | 58 (76.3)** | 93 (35.2) | 18 (47.4) | |
| ≥26 | 40 (26.6)** | 17 (22.4)** | 162 (61.4) | 17 (44.7) | |
| NR | 10 (6.7) | 2 (2.6) | 9 (3.4) | 3 (7.9) | |
| Time of intoxication | |||||
| late night a | 103(72.7) | 51(67.1) | 157 (59.5) | 16 (42.1) | |
| weekend b | 129 (86.0)** | 65 (85.5)** | 129 (48.9) | 15 (39.5) | |
| Severity of intoxication | |||||
| no symptoms | 1 (0.7) | 13(5.0) | 2(5.3) | ||
| mild | 52 (34.7)+ | 37 (48.7) | 101(38.3) | 26(68.4) | |
| moderate | 69 (46.0) | 29 (38.1) | 105(39.8) | 8(21.1) | |
| severe | 25 (16.7) | 9 (11.8) | 40(15.1) | 2(5.3) | |
| fatal | 3 (2.0) | 1 (1.3) | 5(1.9) | 0(0.0) | |
| Way of application | |||||
| oral | 138 (92.0)**/++ | 55 (72.4)** | 67 (25.4) | 22 (57.9) | |
| nasal | 1 (0.7)**/++ | 13 (17.1)** | 70 (26.5) | 5 (13.2) | |
| intravenous | 0** | 1 (1.3)** | 53 (20.1) | 4 (10.5) | |
| inhalation | 1 (0.7) | 3 (3.9) | 15 (5.7) | ||
| NR | 10 (6.7) | 4 (5.3) | 59 (22.4) | 7 (18.4) | |
| Concomitant drug use | |||||
| Monointoxications | 65 (43.3) | 32 (42.1) | 107 (40.5) | 16 (42.1) | |
| only Alcohol | 22 (14.7) | 14 (18.4) | 24 (9.1) | 6 (15.8) | |
| two substances c | 28 (18.7) | 14 (18.4) | 56 (21.2) | 6 (15.8) | |
| > two substances | 35 (23.3) | 16 (21.1) | 77 (29.2) | 11 (28.9) | |
| Coingestion with | |||||
| Alcohol | 36 (24.0) | 17 (22.4) | 61 (23.1) | 12 (31.6) | |
| other amphetamines | 20 (13.3) | 36 (14.7) | 26 (9.8) | 3 (7.9) | |
| Cannabis | 16 (10.7) | 5 (6.7) | 20 (7.6) | ||
| Cocaine | 12 (8.0) | 8 (10.5) | 6 (15.8) | ||
| Benzodiazepines | 7 (4.7)** | 4 (5.3) | 31 (11.7) | 3 (7.9) | |
| GHB/GBL | 6 (4.0) | 5 (6.6) | 9 (3.4) | 1 (2.6) | |
| Heroin | 2 (1.3)** | 1 (1.3)** | 33 (12.5) | 2 (5.3) | |
| other opiates | 6 (4.0) | 2 (2.6) | 15 (5.7) | 2 (5.3) | |
| a 22.00–09.00; b Friday 5 PM - Monday 8 AM; c without alcohol * for p < 0.05, ** for p < 0.01 compared to cocaine; + for p < 0.05, ++ for p < 0.01 compared to amphetamine NR: not reported; GHB: gamma-hydroxybutyrate, GBL: gamma-butyrolactone | |||||
| Table 2: Medical problems associated with abuse of stimulants (monointoxications). | |||||
| MDMA | Amphetamine | Cocaine | Methylphenidate | ||
| Number of cases (%) | 65 (100) | 32 (100) | 107 (100) | 16 (100) | |
| Severity | no symptoms | 1 (1.5) | 9 (8.4) | 2 (12.5) | |
| mild | 28 (43.1) | 20 (62.5)* | 42 (39.2) | 11 (68.7) | |
| moderate | 26 (40.0) | 12 (37.5) | 41 (38.3) | 3 (18.7) | |
| severe | 9 (13.8)+ | 14 (13.1) | |||
| fatal | 1 (1.5) | 1 (0.9) | |||
| Demographics | male | 33 (50.7)++ | 26 (81.3) | 68 (63.5) | 8(50) |
| Mean age [range] | 19.2 [11-62] | 21.8 [16-35] | 28.9 [15-57] | 24.2 [11-50] | |
| ≤25 | 46 (70.8)** | 23 (71.9)** | 38 (35.5) | 6 (37.5) | |
| ≥26 | 15 (23.1)** | 8 (25.0)** | 65 (60.7) | 8 (50.0) | |
| NR | 4 (6) | 1 (3.1) | 4 (3.7) | 2 (12.5) | |
| Cardiopulmonary | HR >100 beats/min | 23 (35.4) | 12 (37.5) | 32 (29.9) | 1 (6.2) |
| HR >140 beats/min | 3 (4.6) | 2 (6.2) | 9 (8.4) | ||
| HR >180 beats/min | 1 (1.5) | 2 (6.2) | |||
| SBP>150 mmHg | 15 (23.1) | 5 (15.6) | 19 (17.8) | 1 (6.2) | |
| SBP>190 mmHg | 1 (1.5) | 2 (6.2) | 3 (2.8) | 1 (6.2) | |
| Chest pain | 1 (1.5)** | 2 (6.2) | 19 (17.8) | ||
| Palpitations | 3 (4.6) | 3 (9.3) | 8 (7.5) | 1 (6.2) | |
| Ischemic signsa | 1 (3.6)* | 1 (3.1)* | 15 (22.1) | 1 (25.0) | |
| Myocardial Ischemia | 1 (1.5) | 3 (2.8) | |||
| Neurologic | Agitation | 14 (21.5) | 8 (25.0) | 28 (26.2) | 4 (25.0) |
| Seizures | 6 (9.2) | 0* | 7 (6.5) | ||
| Confusion/delirium | 1 (1.5) | 2 (6.2) | |||
| Mydriasis | 16 (29.2)* | 3 (9.3) | 13 (12.1) | 1 (6.2) | |
| Dyskinesia | 5 (7.7) | 2 (6.2) | 3 (2.8) | ||
| Tremor | 5 (7.7) | 3 (2.8) | 4 (25.0) | ||
| Myoclonus | 8 (12.3) | 4 (12.5) | 3 (2.8) | ||
| Dysaesthesia | 3 (4.6) | 5 (15.6) | 8 (7.5) | ||
| Psychiatric | Hallucinations | 6 (9.2) | 3 (9.4) | 7 (6.5) | |
| other Psychosis | 3 (4.6) | 4 (3.7) | 1 (6.2) | ||
| Desorientation | 3 (4.6) | 10 (9.3) | |||
| Anxiety/Panic | 9 (13.8)** | 5 (15.6)** | 3 (2.8) | 1 (6.2) | |
| Other | Hyperthermiab | 6 (9.2) | 3 (9.4) | 7 (6.5) | |
| Nausea/Vomiting | 14 (21.5)** | 5 (15.6)* | 4 (3.7) | 1 (6.2) | |
| Excitation | 8 (12.3) | 2 (6.2) | 9 (8.4) | 3 (18.7) | |
| Documented Laboratory Values | 40 (100) | 20 (100) | 60 (100) | 9 (100) | |
| CK>250 U/l | 4 (10.0) | 2 (10.0) | 17 (15.9) | ||
| CK>1500 U/l | 3 (7.5) | 2 (10.0) | 5 (4.7) | 1 (11.1) | |
| Leucocytosisc | 3 (7.5) | 2 (10.0) | 7 (11.7) | 1 (11.1) | |
| Hyponatremiad | 2(5.0) | 1 (1.7) | 1 (11.1) | ||
| Management | Benzodiazepines | 18 (27.7) | 10 (31.2) | 21 (19.6) | 2 (12.5) |
| Other sedatives | 5 (7.7) | 3 (2.8) | |||
| Antihypertensives | 1 (1.5)* | 1 (3.1) | 10 (9.3) | 1 (6.2) | |
| a % of total number of electrocardiograms performed; b > 39 ºC; c > 10 x 109 cells/l; d < 135 mmol/l * for p < 0.05, ** for p < 0.01 compared to cocaine; + for p < 0.05, ++ for p < 0.01 compared to amphetamine HR: heart rate; SBP: systolic blood pressure; CK: creatine kinase; NR: not reported | |||||
There were 433 reports of MDMA abuse, 147 reports of amphetamine abuse, 41 reports of methamphetamine abuse, 667 reports of cocaine abuse, and 122 reports of methylphenidate abuse in 1997–2009. Detailed outcome data was available in 546 (39%) of all reported cases. Characteristics of cases with a known medical outcome are presented in table 1. The total number of reported cases per year for all stimulants was relatively stable over time, however, the relative proportion of individual substances changed: whereas reports for cocaine and MDMA decreased from a peak in 2001–2003, reports for methylphenidate increased starting in 2004 (fig. 1). The number of reported methylphenidate cases exceeded the number of reports related to MDMA abuse in 2009 and was second only to reports on cocaine (fig. 1).

Figure 1
Annual numbers of exposures to stimulants reported to the Swiss Toxicological Information Centre from 1997 to 2009.
Patient characteristics and use patterns are shown in table 1. The number of cases of abuse of methamphetamine (n = 12) and of MDMA-like amphetamine derivatives (CT4 and CT7, n = 6) was very small, and therefore user characteristics for the different amphetamines are only shown for MDMA and amphetamine. MDMA and amphetamine users were significantly younger than cocaine users. Most users of MDMA and amphetamines were aged below 26 years with a similar proportion of males and females, whereas most cocaine users were aged over 25 and typically male. MDMA and amphetamine exposure usually occurred at weekends. In contrast, cocaine exposures were reported relatively more frequently during the week. MDMA and amphetamines were chiefly ingested orally, whereas nasal or intravenous administration was more commonly reported with cocaine use. Methylphenidate exposures also included nasal and intravenous administration in a substantial proportion of cases. Fifteen of the 38 methylphenidate abusers (40%) reportedly used methylphenidate as a prescription drug for attention deficit hyperactivity disorder (ADHD). All stimulants were typically used together with other drugs of abuse, mainly ethanol. Co-ingestion of benzodiazepines or heroin was more frequently reported with cocaine than with MDMA or amphetamine.
Clinical and laboratory findings associated with exposure to different stimulants are shown in table 2. Severity of poisoning was classified as either mild or moderate for all amphetamine and methylphenidate exposures and for the majority of exposures with MDMA or cocaine. However, approximately 15% of MDMA and cocaine mono-exposures with a known medical outcome resulted in severe poisoning, and one fatal case was reported in each of these groups. In all stimulant groups, clinical findings consisted mainly of symptoms and signs of sympathetic nervous system stimulation, such as palpitations, sinus tachycardia, hypertension, and mydriasis. Other frequently observed clinical findings included agitation, disorientation, anxiety, and psychosis. Clinical findings in accordance with serotonergic toxicity, such as gastrointestinal manifestations (nausea and vomiting), and neurological findings (tremor, myoclonus) were more frequently reported in association with exposure to MDMA and amphetamines compared to cocaine. Significant hyperthermia, a potentially life-threatening condition, was commonly reported in abusers of MDMA, amphetamine and cocaine, but not among abusers of methylphenidate (table 2 and 3). Seizures were reported in abusers of MDMA and cocaine, but not in relation to exposure with amphetamines and methylphenidate. Management included clinical observation and administration of benzodiazepines in approximately one third of the patients. Chest pain and/or signs of myocardial ischaemia in the electrocardiogram were significantly more often reported related to cocaine abuse as compared to abuse of MDMA or amphetamine. The characteristics of severe and fatal intoxications are shown in Table 3. Some symptoms and signs such as coma, cerebral haemorrhage, and cerebral oedema occurred only in cases of polydrug abuse. In agreement with a previous study [22] central nervous system depression (coma) was typically observed with co-use of γ-hydroxybutyrate (GHB) or opiates. Compared to the use of only one drug, exposures to more than one drug were associated with more severe toxicity (severe/fatal vs. non-severe) adjusted for age, gender and route of drug administration (adjusted OR [95% CI] = 2.00 [1.15–3.49]; p <0.05). Stratification for the different drug groups showed that this association was only significant for the amphetamine group, where all severe intoxications were reported with combined use. Age (OR [95% CI] = 0.96 [0.56–1.65]), gender (OR [95% CI] = 1.10 [0.64–1.91]) or route of drug administration (OR [95% CI] = 1.24 [0.71–2.18]) were not associated with poison severity in our study sample.
| Table 3: Severe intoxications. | ||||
| MDMA | Cocaine | |||
| Symptoms/pathology | Monointoxication | co-abuse | Monointoxication | co-abuse |
| Total number of cases (%) | 65 (100) | 85 (100) | 107 (100) | 157 (100) |
| Severe intoxications | 9 (14) | 16 (19) | 14 (13) | 22 (14) |
| Fatalities | 1 (2)a | 2 (2)b | 1 (1)c | 4 (3)d |
| Total of severe and fatal cases (%) | 10 (15) | 18 (21) | 15 (14) | 26 (17) |
| Mean age [range] | 23.3 [15-62] | 20.4 [16-32] | 34.1 [24-54] | 28 [14-53] |
| ≤ 25 | 8 | 16 | 2 | 11 |
| ≥ 26 | 2 | 2 | 13 | 15 |
| Male | 4 | 11 | 9 | 20 |
| Severe agitation and/or psychosis | 3 | 5 | 4 | 8 |
| Severe tachycardia (>180 beats/min) | 1 | 1 | ||
| Severe hypertension (SBP >190 mmHg) | 1 | 2 | 3 | 2 |
| Myocardial ischemia | 2 | 4 | 4 | |
| Cerebrovascular ischemia | 1 | |||
| Ischemic colitis | 1 | |||
| Severe rhabdomyolysis (CK >10.000 U/l) | 1 | 1 | 4 | |
| Severe dyskinesia | 1 | 1 | ||
| Multiple seizures | 1 | 1 | 2 | 2 |
| Hyponatremia/SIADH | 1 | 3 | ||
| Liver failure/liver toxicity | 1 | 1 | 2 | |
| Renal failure | 1 | 1 | ||
| Hyperthermia/DIC | 1 | 1 | 1 | 4 |
| Pancreatitis | 1 | 1 | ||
| Leucoencephalopathy | 1 | |||
| Coma | 4 | 8 | ||
| Cerebral edema | 3 | |||
| Cerebral hemorrhage | 3 | |||
| Aspiration pneumonia | 1 | |||
| Ventricular fibrillation | 1 | |||
| a fatality associated with hyperthermia, multiple seizures, renal and liver failure b one fatality associated with hyponatremia/SIADH and cerebral edema, one with hyperthermia/DIC c fatality associated with miocardial ischemia d all fatalities were associated with miocardial ischemia, one also with hyperthermia, seizures, and multiorgan failure DIC: dissiminated intravascular coagulation; SBP: systolic blood pressure; CK: creatine kinase SIADH: syndrome of inappropriate antidiuretic hormone hypersecretion | ||||
We found that the number of reports per year was relatively stable over the study period for all stimulants, but the pattern of substances involved changed over time. Whereas the number of reports for MDMA and cocaine peaked in 2001 and 2003 respectively, the number of yearly reports of methylphenidate abuse started to rise from 2004 onwards. Whether this increase in methylphenidate case reports reflects an increase in methylphenidate abuse in Switzerland [23] or a change in reporting due to heightened awareness remains unclear. Frequent recreational use of methylphenidate was recently noted on the Zurich party scene [24, 25]. An increase in methylphenidate abuse has also been observed in the United States in recent years [1, 2, 26] and abuse of ADHD medications has risen in line with the prescription of these drugs [4]. It is of note that approximately 40% of the methylphenidate abusers in our case series were taking methylphenidate as a prescribed drug, indicating diversion from the intended medical use. These exposures concerned intended use of methylphenidate in higher than prescribed doses or snorting of crushed tablets. Misuse of prescription stimulants was reported by others in 14-22% of ADHD patients, typically those with substance use disorders [27, 28]. To the best of our knowledge there are currently no comparable data available in Switzerland, though our retrospective survey shows a clear pattern of increasing reports of methylphenidate abuse since 2004. While there were no cases of higher than moderate toxicity in our dataset, the increasing number of methylphenidate exposures reported to the STIC in recent years is of concern and requires further investigation regarding both prevalence of abuse and potential medical consequences.
Although the estimated number of adolescent and adult Swiss who report having used cocaine or MDMA at least once in their lifetime increased from 1.6% to 2.8% and from 1% to 1.8% respectively from 1997 to 2007 [9], these apparent increases in use prevalence were not reflected in an increased number of reports to the STIC. In our study most reports relating to amphetamines concerned MDMA. There were only 41 reports of methamphetamine abuse, indicating that abuse of this substance may not be a major issue in Switzerland [24, 25], unlike in countries and regions such as the United States, Asia, and Australia [3, 5, 6].
The majority of amphetamine or MDMA exposures resulted only in mild to moderate toxicity. In agreement with other studies [12, 13] the most frequent clinical signs and symptoms were tachycardia, arterial hypertension, nausea, agitation, and anxiety/panic. Among cases with a known medical outcome, 15% of exposures to MDMA alone and 21% of co-ingestions including MDMA were reported to have resulted in severe toxicity, in three cases resulting in a fatal outcome. MDMA increases antidiuretic hormone secretion which, together with profuse sweating and increased intake of water, may result in hyponatraemia and cerebral oedema [29, 30]. However, hyponatraemia was reported in only two patients with MDMA mono-intoxication (5% of the cases with available laboratory values) and there were no reported cases of brain oedema or significant central nervous system depression when MDMA was used alone. In accordance with a case series of MDMA exposures presenting at the emergency department of the University Hospital of Zurich [12], our data indicate that, in Switzerland at any rate, hyponatraemia with associated cerebral oedema is a very rare complication of MDMA use. In contrast, hyponatraemia was found in 52% of the female and in 22% of the male intoxication cases reported to the California Poison Control System [31]. However, the latter case series also included subjects who ingested other substances besides MDMA.
Among cases with a known medical outcome, 14% of exposures to cocaine alone and 17% of co-ingestions including cocaine were reported to have resulted in severe toxicity, including 5 fatalities. Severe toxicity in these cocaine exposures most frequently resulted from severe agitation, severe hypertension, and myocardial ischaemia.
We observed differences in user characteristics and the pattern of use between the amphetamines and cocaine. Amphetamine – including MDMA exposure – involved a younger population and was mainly reported at weekends, while cocaine users were older and abuse occurred relatively more frequently on weekdays. Parenteral drug use and co-use of heroin was reported more frequently in cocaine users as compared to the amphetamine and MDMA groups. These findings are in line with other reports that amphetamines, including MDMA, are typically used as party drugs [32] while cocaine may, at least in part, be consumed on a more regular basis and by another user population. Different groups of cocaine users have been described, and socially marginalised users who are not in an addiction treatment programme typically administer cocaine by intravenous injection and co-use heroin or multiple substances [7].
Our study has several limitations [33]. First, case reports to the STIC describe the nature of the cases that are reported rather than presenting the true prevalence and characterising medical problems among the stimulant-using population in Switzerland. Data from poison control centres are subject to reporting bias [33]. It is not known how many physicians report their cases to the STIC. Deaths among stimulant users are also underreported because they often occur outside a health care facility. According to mortality counts obtained by us from the Swiss Federal Statistical Office there were 52 deaths associated with cocaine use (International Classification of Diseases (ICD) codes F14.0-14.9) in the 13-year study period, while only 5 were reported to the STIC. However, these deaths also included criminal and suicide cases. No data were available on deaths associated with use of other stimulant drugs. Physicians may not have mentioned all their patients’ symptoms or laboratory data, and underreporting of cases with no or only minor toxicity is likely to have occurred. Second, laboratory confirmation was not available in about two thirds of reported cases, but to minimise misclassification in the case of missing laboratory data we included only cases where the causal relationship between drug use and symptoms was at least likely. Third, it has to be acknowledged that a positive drug screening test for amphetamines does not differentiate between amphetamine, methamphetamine, or MDMA, and therefore misclassification between these groups might have occurred. In addition, a positive urine screen for stimulants may not indicate poisoning but rather exposure, given the length of time needed for detection in urine. Similarly, the clinical data available to the STIC cannot be used to discriminate acute from chronic toxicity associated with stimulant use.
In summary, our study showed an increase in reports of methylphenidate abuse over recent years, indicating the need for further studies. Reported exposures to stimulants in Switzerland are predominantly associated with minor to moderate toxicity, but severe toxicity occurred in approximately 15% of the reported cases of MDMA and cocaine abuse with a known medical outcome. In addition, we documented differences in user characteristics, time of use, and medical complications associated with amphetamines, including MDMA, as compared to cocaine.
1 McCabe SE, Knight JR, Teter CJ, Wechsler H. Non-medical use of prescription stimulants among US college students: prevalence and correlates from a national survey. Addiction. 2005;100:96–106.
2 Kroutil LA, Van Brunt DL, Herman-Stahl MA, Heller DC, Bray RM, Penne MA. Nonmedical use of prescription stimulants in the United States. Drug Alcohol Depend. 2006;84:135–43.
3 McKetin R, Kozel N, Douglas J, Ali R, Vicknasingam B, Lund J, et al. The rise of methamphetamine in Southeast and East Asia. Drug Alcohol Rev. 2008;27:220–8.
4 Setlik J, Bond GR, Ho M. Adolescent Prescription ADHD Medication Abuse Is Rising Along With Prescriptions for These Medications. Pediatrics. 2009;124:875–80.
5 Maxwell JC, Rutkowski BA. The prevalence of methamphetamine and amphetamine abuse in North America: a review of the indicators, 1992–2007. Drug Alcohol Rev. 2008;27:229–35.
6 Degenhardt L, Roxburgh A, Black E, Bruno R, Campbell G, Kinner S, et al. The epidemiology of methamphetamine use and harm in Australia. Drug Alcohol Rev. 2008;27:243–52.
7 Prinzleve M, Haasen C, Zurhold H, Matali JL, Bruguera E, Gerevich J, et al. Cocaine use in Europe – a multi-centre study: patterns of use in different groups. Eur Addict Res. 2004;10:147–55.
8 Andersson B, Hibell B, Beck F, Choquet M, Kokkevi A, Fotiou A, et al. Alcohol and Drug Use Among European 17–18 Year Old Students: Data from the ESPAD Project. Stockholm, Sweden: Swedish Council for Information on Alcohol and Other Drugs (CAN), The Pompidou Group at the Council of Europe and the authors, 2007.
9 SFA. Schweizerische Fachstelle für Alkohol- und andere Drogenprobleme. Berechnungen auf Basis der Schweizerischen Gesundheitsbefragung 2007. Available from http://www.sfa-ispa.ch/ 2009.
10 McCabe SE, Boyd CJ, Teter CJ. Subtypes of nonmedical prescription drug misuse. Drug Alcohol Depend. 2009;102:63–70.
11 Michaud PA, Berchtold A, Jeannin A, Chossis I, Suris JC. Secular trends in legal and illegal substance use among 16 to 20 year old adolescents in Switzerland. Swiss Med Wkly. 2006;136:318–26.
12 Liechti ME, Kunz I, Kupferschmidt H. Acute medical problems due to Ecstasy use. Case-series of emergency department visits. Swiss Med Wkly. 2005;135:652–7.
13 Gray SD, Fatovich DM, McCoubrie DL, Daly FF. Amphetamine-related presentations to an inner-city tertiary emergency department: a prospective evaluation. Med J Aust. 2007;186:336–9.
14 Brody SL, Slovis CM, Wrenn KD. Cocaine-related medical problems: consecutive series of 233 patients. Am J Med. 1990;88:325–31.
15 Williams H, Dratcu L, Taylor R, Roberts M, Oyefeso A. “Saturday night fever”: ecstasy related problems in a London accident and emergency department. J Accid Emerg Med. 1998;15:322–6.
16 Richards JR, Bretz SW, Johnson EB, Turnipseed SD, Brofeldt BT, Derlet RW. Methamphetamine abuse and emergency department utilization. West J Med. 1999;170:198–202.
17 Cregg MT, Tracey JA. Ecstasy abuse in Ireland. Ir Med J. 1993;86:118–20.
18 Guirguis M, Gossweiler B, Kupferschmidt H, Lorent JP, Rauber C, Meier PJ. TOXI – A multifunctional system for poisons information and clinical toxicological data evaluation. J Toxicol Clin Toxicol. 1999;37:405.
19 Liechti ME, Kupferschmidt H. Gamma-hydroxybutyrate (GHB) and gamma-butyrolactone (GBL): analysis of overdose cases reported to the Swiss Toxicological Information Centre. Swiss Med Wkly. 2004;134:534–7.
20 Persson HE, Sjoberg GK, Haines JA, Pronczuk de Garbino J. Poisoning severity score. Grading of acute poisoning. J Toxicol Clin Toxicol. 1998;36:205–13.
21 Hintze J. NCSS and PASS statistical & power analysis software. Kaysville, Utah, USA, 2004.
22 Liechti ME, Kunz I, Greminger P, Speich R, Kupferschmidt H. Clinical features of gamma-hydroxybutyrate and gamma-butyrolactone toxicity and concomitant drug and alcohol use. Drug Alcohol Depend. 2006;81:323–6.
23 Livio F, Rauber-Lüthy C, Biollaz J, Holzer L, Winterfeld U, Buclin T. Methylphénidate et abus. Pediatrica. 2009;20:41–4.
24 Schaub M, Quinteros-Hungerbühler I. Lokale Trendstudie. Zurich: Institut für Sucht- und Gesundheitsforschung Zürich (ISGF), 2010.
25 Kostka R, Monego R, Rüegg S, Suter D, Zeltner C. Monitoringbericht Drogen und Sucht 2010. Zurich, Switzerland: Stadt Zürich, 2010.
26 Klein-Schwartz W, McGrath J. Poison centers' experience with methylphenidate abuse in pre-teens and adolescents. J Am Acad Child Adolesc Psychiatry. 2003;42:288–94.
27 Bright MG. Abuse of medications employed for the treatment of ADHD: results from a lage-scale community survey. Medscape J Med. 2008;10:111.
28 Wilens TE, Gignac M, Swezey A, Monuteaux MC, Biederman J. Characteristics of adolescents and young adults with ADHD who divert or misuse their prescribed medications. J Am Acad Child Adolesc Psychiatry. 2006;45:408–14.
29 Forsling M, Fallon JK, Kicman AT, Hutt AJ, Cowan DA, Henry JA. Arginine vasopressin release in response to the administration of 3,4-methylenedioxymethamphetamine (“ecstasy”): is metabolism a contributory factor? J Pharm Pharmacol. 2001;53:1357–63.
30 Liechti ME. “Ecstasy” (MDMA): pharmacology, toxicology, and treatment of acute intoxication. Dtsch Med Wochenschr. 2003;128:1361–6.
31 Rosenson J, Smollin C, Sporer KA, Blanc P, Olson KR. Patterns of ecstasy-associated hyponatremia in California. Ann Emerg Med. 2007;49:164–71, 71 e1.
32 Dughiero G, Schifano F, Forza G. Personality dimensions and psychopathological profiles of Ecstasy users. Hum Psychopharmacol. 2001;16:635–9.
33 Hoffman RS. Understanding the limitations of retrospective analyses of poison center data. Clin Toxicol. (Phila) 2007;45:943–5.
No funding; no competing interests.