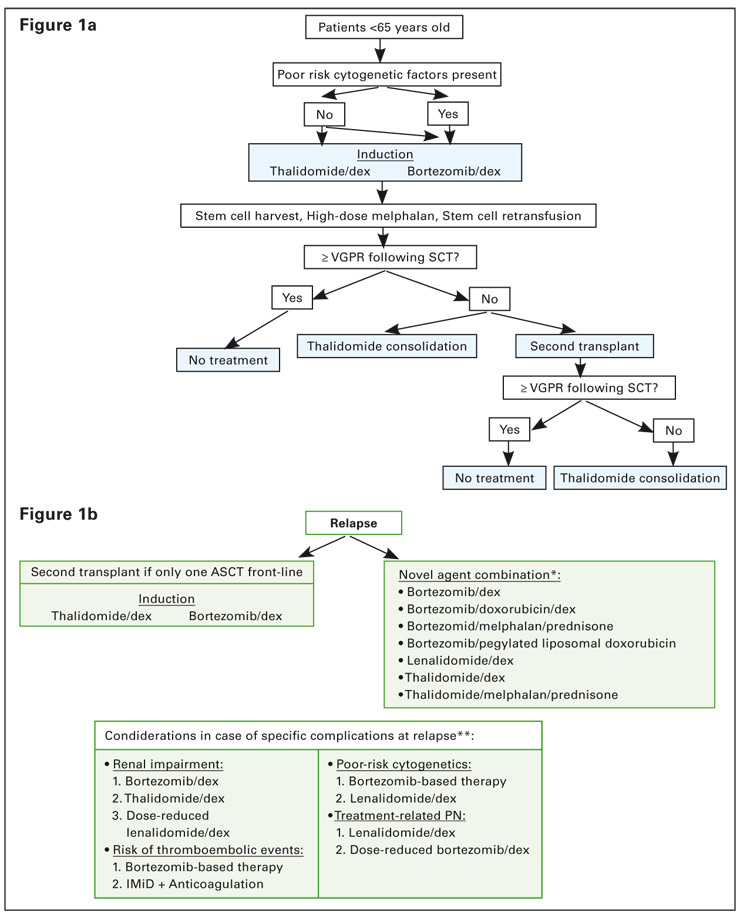
Figure 1
MM treatment tree – transplant setting.
* alphabetical order, † order of preference
DOI: https://doi.org/10.4414/smw.2010.13054
Multiple myeloma (MM) is a B-cell neoplasm that stems from the malignant transformation of plasma cells in the bone marrow. It is characterised by skeletal destruction, renal failure, anaemia and hypercalcaemia. Multiple myeloma is the second most frequent haematological malignancy and its incidence rises with increasing age. Traditionally, multiple myeloma was associated with a poor prognosis with a median survival of 3–5 years from diagnosis. Although the disease remains incurable with conventional therapy, the outlook for patients has improved recently due to advances in active therapy, as well as supportive care [1]. Notably, the last few years have seen the introduction of a number of novel agents, such as thalidomide, bortezomib and lenalidomide, which are increasingly being incorporated into present-day treatment practices.
An expert meeting was convened to discuss current treatment practices in Swiss myeloma centres with a focus on the use of novel agents and to assess the evidence for the use of these agents in different settings. Reports of phase II and phase III studies with the novel agents thalidomide, lenalidomide and bortezomib, either fully published or presented as meeting abstracts between 2005 and 2009, were reviewed with regard to their relevance for clinical practice in Switzerland. These and the considerations from discussions at a meeting among European experts [2] formed the basis for discussions at the expert meeting and for the recommendations that were formulated and are summarised in this article.
Concerning the trials investigating novel agents, there is a lack of head to head comparisons. In addition, cross-trial comparisons can only be made with caution because of substantial differences in trial designs, study populations and outcome criteria. Furthermore, patient numbers in sub-analyses are often limited, thereby complicating the possibility to draw firm conclusions. Taking into account these limitations, an attempt has been made to provide an overview of the data and recommendations for daily clinical practice.
A diagnosis of a symptomatic MM requires the presence of clonal plasma cells in the bone marrow, monoclonal (M-) protein in the serum and/or urine (except in patients with true non-secretory myeloma), and evidence of myeloma-induced clinical symptoms (hypercalcaemia, renal insufficiency, anaemia, or bone lesions). Asymptomatic or smoldering MM is defined by a serum M-protein ≥30 g/L, ≥10% clonal plasma cells in the bone marrow and no organ or tissue damage. Early treatment of high risk asymptomatic MM is currently under investigation [3]. Myeloma is often preceded by a monoclonal gammopathy of undetermined significance (MGUS) state which does not require treatment (table 1) [4, 5].
The following tests are recommended to confirm the diagnosis of multiple myeloma [5, 6]:
– Biological assessments to differentiate symptomatic and asymptomatic MM: haemoglobin (and full blood cell count), serum creatinine and calcium levels
– Detection and evaluation of M-component by serum and urine protein electrophoresis and immunofixation
– Quantification of IgG, IgA and IgM immunoglobulins
– Serum free light chain measurement to identify and monitor non-secretory MM
– Bone marrow biopsy and aspirate to evaluate bone marrow plasma cell infiltration, including cytogenetic examination and immunophenotyping
– Full skeleton X-ray survey to evaluate the extent of lytic bone lesions
– Determination of β2-microglobulin, C-reactive protein (CRP) and lactate dehydrogenase (LDH) levels
| Table 1Diagnostic criteria for monoclonal gammopathy of undetermined significance (MGUS), asymptomatic (smoldering) multiple myeloma and multiple myeloma [4, 5]. | |||
| Monoclonal gammopathyof undetermined significance (MGUS) | Asymptomatic (smoldering) multiple myeloma | Multiple myeloma | |
| Bone marrow plasma cells | <10% | ≥10% | Present |
| and | and/or | and | |
| M-protein | <30 g/L | ≥30 g/L | Present |
| and | and | and | |
| Clinical symptoms (hypercalcaemia, renal insufficiency, anaemia and bone lesions [CRAB]) | Absent | Absent | Present |
| In case of non-secretory multiple myeloma, the criterion of an M-Protein >30 g/L for the diagnosis of symptomatic MM is not mandatory. | |||
The criteria for the assessment of response and progression are based on those developed by the European Group for Blood and Bone Marrow Transplantation (EBMT), commonly known as the Bladé criteria [7]. The EBMT criteria were modified in the International Myeloma Working Group (IMWG) as uniform response criteria which are widely used and are recommended [8]. The modifications included the addition of very good partial response (VGPR) and stringent response criteria. Furthermore, free light chain response and progression criteria for patients without measurable disease have been added.
Clinical relapse is defined by the occurrence of one or more of the following: development of new or increase in soft tissue plasmacytomas or bone lesions, hypercalcaemia, decrease in haemoglobin or rise in serum creatinine [8]. Progressive disease is defined as an increase in 25% in any one or more of the following: serum or urine M-component, bone marrow plasma cell percentage, new bone lesions or soft tissue plasmacytomas, hypercalcaemia. Importantly, the IMWG criteria stipulate that these are also the criteria for progression from CR.
The decision to initiate treatment is determined by the stage of the disease, as defined by the Durie-Salmon (DS) or the International Staging Systems (ISS) (table 2), as well as by the parameters contained in the CRAB criteria (C = increased calcium level; R = impaired renal function; A = anaemia; B = bone lesions). Patients with stage I disease and without symptoms or those with smoldering or indolent myelomas do not require treatment and are generally managed by close observation. Once the disease advances (stage ≥II) or symptoms develop, treatment is initiated.
The decision to follow an intensive treatment pathway consisting of induction treatment followed by high-dose chemotherapy (high-dose melphalan) and stem cell transplantation on the one hand, or a non-intensive treatment option using chemotherapy alone on the other hand, is taken based on the age of the patient, their performance status and the presence of comorbidities. For young patients, generally considered to be those below 65 years of age, and those without significant comorbidities, high-dose therapy followed by autologous stem cell transplantation (ASCT) is considered the standard of care. For patients older than 65 years or those with significant comorbidities, tolerability considerations preclude the use of stem cell transplantation.
| Table 2Staging systems for multiple myeloma: Durie-Salmon staging system [90] and the International staging system [91]. |
| Durie-Salmon staging system |
| Stage I |
| All of the following criteria have to be fulfilled:Haemoglobin >10 g/dLCalcium <3 mmol/LNormal bone structure on conventional X-ray analysis or solitary plasmacytoma onlyLow M-component production ratesIgG <50 g/LIgA <30 g/LUrine light chain M-component on electrophoresis <4 g/24 h |
| Stage II |
| Fulfilling the criteria of neither I nor III |
| Stage III |
| One or more of the following criteria have to be fulfilled:Haemoglobin <8.5 g/dLCalcium >3 mmol/LAdvanced lytic bone lesionsHigh M-component production ratesIgG >70 g/LIgA >50 g/LUrine light chain M-component on electrophoresis >12 g/24 h |
| SubclassificationA: serum creatinine <177 μmol/LB: serum creatinine ≥177 μmol/L |
| International staging system | ||
| Stage | Criteria | Median survival (months) |
| I | serum β2-microglobulin <3.5 mg/L andserum albumin ≥3.5 g/dL | 62 |
| II | serum β2-microglobulin <3.5 mg/L andserum albumin <3.5 mg/dLorserum β2-microglobulin 3.5–5.5 mg/L | 44 |
| III | serum β2-microglobulin ≥5.5 mg/L | 29 |
In the transplant setting, the achievement of a sustained complete response (CR) is generally considered to be associated with prolonged treatment-free intervals [9, 10]. In addition, achieving CR may also have a positive impact on quality of life and may result in prolonged survival. Achieving CR is therefore considered an important treatment goal in young patients to increase overall survival (OS) and improve overall patient outcome.
The group of patients ineligible for transplantation is heterogeneous and treatment goals will depend on the individual situation, as well as patient preference. For some patients, achievement of CR may be the desired goal with the aim of lengthening survival, whereas for others, quality of life considerations may be more important. Nevertheless, studies indicate that also in this patient population the achievement of a sustained CR is associated with improved outcome [11, 12].
Prior to stem cell mobilisation, induction therapy is administered to reduce the tumour burden. Before the arrival of novel agents, the combination of vincristine, doxorubicin and dexamethasone (VAD) was the induction regimen of choice. However, recent studies have shown that this regimen is inferior to induction regimens incorporating novel agents. Two induction regimens are currently widely used in Switzerland and are recommended: the combination of thalidomide and dexamethasone (thal/dex) and the combination of bortezomib and dexamethasone (bortezomib/dex). If poor-risk cytogenetic features are present, bortezomib/dex may be the treatment of choice, whereas in the absence of such factors, both regimens can be recommended. It should be noted that bortezomib/dex is currently not approved as a first line induction regimen, and that thalidomide has no approved indication for MM in Switzerland. Figure 1a shows a possible treatment tree in the transplant setting.

Figure 1
MM treatment tree – transplant setting.
* alphabetical order, † order of preference
Thal/dex was shown to increase response rates compared to VAD in several studies (table 3) [13, 14]. In a retrospective matched case-control analysis that compared thal/dex with VAD, thal/dex resulted in a significantly higher pre-transplant overall response rate (ORR) (76% vs 52%, p <0.001), while CR and near CR (nCR) rates were comparable between the two arms (13% in each arm) [13]. In the final analysis of the phase II Bologna 2002 study with a median follow up of 43 months, thal/dex induction followed by double ASCT resulted in a CR rate of 33% and a median TTP and progression-free survival (PFS) of 68 months and 47 months, respectively. The 5-year projected OS rate was 65% [15]. In another study, response rates to thal/dex and VAD pre- and post-transplant were investigated [14]. While thal/dex resulted in a significantly higher VGPR rate than VAD prior to transplant (35% vs 13%, p = 0.002), there was no significant difference in VGPR between the two regimens six months post-transplant (44% vs 42%, p= 0.87).
The combination of bortezomib/dex as induction therapy was compared with VAD in a randomised phase III study conducted by the French myeloma study group (IFM) [16]. Results following induction treatment demonstrated a significant advantage for bortezomib/dex over the VAD regimen: the ORR was 82% for bortezomib/dex versus 65% for VAD (p <0.0001), with CR and nCR rates of 15% versus 7%, respectively (p = 0.0035) and CR and VGPR rates of 39% versus 16%, respectively (p<0.0001). Post-transplant, 40% of patients receiving bortezomib/dex had CR or nCR versus 22% of patients receiving VAD (p = 0.0001). The CR and VGPR rates were 61% versus 44% (p = 0.0007), respectively. Importantly, CR and VGPR rates remained higher with bortezomib/dex than with VAD post-transplant. With a median follow-up of two years, the median PFS was not reached for bortezomib/dex, while for VAD, median PFS was 28 months. There was a significant difference in 2-year PFS between the two arms (bortezomib/dex 69% vs VAD 60%, p = 0.0115), while 2-year OS was comparable in both arms (90% vs 88%, p = 0.4689) (table 3).
The combination of bortezomib/dex resulted in a significantly higher ≥ VGPR rate than VAD in patients with deletion of chromosome 13 [del(13)] (p <0.0001) and translocation of chromosomes 4 and 14 [t(4;14)] and/or del(17) (p = 0.04) [16], indicating that the bortezomib combination remains effective in patients with unfavourable cytogenetic abnormalities. Based on these results, the bortezomib/dex combination is therefore regarded as the preferred regimen in the presence of unfavourable cytogenetic abnormalities.
Induction treatment is generally administered until best response, which usually corresponds to 3–4 cycles prior to high-dose therapy.
The dosing regimen followed for the bortezomib/dex combination consists of 3–4 21-day cycles with bortezomib at 1.3 mg/m2 on days 1, 4, 8, 11 and dexamethasone at 40 mg on days 1–4 (cycles 1–4) and days 9–12 (cycles 1–2 only) (total dexa-methasone dose = 320 mg/cycle for cycles 1–2 and 160 mg/cycle for cycles 3–4).
Thal/dex is generally administered for four 28-day cycles according to the following schedule:
Thalidomide 100 mg/d for 14 days, then increased to 200 mg/d. Dexamethasone 40 mg/d on days 1–4, 9–12 and 17–20 (odd cycles) and 40 mg/d days 1–4 on even cycles. In some centres, thalidomide is given at 200 mg/d from the start, whereas in others, the dose is escalated from 50 mg/d or 100 mg/d in the first 7–10 days to 200 mg/d within the first three weeks. Thalidomide may also be administered at 100 mg/d throughout. Dexamethasone may be given in the four day blocks for all four cycles. However, in some centres, dexamethasone is administered only once weekly on days 1, 8, 15 and 22 of the 28-day cycle.
This reduced dose dexamethasone schedule is based on the results of a phase III ECOG trial which investigated lenalidomide in combination with high-dose dexamethasone (RD) (dexamethasone 40 mg/d administered on days 1–4, 9–12, 17–20 [total dexamethasone dose = 480 mg per cycle]) versus lenalidomide plus low-dose dexamethasone (Rd) (dexamethasone 40 mg/d administered on days 1, 8, 15, 22 [total dexamethasone dose = 160 mg per cycle]) in 445 patients with newly diagnosed MM [17]. The study was designed to compare the two arms as first-line induction treatment prior to ASCT. The primary endpoint of response rate at four months was higher with RD (79% versus 68%, p = 0.02), but 1-year OS was significantly higher on the Rd arm (96% versus 87%, p = 0.0002). At three years, however, OS was reported to be comparable in both arms (3-year OS 75% for RD and Rd) [18]. In the group of patients receiving lenalidomide and high-dose dexamethasone, significantly more toxicities were observed and the rate of early deaths was also significantly higher. Although thalidomide in combination with low-dose dexamethasone has not been compared to thalidomide plus high-dose dexamethasone in a front-line clinical trial, the results of the ECOG trial may indicate that a low-dose dexamethasone regimen is preferable.
Prior to stem cell collection, patients may receive granulocyte colony-stimulating factor (G-CSF) or G-CSF plus cyclophosphamide to ensure that an adequate number of cells can be harvested. In addition, the combination of vinorelbine plus G-CSF is a feasible option to enable mobilisation and this combination is used in many Swiss centres [19].
Currently, high-dose therapy prior to transplantation consists of melphalan 200 mg/m2, with or without high-dose dexamethasone. Total body irradiation is no longer administered.
The benefit of a second transplantation is currently challenged by the high efficacy of novel agents used as induction therapy followed by one single ASCT and their efficacy as post-ASCT consolidation. In a French, as well as in an Italian randomised trial, only patients achieving less than a VGPR after the first ASCT derived benefit from a second transplant [20, 21]. Therefore, a second transplant is recommended in case patients do not achieve at least a VGPR following the first ASCT.
Currently, there is limited data to suggest a benefit for thalidomide maintenance treatment for all patients after ASCT. In particular, the benefit for thalidomide maintenance therapy for patients who are in CR after autologous transplantation has not been demonstrated. In addition, recent data indicate that thalidomide maintenance therapy may be unfavourable in patients with del(17) [22], and not of benefit in case of del(13) [23]. Therefore, routine thalidomide maintenance therapy can currently not be recommended. Thalidomide may be beneficial as a consolidation treatment in patients who do not achieve at least a VGPR after the second ASCT procedure based on the results by Attal et al. [23], or for patients for whom a second ASCT is not feasible. The duration and the dose of thalidomide in this setting are currently not well defined.
| Table 3Summary of data for recommended induction regimens: thal/dex and bortezomib/dex. | ||||||||
| Regimen | n | Post-induction | Post-transplant | TTP | OS | Reference | ||
| CR + PR | CR + nCR | CR + PR | CR + nCR | |||||
| Bortezomib/dex vsVAD | 240242 | 82%*65% | 15%*7% | 91%91% | 40%*22% | 2-year PFS:69%*60% | 2-year OS:90%88% | [16] |
| Thal/dex vsVAD (retrospective data) | 100100 | 76%*52% | 13%13% | n/a | n/a | n/a | n/a | [13] |
| Thal/dex vsVAD | 100104 | n/a | ≥VGPR35%*13% | n/a | ≥VGPR44%42% | n/a | n/a | [14] |
| Thal/dex | 357 | n/a | n/a | n/a | 33%† | TTP: 68 monthsPFS 47 months | 5-year OS: 65% | [15] |
| *statistically significant difference between arms; †final CR rate following induction and double autologous stem cell transplantation | ||||||||
The optimal induction regimen is currently unknown and a number of studies are ongoing which are investigating three-drug combinations involving one or two novel agents as well as conventional chemotherapy (e.g., bortezomib/thalidomide/dexamethasone [VTD], bortezomib/lenalidomide/dexamethasone [VRD], bortezomib/cyclophosphamide/dexamethasone [VCD], lenalidomide/cyclophosphamide/dexamethasone [RCD], cyclophosphamide/thalidomide/dexamethasone [CTD]). Recent data suggest that three-drug regimens may be superior over two-drug regimens, for example, combinations such as thalidomide, adriamycin, dexamethasone (TAD), CTD or VTD may be superior over thal/dex alone [24, 25, 26]. Similarly, VTD, VCD or the combination of bortezomib, adriamycin, dexamethasone (PAD) may be superior over bortezomib/dex alone [24, 27, 28]. However, long-term follow ups are needed to provide survival data before recommendations regarding three-drug combinations can be given.
One of the agents undergoing investigation in upfront treatment is lenalidomide. Results of the aforementioned phase III ECOG trial, which investigated two different doses of dexamethasone in combination with lenalidomide, found that the combination is active as induction therapy, although CR rates were low [17, 18]. It also should be noted that the patients undergoing transplantation in this trial were not part of a randomised comparison.
Lenalidomide is also being investigated in combination with bortezomib and dexamethasone (VRD) as a front-line treatment. In a phase I/II trial, this combination was found to result in responses in 98% of patients with a 36% CR and nCR rate [29]. Results from prospective randomised trials are needed before recommendations regarding lenalidomide induction therapy can be given.
Open questions remain regarding the optimal number of induction cycles, and further studies examining the most appropriate consolidation treatment and schedule, including allogeneic transplantation, as well as the role of maintenance therapy are needed. The place of allogeneic transplantation in the management of MM remains controversial due to conflicting phase III results [30–33]. The introduction of reduced-intensity conditioning and improvements in supportive care have significantly decreased treatment-related mortality of allogeneic transplantation in myeloma patients during the past decade. Allogeneic transplantation has the potential to induce long-term disease control. It is an option for younger high-risk patients with HLA compatible donors, who are deriving only minor benefit from conventional treatment. At the current time, allogeneic transplantation cannot be recommended as a routine treatment and should be performed in specialised centres only.
Although high-dose therapy followed by ASCT is considered the standard of care for young patients and those without significant comorbidities, the role of transplantation itself, in the era of novel agents, is increasingly being questioned. With the introduction of these more potent agents it is now possible to achieve CR rates with novel agent-based combinations alone that are comparable to those previously achieved with high-dose therapy plus ASCT. Consequently, the benefit of early high-dose therapy plus ASCT as a consolidation has been questioned for patients achieving a CR after modern induction therapy, since these patients might not derive benefit from this procedure. Currently, the strategy of using first-line therapies with novel agents in transplant-eligible patients without proceeding to transplant should only be investigated in well-designed clinical trials.
Finally, further research is needed to define the optimal timing of transplantation, that is either following induction or as a salvage treatment at relapse after first-line therapy with a novel agent-based non-transplant regimen.
Currently, there are two treatment regimens that have become standard for the treatment of patients who are not eligible for transplantation: the combination of melphalan, prednisone and thalidomide (MPT) and the combination of MP and bortezomib (VMP). Figure 2a shows a possible treatment algorithm in the non-transplant setting.
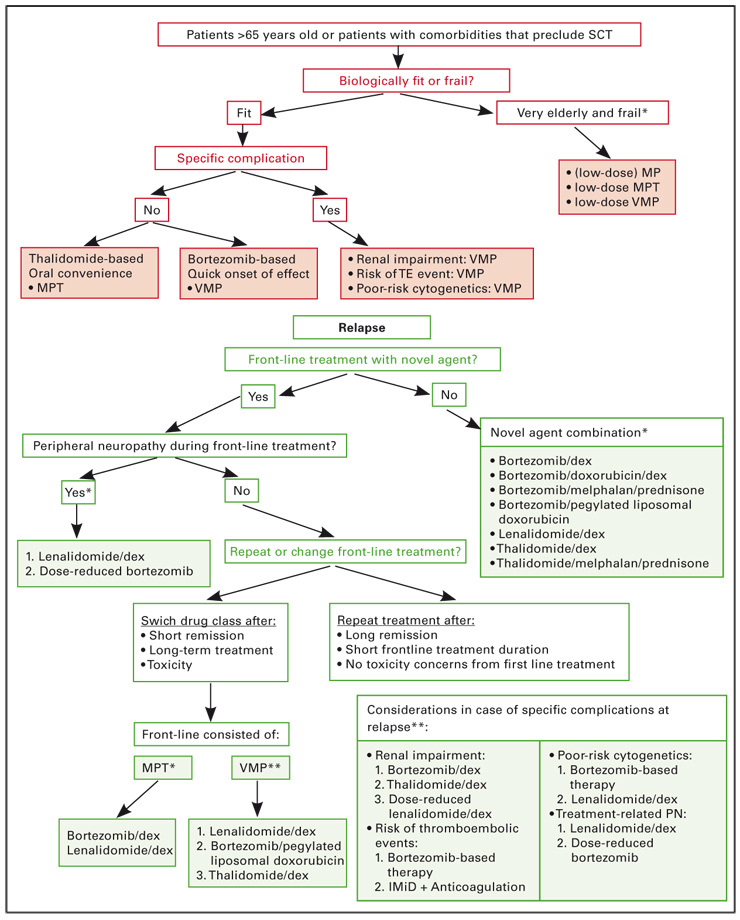
Figure 2
MM treatment tree – non-transplant setting* alphabetical order
The combination of MPT has been compared with MP in five randomised studies [34-38]. In all studies, the addition of thalidomide to MP resulted in a significant improvement in ORR and CR rates, as well as in TTP, PFS or event-free survival (EFS) (table 4). In two of the studies, a significant benefit in terms of OS was also seen [34, 35].
VMP has been shown to be superior over MP in the international phase III VISTA trial [39, 40]. The ORR, determined using the stringent EBMT criteria, was 71% with VMP, compared with 35% with MP, with an immunofixation-negative CR rate of 30% with VMP versus 4% with MP (p <0.000 001). TTP was significantly longer in the VMP arm than in the MP arm (24 months versus 16.6 months, p <0.0 000 001). Although median OS was not reached in either arm after a median follow-up of 25.9 months, VMP demonstrated a significantly superior 3-year OS rate compared with MP: 72% versus 59%, respectively (p = 0.0032) (table 4).
Based on these results, both VMP and MPT are recommended for the treatment of patients not eligible for transplantation. Currently, VMP is approved for this indication in Switzerland, whereas MPT is not.
If MPT is chosen, prophylactic anticoagulation is needed, while with VMP co-administration of antiviral prophylaxis is recommended to prevent the occurrence of Herpes zoster.
Importantly, both MPT and VMP have demonstrated efficacy for the treatment of very elderly patients. MPT was significantly more effective than MP in very elderly patients, as demonstrated by Hulin et al. in patients >75 years of age [35]. Regarding VMP, a subanalysis of the VISTA trial showed that VMP was more effective than MP in patients <75, as well as in those >75 years of age [41].
In the presence of particular disease characteristics, such as cytogenetic abnormalities and renal impairment, the VMP regimen may be appropriate, as indicated by results from a subgroup analysis of the VISTA trial which showed that the regimen is more effective than MP in patients with high-risk disease. In the VMP arm, TTP and OS were found to be similar in patients with high-risk cytogenetic features, such as t(4;14), t(14;16) and del(17), and those with standard-risk features [40]. In addition, the VMP regimen was shown to be more effective than MP in patients with renal impairment in terms of ORR, CR, duration of response and TTP. More patients had reversal of renal impairment with VMP than MP (44% vs 34%) [42]. VMP may also be the regimen of choice in case of a risk of venous thrombolic events (VTEs) as bortezomib is not associated with increasing the risk of this complication, unlike thalidomide and lenalidomide.
Although MP is no longer considered a stan-dard treatment for the non-transplant group, it may be a useful option in very elderly and frail patients. In some cases, it may be administered at a reduced dose.
Treatment is generally administered until a plateau of the paraprotein is reached, unless discontinuation is required due to toxicity. Once CR is reached, two additional cycles of therapy are administered, if not ruled out by tolerability concerns.
In the non-transplant setting, the role of consolidation/maintenance therapy is not well defined and only limited data is currently available. It is not clear if thalidomide treatment is of benefit in this setting and it has been suggested that thalidomide maintenance may play a role in inducing drug-resistant relapses [36]. In addition, data from the MRC Myeloma IX study showed that thalidomide maintenance treatment was associated with an unfavourable outcome in patients with del(17) [22].
| Table 4Summary of phase III data for recommended regimens in front-line non-transplant setting: MPT and VMP. | ||||||
| Regimen | n | CR + PR (%) | CR (%) | TTP/PFS/EFS | OS | Reference |
| VMP vsMP | 337331 | 7135 | 304 | 24 months16.6 months | 3-year OS:72%*59% | [39, 40] |
| Thal/MP vsMP | 129126 | 7648 | 164 | 21.814.5 | 45 m47.6 m | [36] |
| Thal/MP vsMP | 191124 | 7635 | 132 | 27.5 m17.8 m | 51.6 m*33.2 m | [34] |
| Thal/MP vsMP | 113116 | 6231 | 71 | 24.1 m18.5 m | 44 m*29.1 m | [35] |
| Thal/MP vsMP | 363 | 4228 | 6†3† | 20 m18 m | 29 m33 m | [37] |
| Thal/MP vsMP | 152149 | 6647 | 22 | EFS 13 m vs 9 mPFS 13 m vs 10 m | 37 m30 m | [38] |
| † CR + nCR*statistically significant difference in OS between arms | ||||||
A number of studies are currently ongoing to define the role for lenalidomide in the non-transplant setting. The aforementioned phase III ECOG trial, which was designed to investigate lenalidomide in combination with either high- or low-dose dexamethasone as front-line induction therapy prior to ASCT, also included patients who were ≥65 years old (n = 233). Among these, the 1-year survival for patients randomised to Rd was significantly superior to that in the RD group (94% vs 83%, p = 0.004), suggesting that the combination of lenalidomide and low-dose dexamethasone (Rd) may be an effective regimen in elderly patients, who are not candidates for transplantation [17]. It has to be noted that the trial was designed as an induction study with the primary endpoint of response rate at four months and was not intended to examine the efficacy of long-term lenalidomide/dexamethasone, therefore adding complexity to the interpretation of the results. Moreover, the comparison of the two arms in this elderly population was not a prospective randomised comparison. Lenalidomide in combination with MP has shown positive results in a phase I/II trial. In 21 patients, the ORR was 81%, with 47.6% achieving at least a VGPR, median TTP was 28.5 months and 2-year OS 90.5% [43]. An ongoing randomised phase III trial is investigating this combination in comparison with MP, and in another ongoing phase III trial, MPT is being compared with lenalidomide in combination with low-dose dexamethasone.
Other questions in the non-transplant setting concern the role of other novel agent combinations, such as bortezomib/dex, as well as the use of consolidation and maintenance therapy and how to best tailor treatment to the individual patient situation. Adapting treatment to the individual patient situation by adjusting the doses of different agents is of interest to maintain efficacy while improving tolerability, and is discussed further in the section on the management of adverse events.
The decision to initiate treatment for relapse (both in the transplant and non-transplant setting) is, again, based on the CRAB criteria. An increase in the paraprotein level plays a secondary role, especially in early relapse.
In general, the choice of treatment at relapse is influenced by the efficacy of the previous treatment, as well as toxicity considerations. Following a defined course of initial treatment and after a long duration of remission (>6–12 months) in the absence of toxicity, a repetition of the initial therapy is feasible. On the other hand, if the remission duration was short and there are tolerability concerns, then a switch in treatment is indicated.
Figure 1b outlines possible treatment approaches at relapse after front-line ASCT and table 5 provides a summary of the data for the different recommended treatments.
Following relapse after one prior transplantation procedure, a second transplantation at relapse is a feasible option if the remission duration was at least 2–3 years, although it is also feasible to consider a second transplantation already after a remission duration of >12 months. It may be useful to employ a different induction regimen from the one used initially, although retreatment with the initial regimen may be feasible after a long duration of response. If transplantation is not an option at relapse, combination therapy including novel agents may be indicated.
In case of upfront treatment with thalidomide, a change to a bortezomib- or lenalidomide-containing regimen may be useful. A number of bortezomib-based treatment options exist, which have all demonstrated efficacy in the treatment of relapsed/refractory disease. Bortezomib is frequently combined with dexamethasone and the combination has been shown to improve response rates compared to those achieved with bortezomib alone. With bortezomib monotherapy in patients with relapsed MM, an ORR of 43% and a CR and nCR rate of 16% were observed in the phase III APEX trial compared with 18% and 2% , respectively, for dexamethasone; TTP was 6.2 months and OS was 29.8 months with bortezomib versus 3.5 months and 23.7 months with dexamethasone [44]. The difference in survival was significant, despite more than 62% of dexamethasone patients crossing over to receive bortezomib. In a recent report, the addition of dexamethasone to bortezomib in relapsed/refractory disease was found to result in an ORR of 58.3% with a 32.5% CR rate [45]. Median TTP was 10 months and OS, at a median follow up of 14.1 months, was 66.1%. The addition of pegylated liposomal doxorubicin (PLD) to bortezomib has also been found to significantly extend TTP and OS compared to bortezomib alone. TTP was 9.3 months with the combination of bortezomib plus PLD versus 6.5 months with bortezomib alone (p = 0.000004), and the 15 month survival rate was 76% versus 65%, respectively (p = 0.03) [46]. An analysis of the effect of prior thalidomide exposure revealed that response and TTP were comparable in patients with and without prior thalidomide therapy [47]. A large number of studies have examined bortezomib in combination with steroids, conventional chemotherapy or other novel agents and have demonstrated improved efficacy due to additive or synergistic effects. The combination of bortezomib/thalidomide/dexamethasone (VTD) is currently being investigated in a European trial.
Lenalidomide in combination with dexamethasone (lenalidomide/dex) was analysed in two phase III studies that demonstrated significantly improved ORR, CR, TTP and OS for the combination compared to dexamethasone alone (MM009, MM010 studies) [48–50]. Therefore, lenalidomide/dex can also be recommended in the relapsed/refractory setting. The ORR with lenalidomide/dex was 60.6% versus 21.9% with dexamethasone alone (p <0.001) and the CR rate was 15% versus 2% (p<0.001), respectively. TTP with lenalidomide/dex was 13.4 months, compared with 4.6 months with dexamethasone alone (p <0.001) and the median OS was 38 months versus 31.6 months (p = 0.045), despite 47% of patients in the dexamethasone group crossing over to receive lenalidomide/dex [49]. An analysis of the effects of prior thalidomide exposure on response, TTP, and OS with lenalidomide/dex revealed that the combination remained significantly superior to dexamethasone alone regardless of prior thalidomide administration. Of note, the combination of lenalidomide/dex was significantly more effective than dexamethasone alone in all subgroups of patients with prior exposure to thalidomide, even in those who had relapsed on or had never previously responded to thalidomide. However, in patients who were refractory to thalidomide, treatment with lenalidomide/dex was associated with a reduction in CR, ORR, TTP and PFS compared with those with thalidomide-sensitive disease [51]. Lenalidomide is also being investigated in combination with other agents, such as adriamycin, cyclophosphamide, bortezomib and thalidomide. In general, these combinations result in high response rates. For example, with the combination of lenalidomide, adriamycin and dexamethasone, a 73% ORR and a 15% CR rate were reported and 1-year OS was 88% [52].
The combination of VRD is also being investigated in the relapsed/refractory setting and has shown promising results which require further investigation [53]. Lenalidomide is less neurotoxic than bortezomib and thalidomide, and may be particularly useful if peripheral neuropathy is present from front-line treatment with these agents.
In case of upfront treatment with bortezomib, a change to a thalidomide- or lenalidomide-containing regimen may be reasonable. However, following successful therapy with bortezomib, retreatment with a bortezomib-containing combination is feasible [40]. In addition, after the use of bortezomib in second or later treatment lines, retreatment with this agent appears to be a feasible option [54, 55].
In selected younger patients, allogeneic transplantation may be an option in the relapsed/refractory setting, although the place of allogeneic transplantation in the management of MM remains controversial, as discussed above.
Figure 2b outlines possible treatment approaches at relapse in the non-transplant setting.
Retreatment with the previous therapy can be considered if this therapy was effective and if a long duration of response and a treatment-free period was obtained, with acceptable toxicity. Following front-line treatment with VMP, treatment with lenalidomide/dex or thal/dex should be considered, however, alternatively, retreatment with bortezomib appears feasible [40]. Following front-line treatment with MPT, a bortezomib- or lenalidomide-containing regimen may be chosen at relapse. If front-line treatment did not contain a novel agent, then inclusion of a novel agent at relapse may be beneficial.
Peripheral neuropathy (PN) may be the most prominent toxicity present from front-line treatment with thalidomide or bortezomib and, in such cases, switching to a less neurotoxic agent, such as lenalidomide, at relapse is advisable. The combination of lenalidomide/dex has demonstrated efficacy at relapse and can be recommended. Alternatively, in patients with a long duration of response following bortezomib treatment and with only early signs of PN, dose-reduced bortezomib might be an option.
Generally, treatment is administered until a plateau is reached. If a CR is obtained, then two additional cycles are usually administered.
The duration of treatment for bortezomib and lenalidomide as reported in clinical trials in the relapsed/refractory setting differs. Bortezomib is administered for a limited number of distinct treatment cycles, thereby offering patients the opportunity of treatment-free intervals [44], whereas lenalidomide is administered continuously until disease progression (over 3 weeks in cycles of 4 weeks) [48–50]. The dose of dexamethasone in the lenalidomide/dex combination (total dex dose = 480 mg/cycle) may be reduced to a weekly administration to improve tolerability, based on the results of the aforementioned phase III ECOG trial which investigated lenalidomide in combination with high-dose dexamethasone, versus lenalidomide plus low-dose dexamethasone in the front-line setting [17, 18] (total dex dose = 160 mg/cycle). However, the effect of the reduced-dose regimen on survival in the relapsed/refractory setting has not been prospectively investigated. In contrast, the efficacy of low-dose dexamethasone in combination with bortezomib has been demonstrated in prospective trials in the relapsed/refractory setting [56, 57] (total dex dose = 160 mg/cycle).
| Table 5Summary of data for recommended regimens in relapsed/refractory setting. | |||||||
| Treatment | Study details | n | CR + PR | CR + nCR | TTP | OS | Reference |
| Bortezomib vsdex | III | 333336 | 43%*18% | 16%*2% | 6.2 months*3.5 months | 29.8 months*23.7 months | [44] |
| Bortezomib/dex | IIIb | 638 | 67% | 33% (≥VGPR) | Not evaluated | Not evaluated | [92] |
| Bortezomib/dex | retrospective | 192 | 58.3% | 32.5% | 10 months | 66.1%(at 14.1 months) | [45] |
| Bortezomib/pegylated liposomal doxorubicin vsBortezomib | III | 318318 | 52%*44% | 17%13% | 9.3 months*6.5 months | 15 months OS:76%*65% | [46] |
| Bortezomib/doxorubicin/dex | II | 64 | 67% | 25% (≥VGPR) | Not stated | 1-year OS: 66% | [93] |
| Lenalidomide/dex vsDex | III | 353351 | 60.6%*21.9% | 15%*2% (CR) | 13.4 months*4.6 months | 38 months*31.6 months | [48–50] |
| Thal/dex | Systematic review: phase II trials | 451 | 46% | 4% (CR) | weighted median EFS: 8 months | weighted median OS: 27 months | [94] |
| *significant difference between arms | |||||||
Hypercalcaemia is frequently a presenting feature of multiple myeloma which requires prompt attention to minimise renal damage. Hydration should be initiated immediately. Treatment consists primarily of bisphosphonates and, in addition, steroids can be beneficial [5].
Renal impairment/renal failure requires fast action to prevent further decline or to attempt to salvage renal function. Regarding the use of novel agents in patients with this complication, a number of small studies have investigated their use. Thalidomide is a feasible option and published data indicate that it is effective with response rates, and toxicities found to be similar in patients with impaired and those with normal renal function [58]. However, it may be associated with hyperkalemia in some patients [59, 60]. Thalidomide dose reductions are generally not required in patients with renal impairment.
Lenalidomide in combination with dexamethasone has also been studied in patients with, predominantly, mild to moderate renal impairment [61]. Lenalidomide is mainly excreted by the kidneys and therefore dose adjustments in case of renal impairment are mandatory. In patients with moderate renal impairment (30 > CrCl <50 ml/min), lenalidomide should be administered at 10 mg once daily. The dose may be escalated to 15 mg once daily after two cycles if the patient is not responding to treatment and if treatment is tolerated. In patients with severe renal impairment (CrCl <30 ml/min, not requiring dialysis), lenalidomide should be administered at 15 mg every other day. The dose may be escalated to 10 mg once daily if treatment is tolerated. In patients with end stage renal disease (CrCl <30 ml/min, requiring dialysis), lenalidomide should be administered at 5 mg once daily. On dialysis days, the dose should be administered following dialysis. Although patients with a serum creatinine level of 2.5 mg per 100 ml or higher were excluded from the phase III trials in the relapsed/refractory setting and prospective data on the effect of this dose reduction algorithm on outcome in terms of tumour response and survival are currently lacking, lenalidomide may be feasible in patients with moderate renal impairment. In the Swiss prescription information for lenalidomide, use in patients with moderate and severe renal impairment is currently not recommended, due to insufficient data [62].
Bortezomib has a rapid onset of response and, in patients with renal impairment, it has been shown to result in response rates and tolerability comparable to those observed in patients with normal renal function. Importantly, reversal of renal failure has been documented in about 40% of patients with cast nephropathy which may be, in part, attributable to a direct effect of bortezomib on upregulated tubular NFκB receptors [42, 63, 64]. The current prescribing information indicates that data on bortezomib in patients with renal impairment are limited and that dose adjustments should be considered [65]. Pharmacokinetic data indicate that bortezomib elimination is independent of renal clearance [66]. For this reason, and based on recent clinical data concerning efficacy and tolerability, dose adjustments are not deemed necessary [42, 67] and bortezomib can be recommended in patients with renal impairment.
Cytogenetic testing is useful as it provides valuable prognostic information. However, definite treatment recommendations based on cytogenetic risk are currently difficult to give because of a lack of data from randomised trials with the novel agents in this setting. Nevertheless, data from a number of small studies and subgroup analyses provide some indication regarding the efficacy of the various agents. Bortezomib appears to be effective in patients with del(13) and t(4;14), as well as in those with del(17) [16, 39], whereas the efficacy of thalidomide may be reduced in the presence of cytogenetic abnormalities. Regarding lenalidomide, some studies indicate that response and OS are not influenced by the presence of cytogenetic abnormalities, while other studies suggest that the agent may be less effective in this setting [68–70]. In general, based on the limited data available, bortezomib combinations may be the treatment of choice in the presence of cytogenetic abnormalities.
Frail patients, generally considered to be those in poor clinical condition, may require dose modification of the chosen regimen to improve tolerability and enable the administration of the treatment for the indicated time. For example, using the MPT regimen, it may be necessary to adjust the doses of melphalan and/or thalidomide. Similarly, when using the VMP regimen, it may be necessary to adjust the doses of melphalan and/or bortezomib. In addition, a reduced frequency of administration can be considered for bortezomib, as outlined for the VMP regimen in the section below.
This section focuses on the management of the most frequent toxicities seen with novel agents.
PN is a frequent symptom in patients with multiple myeloma. It can be the result of the disease itself, myeloma-associated amyloidosis, or a consequence of comorbidities, such as diabetes. In addition, it can be caused by myeloma treatments, such as thalidomide and bortezomib.
Before initiating treatment, an assessment of the PN baseline status is important in order to correctly monitor evolution of the toxicity. Active management of PN is important to detect (new) symptoms as early as possible in order to reverse them and prevent deterioration. It is useful to ask several simple questions which will establish if any symptoms are present and to assess their severity. Such questions are:
– Do you experience any tingling, numbness or pain in hands and feet?
– Do you experience difficulties in doing up buttons?
If the answer to the first question is affirmative, then these may be indicative of early signs of PN and dose reduction should be considered. If the answer to the second question is positive, this is a sign of more severe PN and treatment should be halted.
Dose modification guidelines to manage PN have been developed and prospectively evaluated for bortezomib [39, 56, 71]. In the presence of grade 1 PN with pain or grade 2 PN with symptoms interfering with function but not with activities of daily living, the dose should be reduced to 1.0 mg/m2. In case of grade 2 PN with pain or grade 3 with symptoms interfering with activities of daily living, treatment should be discontinued until symptoms have resolved. When toxicity has resolved, bortezomib can be re-initiated at a dose of 0.7 mg/m2and the treatment schedule should be reduced to one administration per week. In case of grade 4 PN, bortezomib should be discontinued.
In daily practice, a more cautious approach with earlier dose reduction (or discontinuation) might decrease the occurrence of severe symptoms and avoidable irreversibility, thus being beneficial to the patients. Using prompt dose reduction increases the likelihood of resolution of symptoms. For example, in the VISTA trial, bortezomib dose reduction was applied in 22% of patients; 60% of PN events had completely resolved in a median of 5.7 months, while 79% of PN events had improved by at least one grade in a median of 1.9 months [72].
Notably, for elderly and frail patients, two recent phase III trials demonstrated that administering bortezomib at a reduced frequency, once weekly instead of twice weekly, as part of the VMP regimen, is an effective and well tolerated treatment option. In a trial conducted by the Spanish Myeloma group, patients (n = 260) were randomised to receive six cycles of VMP or bortezomib with thalidomide and prednisone (VTP) [73]. During cycle 1, bortezomib was administered twice weekly and in the subsequent cycles, bortezomib was only administered once weekly at the dose of 1.3 mg/m2. Compared with the results obtained in the VISTA trial, in which bortezomib was administered twice weekly, the weekly administration resulted in a substantial improvement in tolerability. Notably, the incidence of grade 3/4 PN was only 5% with the reduced dose VMP regimen and treatment discontinuations were only seen in 12% (compared with 14% and 34% in the VISTA trial, respectively). Efficacy was maintained with an ORR of 81%, while 22% of patients achieved CR, and OS at two years was 92%.
Similarly, a study conducted by the Italian Myeloma group which examined bortezomib administered weekly in a trial designed to compare VMPT versus VMP in elderly patients (n = 354), found that the weekly administration of bortezomib markedly improved the tolerability of the VMP regimen [74]. The study initially planned to administer 4 cycles of twice-weekly bortezomib, however, following a protocol amendment, patients only received once-weekly bortezomib as part of the VMP and VMPT regimens. A comparison of efficacy and toxicity in patients receiving twice-weekly or once-weekly bortezomib in the VMP arm revealed that a shift from twice-weekly to once-weekly bortezomib dosing reduced the rate of CR from 27% to 20%, but that it also substantially reduced the incidence of sensory neuropathy (14% vs 2%) and the rate of treatment discontinuation (15% vs 4%), while OS at three years was 89%.
Thalidomide-associated PN has been linked to the duration of therapy and in a study that investigated high doses of the agent (up to 800 mg/day), it was recommended to limit thalidomide treatment to less than 6 months to minimise the risk of neurotoxicity [75]. However, at lower doses, thalidomide can be administered over prolonged periods without risking the development of severe neurotoxicity. There is a lack of data regarding the reversibility of thalidomide-induced PN. Prompt dose modification and discontinuation of treatment in the event of PN are important to limit worsening of the complication and improve the likelihood of recovery. The following recommendations have been suggested [76]: In case of grade 1 PN, the dose of thalidomide should be reduced by 50%. In the presence of grade 2 PN, therapy should be withheld until the resolution of symptoms and then restarted at a 50% reduced dose. If grade 3 or 4 PN develop, thalidomide should be discontinued permanently.
PN is uncommon with lenalidomide treatment.
MM itself is associated with a risk of developing venous thrombolic events (VTEs). In addition, thromboembolic events are one of the most significant side effects associated with thalidomide and lenalidomide when these agents are used in combination with steroids or chemotherapy. When choosing thalidomide or lenalidomide, prophylaxis with anticoagulants should always be considered. A risk-adapted strategy may be appropriate to manage this complication. For patients with a low risk of developing VTEs, prophylaxis using aspirin may be sufficient, whereas for patients with a number of risk factors, low-molecular weight heparin should be chosen [77]. Bortezomib is not associated with VTEs. Therefore, in patients with a history of VTEs, a bortezomib-based regimen may be the preferred approach. In addition, there is data to suggest that the incidence of VTEs is reduced with bortezomib [24].
Neutropenia:In case of neutropenia with an ANC between 500–1000/mm3, thalidomide dose should be reduced by 50% or the use of growth factors may be considered. Thalidomide treatment should be stopped, if the ANC falls below 500/mm3 and the use of growth factors should be considered. Once the ANC recovers to >500/mm3, thalidomide may be restarted at a 50% reduced dose [76]. If thalidomide is used in combination with melphalan or other cytotoxic agents, dose reduction should be considered in the latter agents first.
Neutropenia:In case of neutropenia with neutrophils below 0.5 × 109/l, lenalidomide treatment should be interrupted. Once neutrophil counts have normalised, treatment should be continued at a reduced dose [62].
The use of growth factors was shown to be required in 59% of patients in a recently published trial investigating lenalidomide monotherapy (30 mg/d) [78]. Considering the overall burden for the patient, it may be preferable to aim to manage toxicity with dose reduction alone and avoid G-CSF.
Thrombocytopenia:In the event of thrombocytopenia with platelets below 30 × 109/l, lenalidomide treatment should be interrupted and restarted at a reduced dose once platelet counts have recovered [62].
The following recommendations have been developed for the use of bortezomib in combination with MP as part of the VMP regimen [65]: Before the start of a new treatment cycle, the number of platelets should be ≥70 × 109/l and the ANC ≥1.0 × 109/l.
In case of persistent neutropenia of grade 4 or thrombocytopenia with or without bleeding in the previous cycle, a reduction in the dose of melphalan by 25% should be considered. If several bortezomib doses were not administered within a cycle (≥3 doses during twice-weekly treatment or ≥2 doses during weekly treatment), the dose of bortezomib should be reduced to the next dose level (from 1.3 mg/m2 to 1.0 mg/m2, or from 1.0 mg/m2 to 0.7 mg/m2).
If on the day of bortezomib administration (except on the first day), the number of platelets is 30 × 109/l or below, or the ANC is 0.75 × 109/l or below, bortezomib should not be administered. Bortezomib treatment can be restarted once the blood counts have recovered.
In case of grade 4 haematologic toxicity in the relapsed/refractory setting, treatment should be interrupted until symptoms have improved. Treatment can then be re-started at a reduced dose (1.3 mg/m2 reduced to 1.0 mg/m2, 1.0 mg/m2 reduced to 0.7 mg/m2). If toxicity does not improve, discontinuation of the treatment should be considered.
In general, bortezomib-associated thrombocytopenia can be managed by extending the intervals between injections or omitting doses should this be necessary. Due to the cyclic nature of the thrombocytopenia, with recovery of the platelet levels during the rest period of a treatment cycle, intervention is rarely needed.
The following dose modification schedule is being used in some centres: if one dose of bortezomib is omitted, then the next cycle is administered at the normal dose; if two doses are omitted, then the dose of bortezomib is reduced to 1 mg/m2in the following cycle.
With bortezomib use, herpes zoster reactivation has been observed, particularly when no antiviral prophylaxis has been administered. However, this adverse event can be managed with administration of antiviral prophylaxis, as shown from findings in the VISTA trial. When patients did not receive antiviral prophylaxis, the incidence of herpes zoster reactivation was 13% (all grades). In contrast, in patients who received antiviral prophylaxis, the rate dropped to 3% [40]. Therefore, antiviral prophylaxis should be considered in all patients treated with bortezomib. Results from a recent prospective, observational study suggest that acyclovir 400 mg once daily is effective at preventing varicella zoster virus reactivation in patients treated with bortezomib [79]. Alternatively, 500 mg valacyclovir can be administered daily [80]. In addition, valacyclovir at 2 × 500 mg can be used unless severe renal impairment is present [81]. Herpes zoster reactivation has not been reported with thalidomide or lenalidomide use.
The Swiss Agency for Therapeutic Products (Swissmedic) has approved the following novel agents for the treatment of multiple myeloma in Switzerland [82]:
– Lenalidomide (Revlimid) is approved for use in combination with dexamethasone as a treatment for patients with multiple myeloma who have received at least one prior therapy.
– Bortezomib (Velcade) is approved for use in combination with melphalan and prednisone for previously untreated patients with multiple myeloma.
– Bortezomib (Velcade) is approved for patients with relapsed/refractory multiple myeloma who have received at least one prior therapy.
– Pegylated liposomal doxorubicin (Caelyx) is approved for use in combination with bortezomib for patients with progressive multiple myeloma who have received at least one prior therapy and who have either already undergone or are not eligible for bone marrow transplantation.
Thalidomide is currently not approved in Switzerland for its use in multiple myeloma. No public information is available regarding any possible past or ongoing approval processes.
Access to the novel agents for approved indications is possible by submitting an application to the medical examiner (“Vertrauensarzt”/“médecin de conseil”) of the respective insurance company. For use in currently unapproved indications, the application to the medical examiner requires a summary of the arguments that support the use of the agent/combination in the particular situation, including available published evidence. The novel agents fulfil the criteria of an “orphan drug” for myeloma and these criteria can be listed to justify use of these agents.
Although thalidomide is not approved for multiple myeloma, it is widely used in the different treatment stages. Among other options, it is avail-able by “Magistralrezeptur” and is usually reimbursed by health insurance companies.
The availability of novel agents has changed the management of multiple myeloma and substantially improved the situation for many affected patients. Results with novel agents have been reviewed extensively and recommendations for the incorporation of these agents into clinical practice have been developed and in part incorporated into guidelines [2, 6, 83–89].
In our view, based on current data, the main treatment options for induction in the transplant setting are thal/dex and bortezomib/dex. For patients with newly diagnosed disease who are not eligible for transplantation, evidence from clinical trials indicates that MPT and VMP are the new standard treatments. In the relapsed/refractory setting, the efficacy of various combinations incorporating at least one novel agent (thalidomide, bortezomib, lenalidomide) is well documented and these regimens can be recommended. In addition, for agents that are not administered until disease progression, retreatment (mainly as part of a combination regimen) can be an option. The choice of agent in the relapsed/refractory setting will significantly depend on the efficacy and tolerability observed for the prior line of treatment.
Ongoing studies will further define the role of the novel agents in the different treatment stages. Furthermore, studies are needed to define the optimal treatment sequence to establish whether the initial aim should be a stable plateau with a view to initiating more intensive treatment later or whether the main goal should be intense cytoreduction to prolong the time to the next treatment as much as possible. In addition, open questions remain regarding the tailoring of treatments based on individual cytogenetic risk factors. Results from ongoing studies will help to answer these questions.
Finally, a number of newer agents are currently undergoing investigation in clinical trials, such as second generation proteasome inhibitors and immunomodulatory agents. In addition, bendamustine and agents with novel mechanisms of action, for example heat-shock protein 90 (Hsp90) inhibitors and histone deacetylase (HDAC) inhibitors, are being examined and results from ongoing studies will show how these can be incorporated into the management of multiple myeloma.
1 Kumar S, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516–20.
2 Ludwig H, Beksac M, Bladé J, et al. Current multiple myeloma treatment strategies with novel agents: a European perspective. Oncologist. 2010;15:6–25.
3 Mateos MV, López-Corral L, Hernández MT, et al. Multicenter, randomized, open-label, phase III trial of lenalidomide-dexamethasone (Len/dex) vs therapeutic abstention in smoldering multiple myeloma at high risk of progression to symptomatic MM: results of the first interim analysis. Blood. 2009;114 (Abstract 614).
4 Kyle RA, Rajkumar SV. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia. 2009;23:3–9.
5 Taverna C. Multiple Myeloma – erkennen und behandeln. UNI-MED SCIENCE 2009.
6 Harousseau JL, Dreyling M. Multiple myeloma: ESMO Clinical Recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20(Suppl 4):97–9.
7 Blade J, Samson D, Reece D, et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. Br J Haematol. 1998;102:1115–23.
8 Durie BGM, Harousseau J-L, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–73.
9 van de Velde HJ, Liu X, Chen G, et al. Complete response correlates with long-term survival and progression-free survival in high-dose therapy in multiple myeloma. Haematologica. 2007;92:1399–406.
10 Harousseau JL, Attal M, Avet-Loiseau H. The role of complete response in multiple myeloma. Blood. 2009;114:3139–46.
11 Kyle RA, Leong T, Li S, et al. Complete response in multiple myeloma: clinical trial E9486, an Eastern Cooperative Oncology Group study not involving stem cell transplantation. Cancer. 2006;106:1958–66.
12 Harousseau JL, Palumbo A, Richardson P, et al. Superior Outcomes Associated with Complete Response: Analysis of the Phase III VISTA Study of Bortezomib Plus Melphalan–Prednisone Versus Melphalan–Prednisone. Blood. 2008;112 (Abstract 2778).
13 Cavo M, Zamagni E, Tosi P, et al. Superiority of thalidomide and dexamethasone over vincristine-doxorubicindexamethasone (VAD) as primary therapy in preparation for autologous transplantation for multiple myeloma. Blood. 2005;106:35–9.
14 Macro M, Divine M, Uzunhan Y, et al. Dexamethasone + thalidomide (dex/thal) compared to VAD as a pre-transplant treatment in newly diagnosed multiple myeloma (MM): a randomized trial. Blood. 2006;108:22a (Abstract 57).
15 Zamagni E, Testoni N, Terragna C, et al. Incorporation of Thalidomide-Dexamethasone (Thal-Dex) into up-Front Double Autologous Stem-Cell Transplantation (ASCT) for multiple Myeloma: Final analysis of phase II “Bologna 2002” Study. Blood. 2009;114 (Abstract 349).
16 Harousseau JL, et al. VELCADE/dexamethasone (Vel/D) versus VAD as induction treatment prior to autologous stem cell transplantion (ASCT) in newly diagnosed multiple myeloma (MM): updated results of the IFM 2005/01 trial. ASH/ASCO symposium during ASH 2008.
17 Rajkumar SV, Jacobus S, Callander NS, et al. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an open-label randomised controlled trial. Lancet Oncol. 2010;11:29–37.
18 Rajkumar SV, Jacobus S, Callander N, et al. Randomized trial of lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone in newly diagnosed myeloma (E4A03), a trial coordinated by the Eastern Cooperative Oncology Group: Analysis of response, survival, and outcome with primary therapy and with stem cell transplantation. ASH/ASCO symposium during ASH 2008.
19 Bargetzi MJ, Passweg J, Baertschi E, et al. Mobilization of peripheral blood progenitor cells with vinorelbine and granulocyte colony-stimulating factor in multiple myeloma patients is reliable and cost effective. Bone Marrow Transplant. 2003;31:99–103.
20 Attal M, Harousseau JL, Facon T, et al. Single versus double autologous stem-cell transplantation for multiple myeloma. N Engl J Med. 2003;349:2495–502.
21 Cavo M, Tosi P, Zamagni E, et al. Prospective, randomized study of single compared with double autologous stem-cell transplantation for multiple myeloma: Bologna 96 clinical study. J Clin Oncol. 2007;25:2434–41.
22 Morgan GJ, Jackson GH, Davies FE, et al. Maintenance Thalidomide May Improve Progression Free but Not Overall Survival; Results from the Myeloma IX Maintenance Randomisation. Blood. 2008;112 (Abstract 656).
23 Attal M, Harousseau JL, Leyvraz S, et al. Maintenance therapy with thalidomide improves survival in patients with multiple myeloma. Blood. 2006;108:3289–94.
24 Cavo M, Tacchetti P, Patriarca F, et al. Superior Complete Response Rate and Progression-Free Survival after Autologous Transplantation with up-Front Velcade-Thalidomide-Dexamethasone Compared with Thalidomide-Dexamethasone in Newly Diagnosed Multiple Myeloma. Blood. 2008;112 (Abstract 158).
25 Lokhorst H, van der Holt B, Zweegman S, et al. HOVON-50 Final Analysis of Thalidomide Combined with Adriamycine, Dexamethasone (AD) and High Dose Melphalan (HDM). Clin Lymphoma Myeloma. 2009;9 (Suppl.1) (Abstract 46).
26 Morgan GJ, Davies FE, Owen RG et al. Thalidomide Combinations Improve Response Rates; Results from the MRC IX Study. Blood. 2007;110 (Abstract 3593).
27 Knop S, Liebisch P, Wandt H et al. Bortezomib, IV cyclophosphamide, and dexamethasone (VelCD) as induction therapy in newly diagnosed multiple myeloma: Results of an interim analysis of the German DSMM Xia trial. J Clin Oncol. 2009;27(18 suppl) (Abstract 8516).
28 Sonneveld P, van der Holt B, Schmidt-Wolf IGH et al. First Analysis of HOVON-65/GMMG-HD4 Randomized Phase III Trial Comparing Bortezomib, Adriamycine, Dexamethasone (PAD) vs VAD as Induction Treatment Prior to High Dose Melphalan (HDM) in Patients with Newly Diagnosed Multiple Myeloma (MM). Haematologica 2009;94(2 suppl) (Abstract 473).
29 Richardson P, Lonial S, Jakubowiak A, et al. Lenalidomide, bortezomib, and dexamethasone in patients with newly diagnosed multiple myeloma: encouraging efficacy in high risk groups with updated results of a phase I/II Study. Blood. 2008;112 (Abstract 92).
30 Garban F, Attal M, Michallet M, et al. Prospective comparison of autologous stem cell transplantation followed by dose-reduced allograft (IFM99-03 trial) with tandem autologous stem cell transplantation (IFM99-04 trial) in high-risk de novo multiple myeloma. Blood. 2006;107:3474–80.
31 Bruno B, Rotta M, Patriarca F, et al. A comparison of allografting with autografting for newly diagnosed myeloma. N Engl J Med. 2007;356:1110–20.
32 Bruno B, Rotta M, Patriarca F, et al. Nonmyeloablative allografting for newly diagnosed multiple myeloma: the experience of the Gruppo Italiano Trapianti di Midollo. Blood. 2009;113:3375–82.
33 Gahrton G, Bjorkstrand B, Iacobelli S, et al. Tandem Autologous(ASCT)/ Allogeneic Reduced Intensity Conditioning Transplantation (RIC) with Identical Sibling Donor Versus ASCT in Previously Untreated Multiple Myeloma (MM): Long Term Follow up of a Prospective Controlled Trial by the EBMT. Blood. 2009;114 (Abstract 52).
34 Facon T, Mary JY, Hulin C, et al. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99-06): a randomised trial. Lancet. 2007;370:1209–18.
35 Hulin C, Facon T, Rodon P, et al. Efficacy of Melphalan and Prednisone Plus Thalidomide in Patients Older Than 75 Years With Newly Diagnosed Multiple Myeloma: IFM 01/01 Trial. J Clin Oncol. 2009;27:3664–70.
36 Palumbo A, Bringhen S, Liberati AM, et al. Oral melphalan, prednisone, and thalidomide in elderly patients with multiple myeloma: updated results of a randomized, controlled trial. Blood. 2008;112:3107–14.
37 Gulbrandsen N, Waage A, Gimsing P, et al. A randomised placebo controlled study with melphalan/prednisone versus melphalan/prednisone/thalidomide: quality of life and toxicity. Haematologica. 2008;93 (Abstract 209).
38 Wijermans PW, Zweegman S, van Marwijk Kooy M, et al. MP versus MPT in elderly myeloma patients: the final outcome of the HOVON-49 study. Clin Lymphoma Myeloma. 2009;9(Suppl.1) (Abstract 116).
39 San Miguel J, Schlag R, Khuageva N, et al. Bortezomib plus Melphalan and Prednisone for Initial Treatment of Multiple Myeloma. N Engl J Med. 2008;359:906–17.
40 San Miguel J, Schlag R, Khuageva N, et al. Updated follow-up and results of subsequent therapy in the phase III VISTA trial: Bortezomib + Melphalan–Prednisone versus Melphalan–Prednisone in newly diagnosed multiple myeloma. Blood. 2008;112 (Abstract 650).
41 Kropff M, Richardson PG, Schlag R, et al. Similar benefit in patients ≥75 or <75 years with VMP versus MP in front-line MM and bortezomib versus dexamethasone in relapsed MM. Clin Lymphoma Myeloma. 2009;9 (Suppl.1) (Abstract 84).
42 Dimopoulos MA, Richardson P, Schlag R, et al. Bortezomib–Melphalan–Prednisone (VMP) in Newly Diagnosed Multiple Myeloma Patients with Impaired Renal Function: Cohort Analysis of the Phase III VISTA Study. Clin Lymphoma Myeloma. 2009;9 (Suppl.1) (Abstract 166).
43 Palumbo A, Falco P, Falcone A et al. Melphalan, Prednisone, and Lenalidomide for Newly Diagnosed Myeloma: Kinetics of Neutropenia and Thrombocytopenia and Time-to-Event Results. Clin Lymphoma Myeloma. 2009;9:145–50.
44 Richardson PG, Sonneveld P, Schuster M, et al. Extended follow-up of a phase 3 trial in relapsed multiple myeloma: final time-to-event results of the APEX trial. Blood. 2007;110:3557–60.
45 Cuesta A, Galera P, Garcia-Sanchez R, et al. Bortezomib plus dexamethasone in relapsed/refractory multiple myeloma. Evaluation of the efficacy and toxicity. Blood. 2008;112 (Abstract 5180).
46 Orlowski RZ, Nagler A, Sonneveld P, et al. Randomized phase III study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone in relapsed or refractory multiple myeloma: combination therapy improves time to progression. J Clin Oncol. 2007;25:3892–901.
47 Sonneveld P, Hajek R, Nagler A, et al. Combined Pegylated Liposomal Doxorubicin and Bortezomib is highly effective in patients with recurrent or refractory multiple myeloma who received prior Thalidomide/Lenalidomide therapy. Cancer. 2008;112:1529–37.
48 Dimopoulos M, Spencer A, Attal M, et al. Lenalidomide plus Dexamethasone for Relapsed or Refractory Multiple Myeloma. N Engl J Med. 2007;357:2123–32.
49 Dimopoulos MA, Chen C, Spencer A, et al. Long-term follow-up on overall survival from the MM-009 and MM-010 phase III trials of lenalidomide plus dexamethasone in patients with relapsed or refractory multiple myeloma. Leukemia. 2009;23:2147–52.
50 Weber DM, Chen C, Niesvizky R, et al. Lenalidomide plus Dexamethasone for Relapsed Multiple Myeloma in North America. N Engl J Med. 2007;357:2133–42.
51 Wang M, Dimopoulos MA, Chen C, et al. Lenalidomide plus dexamethasone is more effective than dexamethasone alone in patients with relapsed or refractory multiple myeloma regardless of prior thalidomide exposure. Blood. 2008;112:4445–51.
52 Knop S, Gerecke C, Liebisch P, et al. Lenalidomide, adriamycin, and dexamethasone (RAD) in patients with relapsed and refractory multiple myeloma: a report from the German Myeloma Study Group DSMM (Deutsche Studiengruppe Multiples Myelom). Blood. 2009;113:4137–43.
53 Anderson KC, Jagannath S, Jakubowiak A, et al. Lenalidomide, bortezomib, and dexamethasone in relapsed/refractory multiple myeloma (MM): Encouraging outcomes and tolerability in a phase II study. J Clin Oncol. 2009;27:15s (Abstract 8536).
54 Hrusovsky I, Emmerich B, von Rohr A, et al. Bortezomib retreatment in relapsed multiple myeloma (MM): results from a binational, multicenter retrospective survey. Blood. 2008;112 (Abstract 2775).
55 Petrucci MT, Blau IW, Corradini P, et al. Efficacy and Safety of Re-Treatment with Bortezomib (Velcade©) in Patients with Multiple Myeloma: Results from a Prospective International Phase II Trial. Blood. 2008;112 (Abstract 3690).
56 Richardson PG, Barlogie B, Berenson J, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348:2609–17.
57 Jagannath S, Barlogie B, Berenson JR, et al. Updated survival analyses after prolonged follow-up of the phase 2, multicenter CREST study of bortezomib in relapsed or refractory multiple myeloma. Br J Haematol. 2008;143:537–40.
58 Tosi P, Zamagni E, Cellini C, et al. Thalidomide alone or in combination with dexamethasone in patients with advanced, relapsed or refractory multiple myeloma and renal failure. Eur J Haematol. 2004;73:98–103.
59 Harris E, Behrens J, Samson D, et al. Use of thalidomide in patients with myeloma and renal failure may be associated with unexplained hyperkalaemia. Br J Haematol. 2003;122:160–1.
60 Fakhouri F, Guerraoui H, Presne C, et al. Thalidomide in patients with multiple myeloma and renal failure. Br J Haematol. 2004;125:96–7.
61 Weber DM, Spencer A, Wang M, et al. The efficacy and safety of lenalidomide plus dexamethasone in relapsed or refractory multiple myeloma patients with impaired renal function. J Clin Oncol. 2008;26 (Abstract 8542).
62 Revlimid, www.kompendium.ch
63 Dimopoulos MA, Roussou M, Kastritis E, et al. Reversibility of Renal Impairment of Multiple Myeloma Patients Treated with Bortezomib-Based Regimens: Identification of Predictive Factors. Blood. 2008;112 (Abstract 1725).
64 Ludwig H, Adam Z, Greil R, et al. Reversal of acute renal impairment by Bortezomib-Doxorubicin-Dexamethasone (BDD) in Multiple Myeloma (MM). Results from a Phase II Study. Haematologica. 2009;94(s2):154 (Abstract 385).
65 Velcade, www.kompendium.ch
66 Mulkerin D, Remick S, Takimoto C, Ivy P, Karol M, Eton O. Safety, tolerability, and pharmacology of bortezomib in cancer patients with renal failure requiring dialysis: results from a prospective phase 1 study. Blood. 2007;110 (Abstract 3477).
67 San-Miguel JF, Richardson PG, Sonneveld P, et al. Efficacy and safety of bortezomib in patients with renal impairment: results from the APEX phase 3 study. Leukemia. 2008;22:842–9.
68 Avet-Loiseau H, Soulier J, Fermand JP, et al. Impact of Chromosomal Abnormalities Del(13), T(4;14), and Del(17p) and Prior Treatment on Outcomes in Patients with Relapsed or Refractory Multiple Myeloma Treated with Lenalidomide. Blood. 2008;112 (Abstract 3685).
69 Kapoor P, Kumar S, Fonseca R, et al. Impact of risk stratification on outcome among patients with multiple myeloma receiving initial therapy with lenalidomide and dexamethasone. Blood. 2009;114:518–21.
70 Reece D, Song KW, Fu T, Roland B, et al. Influence of cytogenetics in patients with relapsed or refractory multiple myeloma treated with lenalidomide plus dexamethasone: adverse effect of deletion 17p13. Blood. 2009;114:522–5.
71 Richardson PG, Sonneveld P, Schuster MW, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352:2487–98.
72 Mateos MV, Richardson PG, Schlag R, et al. Peripheral neuropathy with VMP in the phase III VISTA study resolves in the majority of patients and shows a rate plateau. Clin Lymphoma Myeloma. 2009;9 (Suppl.1) (Abstract 172).
73 Mateos MV, Oriol A, Martinez J, et al. Bortezomib (Velcade)/melphalan/prednisone (VMP) versus Velcade/thalidomide/prednisonse (VTP) in elderly untreated multiple myeloma (MM) patients. Haematologica. 2009;94(s2):190 (Abstract 471).
74 Palumbo A, Bringhen S, Rossi D, et al. Bortezomib, melphalan, prednisone and thalidomide (VMPT) versus bortezomib, melphalan and prednisone (VMP) in elderly newly diagnosed myeloma patients: a prospective, randomized phase III study. Haematologica. 2009;94(s2): 190 (Abstract 472).
75 Mileshkin L, Stark R, Day B, et al. Development of neuropathy in patients with myeloma treated with thalidomide: patterns of occurrence and the role of electrophysiologic monitoring. J Clin Oncol. 2006;24:4507–14.
76 Ghobrial IM, Rajkumar SV. Management of Thalidomide Toxicity. J Support Oncol. 2003;1:194–205.
77 Palumbo A, Rajkumar SV, Dimopoulos MA, et al. Prevention of thalidomide- and lenalidomide-associated thrombosis in myeloma. Leukemia. 2008;22:414–23.
78 Richardson P, Jagannath S, Hussein M, et al. Safety and efficacy of single-agent lenalidomide in patients with relapsed and refractory multiple myeloma. Blood. 2009;114:772–8.
79 Pour L, Adam Z, Buresova L, et al. Varicella-zoster virus pro-phylaxis with low-dose acyclovir in patients with multiple myeloma treated with bortezomib. Clin Lymphoma Myeloma. 2009;9:151–3.
80 Vickrey E, Allen S, Mehta J et al. Acyclovir to prevent reactivation of varicella zoster virus (herpes zoster) in multiple myeloma patients receiving bortezomib therapy. Cancer. 2009;115:229–32.
81 Valtrex, www.kompendium.ch
82 www.swissmedic.ch
83 Anderson KC, Alsina M, Bensinger W, et al. Multiple myeloma: clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2007;5:118–47.
84 Barosi G, Boccadoro M, Cavo M, et al. Management of multiple myeloma and related-disorders: guidelines from the Italian Society of Hematology (SIE), Italian Society of Experimental Hematology (SIES) and Italian Group for Bone Marrow Transplantation (GITMO). Haematologica. 2004; 89:717–41.
85 Harousseau JL, Moreau P. Autologous hematopoietic stem-cell transplantation for multiple myeloma. N Engl J Med. 2009;360:2645–54.
86 Palumbo A, Sezer O, Kyle R, et al. International Myeloma Working Group guidelines for the management of multiple myeloma patients ineligible for standard high-dose chemotherapy with autologous stem cell transplantation. Leukemia. 2009;23:1716–30.
87 Patriarca F, Petrucci MT, Bringhen S, et al. Considerations in the treatment of multiple myeloma: a consensus statement from Italian experts. Eur J Haematol. 2009;82:93–105.
88 San Miguel JF. Relapse/Refractory myeloma patient: potential treatment guidelines. J Clin Oncol. 2009;27:5676–7.
89 Smith A, Wisloff F, Samson D. Guidelines on the diagnosis and management of multiple myeloma. Br J Haematol. 2005;132:410–51.
90 Durie BG, Salmon SE. A clinical staging system for multiple myeloma. Correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival. Cancer. 1975;36:842–54.
91 Greipp PR, San Miguel J, Durie BG, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23:3412–20.
92 Mikhael JR, Belch AR, Prince HM et al. High response rate to bortezomib with or without dexamethasone in patients with relapsed or refractory multiple myeloma: results of a global phase 3b expanded access program Br J Haematol. 2009;144:169–75.
93 Palumbo A, Gay F, Bringhen S, et al. Bortezomib, doxorubicin and dexamethasone in advanced multiple myeloma. Ann Oncol. 2008;19:1160–5.
94 von Lilienfeld-Toal M, Hahn-Ast C, Furkert K, et al. A systematic review of phase II trials of thalidomide/dexamethasone combination therapy in patients with relapsed or refractory multiple myeloma. Eur J Haematol. 2008;81:247–52.
Janssen-Cilag AG, Baar, Switzerland supported an expert meeting and editorial assistance to facilitate the development of the treatment recommendations and the writing of this manuscript. The recommendations in this manuscript reflect the opinion of the authors.