The stress hormone copeptin: a new prognostic biomarker in acute illness
DOI: https://doi.org/10.4414/smw.2010.13101
Summary
Stress is defined as anything that throws the body out of homeostatic balance; for example an acute illness. Any stressor which activates the hypothalamo-pituitary-adrenal (HPA) axis leads to an increase in concentrations of the adrenal stress hormone, cortisol. One of the major hypothalamic stress hormones, which is stimulated by different stressors, is vasopressin (AVP). However, measurement of circulating AVP levels is challenging because it is released in a pulsatile pattern, it is unstable and is rapidly cleared from plasma. AVP derives from a larger precursor peptide (pre-provasopressin) along with copeptin which is released in an equimolar ratio to AVP and is more stable in the circulation and easy to determine. Copeptin levels were found to closely mirror the production of AVP. We have shown that copeptin more subtly mirrors the individual stress level compared to cortisol. Due to the positive association of copeptin with the severity of illness and outcome, copeptin has been proposed as a prognostic marker in acute illness.
The prognostic accuracy of copeptin has been analysed in sepsis, pneumonia, lower respiratory tract infections, stroke and other acute illnesses. Thereby, copeptin was found to accurately mirror disease severity and to discriminate patients with unfavourable outcomes from patients with favourable outcomes. Copeptin improves the prognostic information provided by commonly used clinical scoring instruments. Importantly, interpretation of copeptin levels must always consider the clinical setting. An accurate prognostic assessment has the potential to guide interventions and effectively plan and monitor rehabilitation and, thus optimise the management of individual patients and the allocation of limited health care resources. Future intervention studies must prove the value of copeptin in clinical decision making and in improving the overall medical management of patients with acute illnesses.
Definition of stress, stressor and stress response
Stress is an aspect of our daily lives and yet there is considerable ambiguity in the meaning of this word.
There are numerous different definitions of “stress”. According to Robert Sapolsky, “stress is anything that throws the body out of homeostatic balance” – for example, an injury, an illness, or subjection to extreme heat or cold.
Stress thus occurs when the body is exposed to a “stressor” which threatens homeostasis, and the “stress response” is the attempt of the body to counteract the stressor and re-establish homeostasis (table 1). A hallmark of the stress response is the activation of the autonomic nervous system and, most importantly, the hypothalamo-pituitary-adrenal (HPA) axis [1]. The hormonal cascade, initiated by a stressor through brain stem and limbic pathways, involves the release of corticotropin-releasing hormone (CRH) from pravocellular neurons in the paraventricular nucleus of the hypothalamus. CRH stimulates the release of adrenocorticotropic hormone (ACTH) from the anterior pituitary gland. Another hypothalamic hormone which is stimulated by different stressors is vasopressin (AVP). AVP seems to exert a potentiating action on CRH and these two agents together are considered the main secretagogues of ACTH [2–6]. ACTH, in turn, stimulates the adrenal cortex to produce cortisol (fig. 1). Many factors influence the pattern and magnitude of the response to a stressor, including the duration of the stressor exposure (acute versus chronic), the type of stressor (physical versus psychological), the stress context, age and gender. The variety of unique stress situations is not well served by a single mediator. Rather, the combination of multiple mediators (e.g., AVP, CRH, cortisol, noradrenalin, orexin, urocortins, dopamine or serotonin) address the specific aspects of a stressor [7].
|
Table 1
|
|
Terms
|
Explanations
|
| Homeostasis |
Stability |
| Stress |
Disruption of homeostatic balance |
| Stressor |
A specific threat to the body |
| Stress response |
The bodies attempt to deal with the stressor |
| Stress hormones |
Mediators which address the specific aspects of a stressor |
| Stress level |
Degree of disruption of the homeostasis |
How to measure the activation of the HPA axis
Cortisol is the classical stress hormone at a peripheral level and is easy to measure. However, the
assessment and interpretation of cortisol levels to assess the integrity of the HPA-axis is dependent on an intact anterior pituitary and adrenal gland, respectively. For a direct assessment of stress at the perception level (i.e., the brain) the measurement of CRH or AVP would be important. Unfortunately however, the measurement of circulating CRH or AVP levels is challenging. Both, CRH and AVP are released in a pulsatile pattern, are unstable especially at room temperature, and are rapidly cleared from plasma within minutes [8–11]. AVP derives from a larger precursor peptide (pre-provasopressin) along with two other peptides, neurophysin II and copeptin (fig. 2). Copeptin is released in an equimolar ratio to AVP, is more stable in the circulation and easy to determine. Copeptin levels have been found to closely mirror the production of AVP [12]. The incubation time for copeptin measurement is 20 minutes and therefore results for the clinician are readily accessible. The measurements can be done on the “Kryptor”; a machine which is already available in many Swiss hospitals.
To evaluate the discriminatory value of copeptin compared to cortisol to assess different stress levels, an observational study was perfomed including patients with presumed increasing levels of stress. It was hypothesised that copeptin, as a brain-derived stress hormone, provides a more direct and subtle measure of the individual stress level. We analysed three groups: Group A (no stress) contained 20 healthy control subjects, without apparent stress. Group B (moderate stress) consisted of 25 patients consecutively recruited from a wide and representative variety of patients having been hospitalised on the medical ward for at least 48 hours. In Group C, 29 stable surgical patients were consecutively recruited undergoing elective coronary bypass grafting under general anesthesia. Peak cortisol levels are achieved in the immediate postoperative period, around the time of extubation [13, 14]. Therefore, large surgery can serve as a standardised physiological model for studying major stress [15].
Indeed, copeptin showed a gradual increase with increasing levels of stress and, in contrast to cortisol levels, differentiated between healthy control subjects without apparent stress and medical patients with a moderate degree of stress [16]. In addition, copeptin showed a more pronounced increase upon major stress compared to cortisol (the increase in cortisol was 265 ± 34% and in copeptin 1430 ± 157%, p <0.001) [16]. This small study was, however, observational in nature, and future studies are needed to support this observation.
Implications of copeptin for the prognosis of acute illness
Based on these findings, we hypothesised that copeptin as a stress hormone is a good prognostic measure to predict outcome in acute illness. The rationale for a presumed prognostic value of stress-hormones such as copeptin is partly due to the positive correlation of the individual stress level with the magnitude of the stressor or, in other words, the severity of the illness. However, copeptin levels are not only influenced by the stressors per se, but also by genetic and epigenetic factors (e.g., age, comorbidity). As a result, if the so-called homeostasis is massively disturbed and the individual threshold to ensure homeostasis is crossed, it is most likely that the recovery is incomplete and results in a less favourable outcome (fig. 3). In addition, prolonged and extensive high levels of stress hormones not only reflect the actual damage but may also directly harm the recovery process. In animal studies, for example, manipulation of blood corticosteroid concentrations during or after an ischaemic event altered infarct size [17–19]. It has been suggested that exposure to elevated glucocorticoid concentrations increases stroke-induced neuronal death.
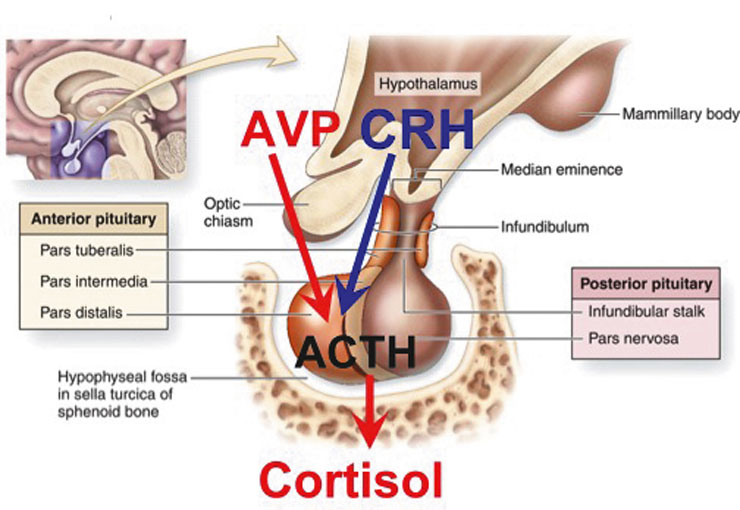
Figure 1
The “Stress-Axis” and Argininvasopressin (AVP)
The hormonal cascade, initiated by a stressor through brain stem and limbic pathways, involves the release of corticotropin-releasing hormone (CRH). AVP seems to exert a potentiating action on CRH and these two agents togetherare considered the main secretagogues of ACTH. ACTH, in turn, stimulates the adrenal cortex to produce cortisol.
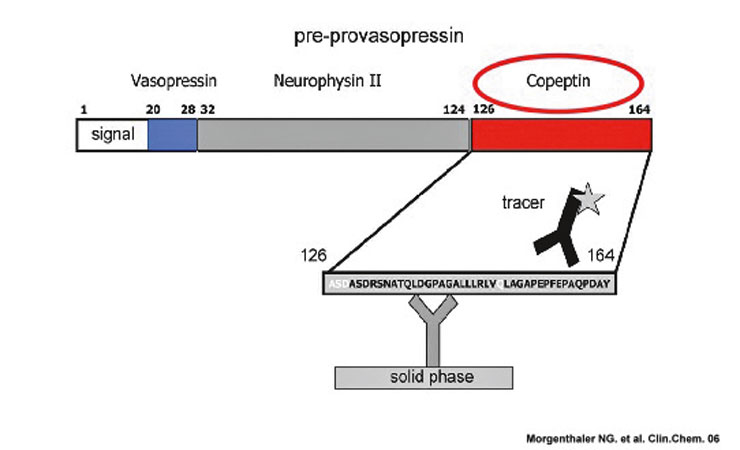
Figure 2
Preprovasopressin and Copeptin
Together with AVP, copeptin is derived from a 164-amino acid precursor termed preprovasopressin, which consists of a signal peptide, AVP, neurophysin II and copeptin. Copeptin is the C-terminal part of the pro-AVP.
Copeptin is measured by a sandwich immunoassay: One polyclonal antibody of human preprovasopressin is bound on the solid phase; a second polyclonal antibody directed to amino acids 149–164 of the preprovasopressin is labelled for chemi-luminance detection.
Copeptin as prognostic marker in sepsis
The early phase of septic shock and experimental endotoxemia is characterised by a marked increase in plasma vasopressin levels [20, 21].
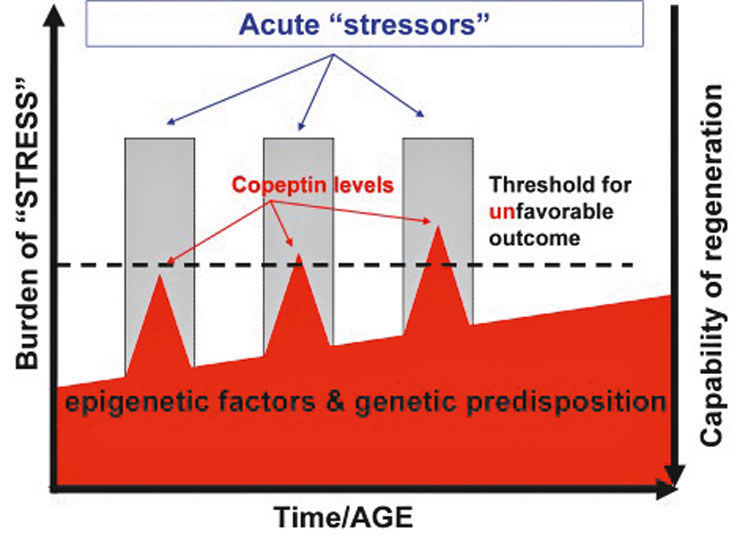
Figure 3
>Hypothesis: why copeptin might be a prognostic marker in acute illness
Our hypothesis is that copeptin as a stress marker mirrors the individual stress level. There is an individual “stress burden”, which is defined by genetic and epigenetic factors (such as co-morbidities). This stress burden changes during life. An identical stressor (for example a stroke) can provoke different copeptin concentrations depending on the magnitude of the stressor but also on the baseline stress burden. Possibly, there is a threshold which indicates how high the stress level can rise before the point of no return is reached. If a stressor leads to an increase which crosses the threshold, the homeostasis can not be accomplished by the stress response resulting in unfavourable outcome.
We therefore investigated copeptin and its prognostic accuracy in 101 consecutive critically ill patients admitted to the medical intensive care unit (ICU) of the University Hospital of Basel, Switzerland. In critically ill patients on admission, there was a stepwise increase of copeptin levels from patients without infection (i.e., SIRS) to patients with sepsis, severe sepsis and septic shock. The median copeptin value on admission in the group of non-survivors was significantly higher compared to the group of survivors. The optimal cut-off, which is about 20 times the median of the normal population, had a sensitivity of 61.5% and a specificity of 83.8%. In comparison, the standard clinical score in critically ill patients, i.e., the APACHE II score, gave similar values compared to copeptin. In this setting of patients with sepsis and septic shock, elevated copeptin levels are due to an insufficient haemodynamic response and due to activation of the HPA axis [20] .
Copeptin as a prognostic marker in lower respiratory tract infections including pneumonia
The development of a septic infection is a continuum and, in the majority of cases, a sequela of lower respiratory tract infections (LRTI). Tissue damage induced by mostly viral acute bronchitis increases susceptibility for a bacterial super-infection of the pulmonary parenchyma, which can finally lead to community acquired pneumonia (CAP). As expected, copeptin levels were significantly higher in patients with LRTI compared to healthy controls, with highest levels in patients with CAP [22]. Copeptin levels increased with increasing severity of CAP, as defined by the gold standard clinical score, which is the pneumonia severity index (PSI). In patients who died, copeptin levels on admission were significantly higher compared to levels in survivors [22]. A multivariate analysis model including the PSI, procalcitonin, C-reactive protein and copeptin to predict survival showed that the PSI (p<0.01) and copeptin (p<0.02), but not procalcitonin (p = 0.5) and C-reactive protein (p = 0.7), were independent predictors of outcome. In this study, the combination of copeptin and the PSI score did not increase the prognostic accuracy of the PSI score alone. The PSI score, however, is only validated for CAP and not for all LRTIs, and only for mortality and not other serious adverse events requiring hospitalisation. Furthermore, it is heavily age-dependent and its complexity jeopardises its dissemination and implementation in everyday practice. In this context, a new measurable biomarker, like copeptin, mirroring distinct pathogenetic mechanisms to predict severity and outcome may improve prognostication of patients.
Interestingly, patients with positive blood cultures had significantly higher copeptin levels compared to patients with negative blood cultures. Thus, copeptin levels seemed to mirror the inflammatory cytokine response and thus the severity of LRTI, the presence of haemodynamic disturbances and therefore the individual stress level.
In patients with acute exacerbations of chronic obstructive pulmonary disease (COPD), copeptin concentrations were also shown to be predictive for long-term clinical failure independent of age, co-morbidity, hypoxemia and lung functional impairment in multivariate analysis [23]. The combination of copeptin and previous hospitalisation for COPD increased the risk of poor outcome.
Copeptin as a prognostic marker in heart disease
As neurohormonal activation plays a crucial role in the pathophysiology of heart disease [24], new prognostic candidate peptides, such as copeptin, were considered as potentially interesting prognostic biomarkers in patients with heart disease. In two studies, 268 and 137 patients with hearth failure and poor long-term prognosis had higher copeptin levels compared to patients with a favourable outcome [25, 26]. The combination of copeptin with brain-natriuretic-peptide (BNP), the standard biomarker for the diagnosis of heart failure, was able to further improve outcome prediction [25].
The prognostic accuracy of copeptin after acute myocardial infarction (AMI) was examined in 980 consecutive patients [27]. Copeptin levels were higher in patients who died or were readmitted with heart failure compared with survivors. In a multivariate analysis, copeptin was an independent predictor for death or heart failure. In a recently published prospective study, the value of a dual marker strategy using troponin T, a marker of cardiac necrosis, and copeptin for rapid rule-out of AMI was reported. The combination of troponin T and copeptin resulted in a high diagnostic accuracy in the diagnosis of AMI already at presentation to the emergency department. An algorithm based on the combination of troponin T and copeptin ruled out AMI with a sensitivity of 98.8% and a negative predictive value of 99.7%. Based on these studies, copeptin was recently listed amongst potential cardiac biomarkers by the National Academy of Clinical Biochemistry [28].However, confirmation with larger studies is warranted before copeptin can be adopted into clinical practice.
Copeptin as prognostic marker in ischaemic stroke
Many patients fear the disabling consequences of stroke even more than those of coronary heartdisease (CHD), since stroke is the primary cause of long term disability [29]. However, stroke risk factors and outcome predictors have generally received not as much attention. Despite falling rates of CHD mortality, stroke mortality has declined little since 1990 [30]. Thus, there is a need to develop a credible evidence base of prognostic information for outcomes that are meaningful to patients and clinicians, including level of independency. However, even validated clinical prognostic models are not precise enough to perfectly predict outcome in individual patients with stroke [31]. Adding blood markers as “biomarkers” might improve the prognostic accuracy of clinical prognostic models. Many publications have reported an association of different biomarkers (e.g., C reactive protein, matrix metalloproteinase 9, brain natriuretic peptide) with stroke severity or outcome. However, none of these publications has reported that the analysed bio-marker provided additional information to established clinical variables (e.g., age, blood pressure, nicotine, dyslipidemia, co-morbidities such as heart failure and lesion size/infarct volume) or whether the biomarker increased the predictive power of validated clinical prognostic scores [32] such as the National Institute of Health Stroke Scale (NIHSS) Score.
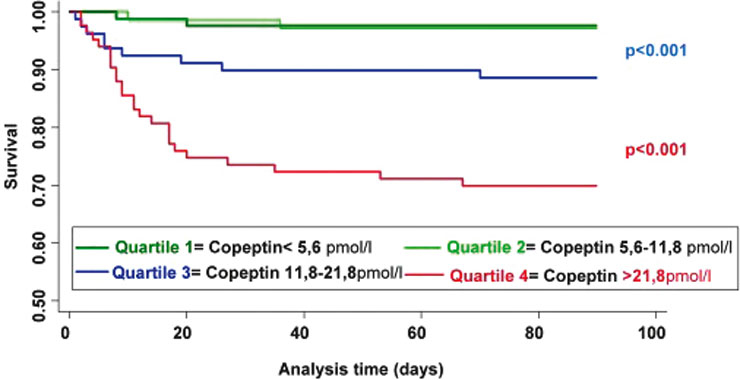
Figure 4
Kaplan Meier survival curves for copeptin in ischaemic stroke
The time to death was analysed by Kaplan Meier survival curves based on copeptin quartiles. Patients in the lower two quartiles (copeptin <5.6 pmol/L and copeptin between 5.6 and 11.8 pmol/L, respectively) had a minimal risk of death, in contrast to patients with copeptin levels in the 3rd and 4th quartile (copeptin between 11.8 and 21.8 pmol/L and copeptin >21.8 pmol/L, respectively), (p <0.0001) (fig. 4).
Activation of the HPA axis is among the first measurable physiological response to cerebral ischaemia [33–36]. Pronounced activation of the HPA-axis and consecutive disruption of the neuroendocrine loops are suggested to aggravate cerebral ischaemia [17, 37]. Based on the findings of a small study, which reported increased AVP levels in patients with ischaemic stroke [38], correlated with stroke severity, we hypothesised that copeptin might be a new prognostic marker in ischaemic stroke [39].
We therefore conducted a prospective cohort study at the University Hospital Basel, Switzerland. From November 2006 to November 2007, 362 patients presenting with an acute ischaemic cerebrovascular event confirmed by CT and/or MRI were included. The primary end point of this study was favourable functional outcome of stroke patients after 90 days from baseline, defined with a modified Rankin Scale (mRS) score of 0 to 2 points. Secondary end point in stroke patients was death from any cause within the 90-day follow-up.
In this prospective, observational study, we found copeptin to be a novel, strong and independent prognostic marker for functional outcome and death in patients with ischaemic stroke. The prognostic accuracy of copeptin in stroke patients was superior to that of other commonly measured laboratory parameters, such as blood glucose, C-reactive protein (CRP) and white blood cell count (WBC), as well as clinical measures (e.g., blood pressure, temperature). It was in the range of the commonly used clinical NIHSS score. Importantly, copeptin was also able to improve the prognostic accuracy of the NIHSS score. The combination of the clinical score with the biomarker revealed a significantly higher area under the curve (AUC) of 0.79 to predict functional outcome, if compared to the clinical score or the marker alone. The combined AUC of 0.89 predicting death was even higher [40]. Furthermore, time to death was analysed by Kaplan Meier survival curves. Patients in the lower two quartiles had a minimal risk of death, in contrast to patients with copeptin levels in the 3rd and 4th quartile (p <0.0001, see fig. 4).
To strengthen the assumption that copeptin is an accurate marker in the very acute phase, we analysed the subgroup of patients with their symptom onset between 0–3 hours (n = 78) and the prognostic accuracy for functional outcome and mortality was similar to the overall sample [40].
We conclude that copeptin may allow improved risk stratification and allocation of targeted therapies for stroke patients and other acute illnesses (table 2) in the future, if these results are externally confirmed in the different settings.
|
Table 2Copeptin levels in different diseases. |
|
Disease
|
Median Copeptin pmol/l (range)
|
| Ischaemic stroke |
Survivors: 9.5 (5.3–19.1)Non-survivors: 35.6 (19.4–93.7) |
| Acute myocardial infarction |
Survivors: 6.5 (0.3–267.0)Non-survivors: 18.5 (0.6–441.0) |
| Heart failure |
Survivors: 21 (8–45)Non-survivors: 46 (19–126) |
| Community-acquired pneumonia |
Survivors: 24.3 (10.8–43.8)Non-survivors: 70.0 (28.8–149.0) |
| COPD acute exacerbation |
Survivors: 12.6 (5.4–27.0)Non-survivors: 42.0 (13.5–103.2) |
| Septic Shock |
Survivors: 59.1 (8.45–386)Non-survivors: 144 (46.5–504) |
| Adapted from Morgenthaler et al. Trends Endocrnol Metab. 2008;19(2):43–9. |
Limitations of copeptin used as a single biomarker
Importantly, biomarkers such as copeptin levels must always be evaluated in the context of a careful clinical assessment. Furthermore, as for all biomarkers, there are limitations of copeptin. Firstly, drugs may suppress the up-regulation of copeptin. For example, in a study in healthy volunteers, copeptin was inhibited in a dose-dependent way upon prednisone treatment [41], suggesting that corticosteroids influence copeptin levels. Secondly, it has been reported that copeptin levels are higher in patients with renal insufficiency [42]. Other false-positive and false-negative results may occur [43]. Knowledge of assay characteristics, particularly the functional assay sensitivity, strengths, pitfalls and optimal cut-off ranges in a predefined clinical setting, are prerequisites for its optimal use in clinical routine.
Importantly, the complexity of every disease is high and the use of a single biochemical marker may always oversimplify prognostic assessment. It is therefore customary to base a prognostic assessment and treatment decisions on several parameters that each mirror different pathophysiological aspects. In this context, copeptin appears to have an interesting potential as a new prognostic biomarker and also makes it a promising candidate for a multimarker panel.
However, the utility of biomarkers is defined by the degree they improve clinical decision-making and add timely information beyond that of readily available information from clinical examination [44].
Outlook
From a public health point of view, accurate prognosis helps to ensure availability of adequate resources to meet the needs of numerous patients.
Several larger series systematically examining copeptin as a prognostic marker in different diseases are needed. The multicentre CoRisk study (Copeptin for risk stratification in stroke) is currently validating the concept of copeptin as a prognostic marker in stroke in a larger group of patients. An initial analysis showed promising results [45]. Future intervention trials are needed to prove the value of copeptin in improving the overall medical management of patients.
There are numerous other regions of interest. Among others, copeptin might prove to be a helpful prognostic marker in patients with an acute ischaemic attack, implementing cut-off levels to identify patients at low versus high risk for cerebrovascular re-events. In general, the emergency setting seems to be the ideal environment where the measurement of copeptin might provide information for risk stratification in all different kinds of acute illnesses.
Correspondence:
Mira Katan, MD
Department of Neurology
Division of Stroke
Columbia University
710W 168th Street
New York NY 10032
USA
mk3270@columbia.edu
References
1 McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87(3):873–904.
2 Charmandari E, Tsigos C, Chrousos G. Endocrinology of the stress response. Annu Rev Physiol. 2005;67:259–84.
3 Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. 1992;267(9):1244–52.
4 Volpi S, Rabadan-Diehl C, Aguilera G. Vasopressinergic regulation of the hypothalamic pituitary adrenal axis and stress adaptation. Stress. 2004;7(2):75–83.
5 Aguilera G, Subburaju S, Young S, Chen J. The parvocellular vasopressinergic system and responsiveness of the hypothalamic pituitary adrenal axis during chronic stress. Prog Brain Res. 2008;170:29–39.
6 Tanoue A, Ito S, Honda K, Oshikawa S, Kitagawa Y, Koshimizu TA, et al. The vasopressin V1b receptor critically regulates hypothalamic-pituitary-adrenal axis activity under both stress and resting conditions. J Clin Invest. 2004;113(2):302–9.
7 Joels M, Baram TZ. The neuro-symphony of stress. Nat Rev Neurosci. 2009;10(6):459–66.
8 Petraglia F, Genazzani AD, Aguzzoli L, Gallinelli A, de Vita D, Caruso A, et al. Pulsatile fluctuations of plasma-gonadotropin-releasing hormone and corticotropin-releasing factor levels in healthy pregnant women. Acta Obstet Gynecol Scand. 1994;73
(4):284–9.
9 Evans MJ, Livesey JH, Ellis MJ, Yandle TG. Effect of anticoagulants and storage temperatures on stability of plasma and serum hormones. Clin Biochem. 2001;34 (2):107–12.
10 Latendresse G, Ruiz RJ. Bioassay research methodology: measuring CRH in pregnancy. Biol Res Nurs. 2008;10 (1):54–62.
11 Struck J, Morgenthaler NG, Bergmann A. Copeptin, a stable peptide derived from the vasopressin precursor, is elevated in serum of sepsis patients. Peptides. 2005;26(12):2500–4.
12 Struck J, Morgenthaler NG, Bergmann A. Copeptin, a stable peptide derived from the vasopressin precursor, is elevated in serum of sepsis patients. Peptides. 2005;26(12):2500–4.
13 Donald RA, Perry EG, Wittert GA, Chapman M, Livesey JH, Ellis MJ, et al. The plasma ACTH, AVP, CRH and catecholamine responses to conventional and laparoscopic cholecystectomy. Clin Endocrinol. (Oxf) 1993;38(6):609–15.
14 Udelsman R, Norton JA, Jelenich SE, Goldstein DS, Linehan WM, Loriaux DL, et al. Responses of the hypothalamic-pituitary-adrenal and renin-angiotensin axes and the sympathetic system during controlled surgical and anesthetic stress. J Clin Endocrinol Metab. 1987;64(5):986–94.
15 Widmer IE, Puder JJ, Konig C, Pargger H, Zerkowski HR, Girard J, et al. Cortisol response in relation to the severity of stress and illness. J Clin Endocrinol Metab. 2005;90(8):4579–86.
16 Katan M, Morgenthaler N, Widmer I, Puder JJ, Konig C, Muller B, et al. Copeptin, a stable peptide derived from the vasopressin precursor, correlates with the individual stress level. Neuro Endocrinol Lett. 2008;29(3):341–6.
17 Sapolsky RM, Pulsinelli WA. Glucocorticoids potentiate ischemic injury to neurons: therapeutic implications. Science. 1985;229(4720):1397–400.
18 Morse JK, Davis JN. Regulation of ischemic hippocampal damage in the gerbil: adrenalectomy alters the rate of CA1 cell disappearance. Exp Neurol. 1990;110(1):86–92.
19 Smith-Swintosky VL, Pettigrew LC, Sapolsky RM, Phares C, Craddock SD, Brooke SM, et al. Metyrapone, an inhibitor of glucocorticoid production, reduces brain injury induced by focal and global ischemia and seizures. J Cereb Blood Flow Metab. 1996;16(4):585–98.
20 Morgenthaler NG, Muller B, Struck J, Bergmann A, Redl H, Christ-Crain M. Copeptin, a Stable Peptide of the Arginine
Vasopressin Precursor, Is Elevated in Hemorrhagic and Septic Shock. Shock 2007.
21 Lindner KH, Strohmenger HU, Ensinger H, Hetzel WD, Ahnefeld FW, Georgieff M. Stress hormone response during and after cardiopulmonary resuscitation. Anesthesiology. 1992;77(4):662–8.
22 Muller B, Morgenthaler N, Stolz D, Schuetz P, Muller C, Bingisser R, et al. Circulating levels of copeptin, a novel biomarker, in lower respiratory tract infections. Eur J Clin Invest. 2007;37(2):145–52.
23 Stolz D, Christ-Crain M, Morgenthaler NG, Leuppi J, Miedinger D, Bingisser R, et al. Copeptin, C-reactive protein, and procalcitonin as prognostic biomarkers in acute exacerbation of COPD. Chest. 2007;131 (4):1058–67.
24 Schrier RW, Abraham WT. Hormones and hemodynamics in heart failure. N Engl J Med. 1999;341(8):577–85.
25 Stoiser B, Mortl D, Hulsmann M, Berger R, Struck J, Morgenthaler NG, et al. Copeptin, a fragment of the vasopressin precursor, as a novel predictor of outcome in heart failure. Eur J Clin Invest. 2006;36(11):771–8.
26 Gegenhuber A, Struck J, Dieplinger B, Poelz W, Pacher R, Morgenthaler NG, et al. Comparative evaluation of B-type natriuretic peptide, mid-regional pro-A-type natriuretic peptide, mid-regional pro-adrenomedullin, and Copeptin to predict 1-year mortality in patients with acute destabilized heart failure. J Card Fail. 2007;13(1):42–9.
27 Khan SQ, Dhillon OS, O’Brien RJ, Struck J, Quinn PA, Morgenthaler NG, et al. C-terminal provasopressin (copeptin) as a novel and prognostic marker in acute myocardial infarction: Leicester Acute Myocardial Infarction Peptide (LAMP) study. Circulation. 2007;115(16):2103–10.
28 Tang WH, Francis GS, Morrow DA, Newby LK, Cannon CP, Jesse RL, et al. National Academy of Clinical Biochemistry Laboratory Medicine practice guidelines: Clinical utilization of cardiac biomarker testing in heart failure. Circulation. 2007;116 (5):e99–109.
29 Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, et al. Heart disease and stroke statistics – 2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119 (3):e21–181.
30 Cooper R, Cutler J, Desvigne-Nickens P, Fortmann SP, Friedman L, Havlik R, et al. Trends and disparities in coronary heart disease, stroke, and other cardiovascular diseases in the United States: findings of the national conference on cardiovascular disease prevention. Circulation. 2000;102(25):3137–47.
31 Counsell C, Dennis M, McDowall M. Predicting functional outcome in acute stroke: comparison of a simple six variable model with other predictive systems and informal clinical prediction. J Neurol Neurosurg Psychiatry. 2004;75(3):401–5.
32 Whiteley W, Chong WL, Sengupta A, Sandercock P. Blood markers for the prognosis of ischemic stroke: a systematic review. Stroke. 2009;40 (5):e380–9.
33 Fassbender K, Schmidt R, Mossner R, Daffertshofer M, Hennerici M. Pattern of activation of the hypothalamic-pituitary-adrenal axis in acute stroke. Relation to acute confusional state, extent of brain damage, and clinical outcome. Stroke. 1994;25
(6):1105–8.
34 Johansson A, Olsson T, Carlberg B, Karlsson K, Fagerlund M. Hypercortisolism after stroke--partly cytokine-mediated? J Neurol Sci. 1997;147(1):43–7.
35 Slowik A, Turaj W, Pankiewicz J, Dziedzic T, Szermer P, Szczudlik A. Hypercortisolemia in acute stroke is related to the inflammatory response. J Neurol Sci. 2002;196(1–2):27–32.
36 Johansson A, Ahren B, Nasman B, Carlstrom K, Olsson T. Cortisol axis abnormalities early after stroke – relationships to cytokines and leptin. J Intern Med. 2000;247(2):179–87.
37 Sapolsky RM. Stress, Glucocorticoids, and Damage to the Nervous System: The Current State of Confusion. Stress. 1996;1(1):1–19.
38 Joynt RJ, Feibel JH, Sladek CM. Antidiuretic hormone levels in stroke patients. Ann Neurol. 1981;9(2):182–4.
39 Barreca T, Gandolfo C, Corsini G, Del Sette M, Cataldi A, Rolandi E, et al. Evaluation of the secretory pattern of plasma arginine vasopressin in stroke patients. Cerebrovasc Dis. 2001;11(2):113–8.
40 Katan M, Fluri F, Morgenthaler NG, Schuetz P, Zweifel C, Bingisser R, et al. Copeptin: a novel, independent prognostic marker in patients with ischemic stroke. Ann Neurol. 2009;66(6):799–808.
41 de Kruif MD, Lemaire LC, Giebelen IA, Struck J, Morgenthaler NG, Papassotiriou J, et al. The influence of corticosteroids on the release of novel biomarkers in human endotoxemia. Intensive Care Med. 2008;34(3):518–22.
42 Bhandari SS, Loke I, Davies JE, Squire IB, Struck J, Ng LL. Gender and renal function influence plasma levels of copeptin in healthy individuals. Clin Sci. (Lond) 2009;116(3):257–63.
43 Christ-Crain M, Muller B. Procalcitonin in bacterial infections – hype, hope, more or less? Swiss Med Wkly. 2005;135(31-32):451–60.
44 Marshall JC. Biomarkers of Sepsis. Curr Infect Dis Rep. 2006;8(5):351–7.
45 G. M. De Marchis, M. Katan, A. Weck, H. P. Mattle, C. Breckenfeld, Ph. Schütz, Ch. Foerch, et al. Validation of Copeptin as prognostic marker in ischemic stroke. Abstract; Meeting of the European Neurological Society 2010.



