Superparamagnetic nanoparticles – a tool for early diagnostics
DOI: https://doi.org/10.4414/smw.2010.13081
M
Hofmann-Amtenbrink, H
Hofmann, X
Montet
Summary
Nanoparticles show several interesting new physical and biological properties and therefore play an increasing role in pharmaceutics and medicine. For more than 30 years this research field has been developing slowly but steadily from physical and biological interest (bench) to applications in clinics (bedside). However, many of these particles for biomedical applications are still in the pre-clinical or clinical phase. Combined with drugs or genes these nanoparticles may change the viability of or the transcription processes in cells, which make them interesting for the pharmaceutical industry, cell biology and diagnostics.
Because most of the application of superparamagnetic nanoparticles as therapeutic tool, like non-viral vector, drug delivery, are still far from clinical use, this review will concentrate on superparamagnetic nanoparticles as versatile agent for early diagnosis, including the use of such particles as contrast agent for MR imaging and as vehicle for the detection of biomarkers.
Introduction
Nanoparticles show several interesting new physical and biological properties and therefore play an increasing role in pharmaceutics and medicine. For more than 30 years this research field has been developing slowly but steadily from physical and biological interest (bench) to applications in clinics (bedside). However, many of these particles for biomedical applications are still in the pre-clinical or clinical phase. Combined with drugs or genes these nanoparticles may change the viability or the transcription processes in cells, which make them interesting for the pharmaceutical industry, cell biology and diagnostics. They are: a) offering more specific experiments in the discovery and development of a drug as they can interact with cells in an unspecific or in a specific way [1], b) useful as a versatile tool in early diagnostics, often combined in screening novel drugs, tracking cells such as stem cells [2, 3] or adsorb at biomarkers, genes etc., and c) may act as a delivery vehicle for therapeutics which can be modified to be targeted to specific cells or regions of disease and to minimise secondary systemic negative effects [4] and finally d) act physically by heating up the surrounding tissue by use of an external field [5, 6]. Some of these particles may even serve in a combined way in all three fields and can be useful to determine a certain individual’s susceptibility to different disorders.
Due to their size, their size-dependent properties and the option of non-invasive application these materials are promising candidates for research, diagnostic and therapeutic applications in various fields such as cancer, neurodegenerative diseases (e.g., multiple sclerosis, stroke), inflammatory diseases (e.g., rheumatoid arthritis, atherosclerosis) and others. Properties only existing on the nanoscale level, such as the change of ferromagnetic to superparamagnetic behaviour in nanoparticles [7] or the increased intensity and long term stability of fluorescent light emission of semiconductor crystals [8] (quantum dots), increase the interest of clinicians and industry in these materials.
Nanoparticles can be purely polymeric or have an inorganic core that can be coated by inorganic or organic shells. Those nanoparticles which are used in biomedical applications include block ionomer complexes, liposomes [9, 10], polymeric micelles, dendrimers, solid polymeric nanoparticles designed from synthetic or natural polymers [11], inorganic nanoparticles based e.g., on iron oxide [12], nanorods made e.g., by carbon [13] and quantum dots [14] or metals and metal alloys [15]. Water-soluble synthetic polymers (dendrimers) are an example of organic nanoparticles tested in pre-clinical models for the delivery of drugs, genes, and imaging agents as they show a rich versatility for tailoring their binding properties to several requirements, among them facilitation of cellular uptake of drugs (e.g., cancer drugs) [16–18]. Magnetic properties, corrosion resistance and toxicity are the key parameters in the design of magnetic nanoparticles for medical application. Materials that have high saturation magnetisation and therefore a high magnetic susceptibility are ideal candidates for MRI contrast agents. Common magnetic materials contain Fe, Co, Ni, Mn, Cr or Gd and can be used in form of metals, metal alloys or metal oxides. Metals and alloys, such as Fe, FeCo, FePt, are among magnetic materials, those with the highest magnetisation. Unfortunately all these materials are susceptible to oxidation and dissolution. Therefore, toxicity is an important issue and the particles can only be used if a dense coating on the particle surfaces is applied. In contrast to metals, metal oxide materials are generally chemically stable yet have acceptable magnetisations. The inorganic particles may have inorganic coatings, for example, of silica, gold or organic coatings such as those mentioned before. All such particles with sizes less than about 100 nm (nanoparticles) will be taken up by mononuclear phagocytes, macrophages and the reticuloendothelial system (RES) in the blood and organs if they are not specially coated by a hydrophilic surface to escape an uptake within the first minutes after injection. For further recognition in specific cells the nanoparticles are functionalised with biological signals, which can be chemically conjugated onto the surface. Used as vehicles for drug delivery these particle systems are finally loaded with therapeutic agents whereby the drug-loading capacity depends on the nanoparticle system. The advantage of modern nanoparticle systems is that they can remain in the blood as a blood pool agent for imaging, or their bioactive component can be transported to certain organs or tissue important for therapeutic delivery. These particle systems can also be targeted to the surface of a specific cell or to a specific cell compartment by entering the cell, a demanding property for the design and further development of specific drugs, for gene/plasmid delivery and the deeper knowledge of cell activities.
Especially superparamagnetic particles based on the specific physical properties of iron oxide at the size of 5 to 20 nm are a key class of nanoparticles, which have the potential to revolutionise clinical diagnostic and therapeutic techniques. Widder et al. created the concept of using magnetic nano- or microparticles in the late 1970’s [19, 20]. As most of the application of superparamagnetic nanoparticles as a therapeutic tool, such as non-viral vector drug delivery, is still far from clinical use, this review will concentrate on superparamagnetic nanoparticles as versatile agents for early diagnosis, including the use of such particles as a contrast agent for MR imaging and as a vehicle for the detection of biomarkers.
Superparamagnetic ironoxide nanoparticles
Superparamagnetic iron oxide nanoparticles (SPION) belong to the class of nanoparticles based on an iron oxide core and are coated by either inorganic materials (silica, gold) or synthetic/natural organic materials (phospholipids, fatty acids, polysaccharides, natural polymers such as dextran or chitosan, biodegradable polymers such as polyethylglycol PEG, non degradable polymers such as like polyvenylalcohol PVA, peptides or other surfactants) [21–23]. In the last years various classes of superparamagnetic iron oxide particles have been developed and mentioned in the widespread literature. These SPIONs are often classified by their size, which includes the iron oxide core and the coating/functionalisation, called hydrodynamic diameter, amongst others by Bowen et al. 2002 [24]; Mornet et al. 2005 [25]; Roch et al. 2005 [26]; Corot et al. 2006 [27], Thorek et al. 2006 [28]. The largest class can be used orally and consists of sizes between 300 nm and 3.5 μm; the most used SPIONs of sizes between 60–150 nm are already used as MRI agents or are in development for drug delivery [29], whilst the ultra small SPIONs (also called USPIO) of about 10–40 nm are of interest for gene delivery [30, 31].
Magnetic properties
Magnetic materials can be classified amongst others by their susceptibility to magnetic fields into diamagnetic materials with weak repulsion from an external magnetic field (negative susceptibility), paramagnetic materials showing small and positive susceptibility, and ferromagnetic materials exhibiting a large and positive susceptibility to magnetic fields, these being known as magnets in daily life (“horseshoe magnets”). The magnetic properties of materials in the first two classes do not persist after removing the external magnetic field, while ferromagnetic materials, exhibiting strong attraction to magnetic fields, remain magnetised even after the external field is removed. Nanoparticles consisting of iron oxide phases γ-Fe2O3 (Maghemite) and/or Magnetite (Fe3O4) with sizes <20 nm or pure iron of 3 nm lose their ferromagnetic behaviour and show paramagnetic behaviour, which means that no hysteresis or magnetism remains after removing the magnetic field. In contrast to normal paramagnetic material these materials show a high saturation magnetisation, which means that a new combination of magnetic behaviour exists at the nano scale: superparamagnetism. Typically, Ms values of magnetite nanoparticles are in the range of 30–65 emu/g, which is lower than the 90 emu/g reported for their ferromagnetic bulk form. The superparamagnetic behaviour is characterised by a typical relaxation time τ; the time which the systems needs to achieve zero magnetisation after an external magnetic field is switched off:
τ = τ.exp(KV⁄kBT) (1)
τwhere is the characteristic time (10–9 s), K is the anisotropy energy (20 000 J/m3 for iron oxide) and V is the volume of the particle: kB is the Boltzmann constant, T is the temperature. Typical relaxation times for particles with a diameter of 4 to 10 nm, as used in the case of contrast agent or for other medical applications, are 10–8 to 10–6 s. Another effect for which these very small superparamagnetic particles are of interest is the presence of energy absorption due to Néel relaxation. As the magnetic domains fixed within the particles have to be reoriented very quickly in an alternating (AC) magnetic field, the energy loss of the particles is used to heat the surrounding environment which can, for example, be the cells of a cancer tissue (further details on the physical background of superparamagnetic particles are given by Rosensweig [32]).
Using a permanent magnetic field, the particles are magnetised up to saturation magnetisation leading to a magnetic dipol-dipol interaction between the particles in such a way that the particles are aligned following the field lines of the permanent external magnet. In a permanent magnetic field, which shows a filed gradient in z direction, a force Fm acts on the magnetised particles:
Fm (z) = 4π/3 r3
mMs(dBz / dz) (2)
Where rm is the radius of the magnetic part of the particle, Ms the saturation magnetisation of the particle and dB
z
/ dz the magnetic flux gradient. This magnetic force leads to an acceleration of the particles in the direction of increasing field strengths. In a liquid, the movement is hindered by a viscous drag (stoke Force, Fs), expressed by the following equation:
Fs = 6πηrhv (3)
where η is the viscosity of the liquid, rh is the hydrodynamic radius of the particle (radius including inorganic core and the polymer coating) and n is the velocity of the particles. A large hydrodynamic radius of the particles, which is often much larger than the magnetic diameter of the particles, especially if the particles are coated and functionalised with various polymers and biomolecules, makes it necessary to generate a strong force, i.e., a high gradient on the magnetic particle to be moved within or against a fluid pressure such as is present in the lymphatic or blood system, in tissue like muscles or cartilage or in organs. For this reason, it is necessary to use high-gradient magnets for both delivery of therapeutics as well as for separation of biomolecules and if possible to increase a) the susceptibility of the nanoparticles by the choice of the material and b) to increase the amount of magnetic particles per delivery system. Both options are limited, either because only iron oxide is biocompatible nanostructured material or because the size of particles may create negative side effects such as embolic events if it exceeds a certain amount. In case of intracellular actions like magnetic transfection of a cell, the particles should even enter the cell, which is only possible by having small particle sizes [28] or by using transfection agents [33, 34]. Typical particle sizes for transport and in-vitro separation are 60 to 150 nm hydrodynamic radius. Such particles include up to 100 iron oxide crystallites, and transport velocities of 5 to 20 micrometer per second are possible.
The magnetic properties of SPION can only be used properly if the particles are of colloidal stability, which is an unaccomplished need in application using physiological fluids. In such fluids an attractive constant van der Waals force is present whilst particles, depending on their coating and functionalisation, show electrostatic and steric repulsive forces, which vary with the ionic strengths, the pH-value and the type and conformation of the adsorbed polymers and biological molecules. In cell media or similar liquids, which have high ionic strength, only steric stabilisation of the coating could avoid agglomeration.But thus the question is posed about the secondary effects of these particles on their passage through the human body. Nanoparticles have to be highly specific, efficient, and should be rapidly internalised by the target cells, which is limited by several factors [35]: “(i) nanoparticle aggregation (nanoparticles have a large surface/volume ratio and tend to agglomerate); (ii) the short half-life of the particles in blood circulation (when nanoparticles agglomerate, or adsorb plasma proteins, they are quickly eliminated from the blood circulation by macrophages of the mononuclear phagocyte system before they can reach the target cells); (iii) the low efficiency of the intracellular uptake of nanoparticles; and (iv) nonspecific targeting.” The coating and functionalisation of such particles described in the last section is therefore decisive for their specific application and their biocompatibility in the human body.
Particles can also agglomerate through the contact with, for example, proteins and in doing so may provoke changes regarding cell internalisation or attachment and cytotoxicity. Therefore synthesis and coating of nanoparticles become a determining step in the further use of nanoparticles in-vivo and in-vitro. The biocompatibility of such material is not only related to their nano-size nature, but especially to their surface properties, which are the determining factors for cell uptake and cytotoxicity, whereas the type of particle does not seem to play such an important role (see Petri-Fink et al. [37]). An overview on some current polymer coating materials is shown in the article of A.K. Gupta and M. Gupta, 2005 [31], as well as other references dealing with further polymer coatings, amongst others polysaccharides (dextran, starch and chitosan) [26, 36], polyvinyl alcohol [37, 38] poly(l-lysine) (PLL) [3], starch derivatives with phosphate groups binding mitoxantrone as chemotherapeutic [39], and inorganic coatings like silica [40] or gold [41]. All these coatings serve further functionalisation of the particles by ligands such as, for
example, peptide sequences conjugated to the nanoparticle surface to facilitate receptor-mediated endocytosis or phagocytosis [42] or proteins and antibodies to bind to biological receptors [43].
Applications in imaging
For many years SPIONs have been used in diagnostics as a contrast agent in magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA) [27, 44–46]. Various SPION, mostly coated with dextran, are already on the market or in clinical trials. Several review articles give an overview of the agents used, such as Corot et al. 2006 [27], who mentions the various polymers used as coatings, such as different varieties of dextran (dextran, carboxymethylated dextran, carboxydextran), starch, PEG, arabinogalactan, glycosaminoglycan, organic siloxane, sulphonated styrene-divinylbenzen, and gives an overview on applications showing that the hydrodynamic diameter of SPION based agents, given intravenously, may vary between 20 and 180 nm (see also Sun et al. [47]). Another overview on the application of superparamagnetic iron oxide- based contrast agents has been given by Wang et al. (2001) [48], who distinguish between agents used as oral SPION, containing larger particles than injectable agents; standard SPION, taken up by the RES in liver and spleen and therefore used for such organs; ultra small SPION with prolongated blood half time which because of this can be used for the imaging of lymph nodes and bone marrow; and monocrystalline SPION, which are much smaller than other SPION. These particles may be used in cellular and molecular imaging. Several forms of ultra small superparamagnetic iron oxides (USPIO) have undergone clinical trials, one of the most notable being Combidex©, which is in late stage clinical trials for use in the detection of lymph node metastases [49]. Various agents are on the market and act as bowel contrast agents (i.e., Lumiren® and Gastromark®) and liver/spleen imaging agents (i.e., Endorem© and Feridex IV© [50], or according to Stoll et al. (2009) [54], especially for USPIO (i.e., Sinerem© (Guerbet) and Supravist©(Bayer Schering Pharma)), or superparagmetic iron oxide (SPIO) contrast media like Resovist© (Bayer Schering Pharma).
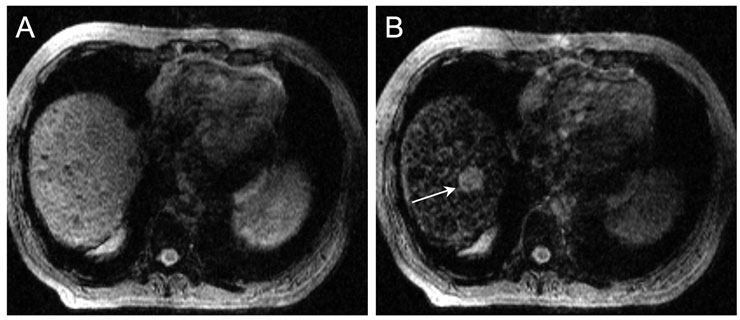
Figure 1
Iron oxide nanoparticles as RES contrast media for MRI.
MR images of the hepatic dome before (A) and after injection of iron oxide nanoparticles (Ferumoxide: 1.4 ml) (B). The lesion (arrow) is clearly visible after injection of the iron oxide nanoparticles, but not before. The increase of contrast between the lesion and the liver is due to a darkening of the normal liver. Moreover, please note the heterogeneous aspect of the liver, corresponding to cirrhosis. In this context, the hepatic nodule is highly suspect of a hepatocellular carcinoma.
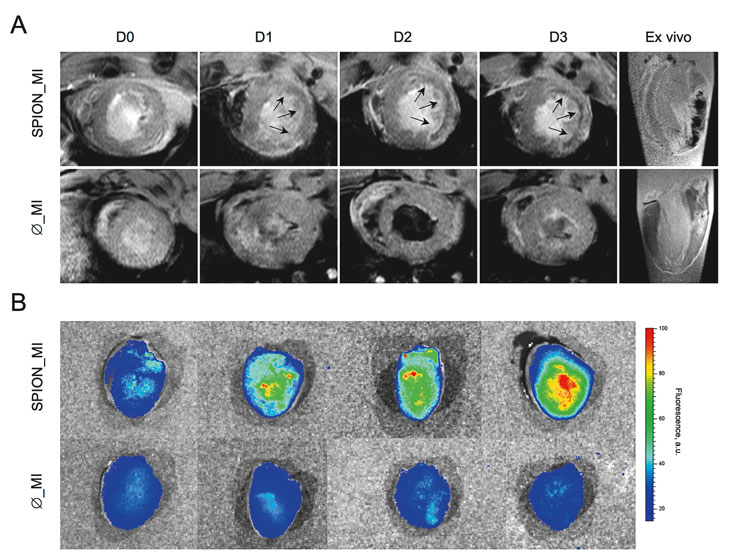
Figure 2
In vivo and ex vivo imaging.
In vivo MRI of the infarcted group is presented in (A). The first line corresponds to a representative rat previously injected with SPION and clearly shows the appearance over time (day [D] 0 to day 3) of a hypointense (black) signal in the myocardial infarction area (arrows). The appearance of the hypointense signal is due to the superparamagnetic iron oxide. The second line corresponds to a representative rat without previous injection of SPION. These rats showed no hypointense signal. Ex vivo MRI (last column) also depicts a clear hypointense signal in the SPION-injected rat, whereas no hypointense signal was seen in the non-injected rats thus confirming the in vivo results.
Ex vivo reflectance fluorescence (B) also confirmed the MR results. A representative image of the injected group shows a clear increase in fluorescence over time, whereas the non-injected groups do not show any fluorescent signal.
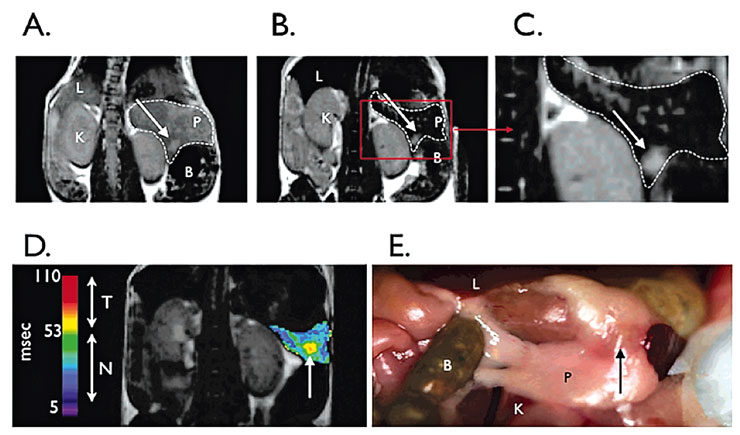
Figure 3
Magnetic resonance imaging (MRI) on T2-weighted sequences of pancreas and tumour.
Preinjection (A) and postinjection (B) of pancreas and tumour. (C) Image from B at higher magnification. Bombesin-targeted nanoparticles permit tumour (arrows) to be seen. Arrow indicates bulk tumour. (D) Postcontrast agent, colorised T2 map of a pancreas with implanted tumour. Tumour has higher T2s than normal pancreas. Abbreviations: L, liver; P, pancreas; K, kidney; B, bowel. (E) Photograph of partially dissected mouse with pancreas and tumour visible.
Contrast agents have to fulfil the requirements of improved enhancement of signals. In contrast to computer tomography (CT) the magnet resonance imaging (MRI) requires a much lower dose of contrast agent. Negative enhancers – like most of the diamagnetic materials – have only a negligible effect on the MR signals, whilst agents containing paramagnetic materials like iron or lanthanide (gadolinium, manganese, dysprosium) show positive enhancement by their unpaired electrons resulting in a positive susceptibility. Iron oxide nanoparticles in a size of 5 to 10 nm induce large magnetic moments altering the magnetic field in a tissue over time and space, and thus create a large magnetic heterogeneity through which water molecules diffuse. This induces dephasing of the magnetic moments of protons creating data for MR images [50], and the T2 relaxation times of water are shortened [52]. It is the significant capacity of superparamagnetic nanoparticles to enhance T1 but also T2/T2* relaxation, and by doing so to reduce the T2/T2* relaxation time (see Wang 2001, [48]). SPIO particles may decrease T2* by magnetic susceptibility effect and T2 by dipole-dipole interaction or scalar effect between protons and magnetic centre [53]. A large magnetisation difference occurs as a result of the non-homogeneous distribution of superparamagnetic particles, which gives rise to local field gradients that accelerate the loss of phase coherence of the spins contributing to the MR signal. By this, both T1 and T2 relaxation time is shortened, however the changes induced are dependent on the particles used – their size, size distribution and coating – and their behaviour in acting either at the cell surface (extracellular) or within the cell (intracellular) [54]. The signal enhancement by using iron oxide is higher than for gadolinium chelates [50], the latter belonging to the first generation of MR contrast agents and acting only extracellularly [55] as unspecific agents [56], whilst iron oxide nanoparticles may act in both ways.
The transport of most of the SPION contrast agents is by intravenous administration, which determines the size of the nanoparticles. For successful delivery the particles have to pass the vascular capillary wall. Whilst Gd ion-polymer complexes, for example, are of small size (≤10 nm), those of coated iron oxides can be up to 100 nm and therefore act differently according to the size distribution and the polymer coatings of the agent used. Depending on their size, charge and the configuration of the coating, these particles are metabolised by the reticuloendothelial system (RES) consisting of monocytes and macrophages. These cells, accumulating in lymph nodes and the spleen as well as in the liver (Kupffer cells), are favoured for the uptake of SPION and affect the delivery time and the diffusion to certain tissues. Smaller particles generally circulate longer and are taken up by cells of the lymphatic system and bone marrow whilst particles >50 nm are taken up by liver cells [56]. If particles are not entirely captured by the liver and spleen, they are widely evaluated as potential markers of inflammation for the diagnosis of inflammatory and degenerative disorders associated with high macrophage phagocytic activity such as plaque imaging or brain ischaemia [17]. SPION are also used in liver tumour detection as the tumour lesions exclude the uptake of particles (tissue in MRI remains bright, because they are lacking Kupffer cells), whilst the macrophages in the normal liver tissue will take up the particles and darken the image of the tissue [12]. See also figure 1, reprinted with permission [58]. Beside the classical fields of SPION use in MRI, imaging of the gastrointestinal tract (GI tract), liver and spleen imaging, lymph nodes imaging, blood pool agent [50] and imaging of inflammatory events (arthritis or other musculoskeletal disorders [55, 59]), the high sensitivity of iron oxide nanoparticles and the capability of such nanoparticles to enter the cells open novel applications, for example, the tracking of various kinds of stem cells transplanted into organs such as the brain [27], to demonstrate macrophage activity within atherosclerotic plaque [60] and in molecular imaging.
Imaging of atherosclerosis and inflammation
Research on the use of SPION for the detection of atherosclerotic plaques by MRI has been undertaken by von zur Mühlen et al. [61]. Monocytes, as precursor cells of macrophages, promote atherosclerosis by secreting mediators and a highly active state of phagocytosis and secretion of cytokines and chemokines. Before rupture atherosclerotic lesions show an accumulation of macrophages. The phagocytosis of SPION by macrophages may therefore act as a potential marker of inflammation for plaque imaging. For the study, MAC-1 expressing Chinese hamster ovary (CHO) cells, expressing MAC-1 either in a native, low affinity state (wildtype, WT) or a high affinity state (GFFKR-deleted cells, DEL) were used to simulate the type of activated macrophages found in atherosclerotic plaques. CHO-cells not expressing MAC-1 were used as controls (NCHO). These cells were grown for 24 h at 38 °C in a cell incubator, washed and then either incubated with or without CD11b-blocking monoclonal antibody 2LPM 19c and finally amino PVA-SPION were added to the cells and incubated for another 24 hours. Cell growth and viability was not influenced by the incubation with SPION (details of the study are shown in ref. [14]). Those cell lines expressing the MAC-1–receptor showed higher signal values compared to natural CHO cells, and the activated receptor in the DEL cell lines raise the signal extinction up to four pixels. It could also be shown that MAC-1 expressing cells incubated with CD11b-antibody constantly show lower signal extinction than those without CD11b-antibody. The studies identified the integrin MAC-1 (CD 11b/CD18) as a mediator for the SPION endocytose into monocytes/macrophages and showed that MAC-1 is a central mediator of inflammation. Dextran-coated particles which are mostly used as contrast agents are commonly attached to cells but not taken up by them, while SPION coated with amino-functionalised polyvinyl alcohols interact with different cells [62, 23] and therefore underline a receptor-based uptake of SPION by cells, which could be further supported through these experiments.
Moreover, mobilisation of monocytes/macrophages in acute myocardial infarction (MI) has also been proved [63]. Briefly, monocytes/macrophages were loaded by a simple intravenous injection of SPION. After complete disappearance of the SPION from the blood, an acute MI was created in rats by ligating a coronary artery. The arrival of monocytes/macrophages was then tracked by magnetic resonance imaging, see figure 2, reprinted with permission [63].
Molecular imaging
Molecular imaging is an integrated discipline defined by H.R. Herschman (2003) [64] as “non-invasive, quantitative and repetitive imaging of targeted macromolecules and biological processes in living organisms. Wickline et al. (2003) [65] state that molecular imaging can be used “to improve diagnostic accuracy and sensitivity by providing an in-vivo analogue in immunocytochemistry or in situ hybridization”. The recent demands in a more “personalised medicine” demand deeper insight into the whole chain of a disease, starting with the early detection of the disease and continuing to a more accurate prognosis, a treatment which is focused to distinguish between responder and non-responder and the capability to monitor a therapy und to better understand the interactions of cells and biomolecules, being the possible determinating step in the treatment. Therefore, it is important to seek non-invasive methods by which the effects of diseased tissue on the surrounding healthy environment can be visualised and described in situ and in vivo. In recent years progress has been made in the field of cellular and molecular imaging technologies that can depict and identify specific molecules which are only associated with certain diseases, especially those which show inflammatory events. Although various imaging modalities are valuable for molecular imaging such as ultrasound or magnetic resonance (MR) [66] or by nuclear constructs used in PET (see [66]), or by optical imaging [67], the following description will focus only on MR imaging with SPION. SPION can be functionalised with antibodies or fragments of it, peptides or proteins or small molecules in a way that they have a high affinity to the targeted cell. For those cases, in which the SPION are used as “single” or “clusters” of only a few nanoparticles, it is important that the longitudinal relaxivity per molecular binding side for the complex is maximised to receive high contrast enhancement even though the number of particles at the targeted side is low (see [66]). Depending on requirements in research it is even possible to visualise single cells. In comparison with gadolinium containing chelates, the SPION show a more extensive shortening of T1 and T2 relaxation time, which results in higher sensitivity in imaging when using SPION and the visualisation of single iron loaded cells at clinical field strengths [54, 68].
Molecular imaging has been applied in a variety of fields, among them oncology where it has been proved to enhance the detection of specific tumours by using over-expressed cellular markers as molecular targets. See figure 3 for an example of pancreas tumour imaging, reprinted with permission from [69].
Intercellular tracking of biomarkers
Recently, SPION for use in targeting and magnetic extraction of cellular compounds and their chemical, optical and physical analysis, including proteomics, were developed [70]. This use of functional nanoparticles enables the identification of their specific interaction partners in a physiological environment, such as the entire intact cell. Proteomic analysis of diseases, where the immobilised interaction partner reacts with the target in physiological conditions, is possible, as well as synthesis of peptide and molecule libraries for the determination of interaction partners. In contrast to all other techniques, interaction partners can be identified in physiological conditions in the cell.
Functionalised nanoparticles smaller than 100 nm carrying simultaneously specific organelle addressing peptides, a cyclic RGD peptide and a fluorescent dye are able to localise specifically at the target site. Exemplarily, nuclei and mitochondria were studied and in situ affinity chromatography was performed. In order to study particle-organelle interactions, the cell lysate was separated on a magnetic separator after the particle incubation on live cells and lysation of the cells. This cell separator has a strong magnetic field gradient of several hundred T/m. After protein electrophoresis, the analysis by mass spectroscopy revealed mitochondrial and nuclear proteins in magnetically enriched fractions.
To investigate how functionalised SPION interact with cell organelles, maleimide-PEG-NHS were covalently bound to amine groups of aminopropylsilica surface of the particle core, generating PEG-SPION leaving maleimide group of the PEG for coupling of the fluorophores and peptides via thiol group. In order to assess the efficiency of mitochondrial targeting, three types of functionalised particles were prepared: coumarin (c[RGDfK-(Ac-SH)] peptide, mentioned below as cRGD) and/or N-terminal 21 residues peptide from mitochondrial 3-oxoacyl-Coenzyme A thiolase (mentioned below as MTP) giving rise to the MTP-SPION, MTP-cRGD-SPION and cRGD-SPION, respectively. The evaluation of nuclear targeting employed two types of nanoparticles: the NTP-SPION were functionalised by FITC and PKKKRKV-GC peptide representing SV40 large T-antigen nuclear localisation signal peptide (NTP), and the NTP-cRGD-SPION contained conjugated cRGD peptide in addition. After the final synthesis step, functionalised particles showed a size distribution in the range of 70–100 nm.
After incubation with the nanoparticles, the cells were lysed and mass spectrometric analysis of the proteins associated to magnetically sedimented complexes was performed. The proteins detected on the surface of MTP-cRGD-SPION can be subdivided into several classes: i) proteins associated directly with the transport of the nanoparticles to their target site (heat shock proteins); ii) proteins associated with the transport of the particles to the mitochondrial outer membrane; iii) proteins associated with the membrane of the mitochondria (e.g., ATP synthase); iv) proteins associated to the processing of the arriving signal in the mitochondria (e.g., hsp 60); v) proteins suggested as associated to the mitochondria, but final proof lacking. Proteins belonging to the second part of glycolysis (GAPDH, enolase, Pyruvate kinase) were identified proteins. With this investigation, a novel tool for the investigation of not only the up-take mechanism but also for biomarker detection and screening of peptides and drugs was developed.
Conclusion and outlook
Superparamagnetic iron oxide nanoparticles (SPION) are established contrast agents for a few applications such as imaging of liver or lymph nodes. The same particles, embedded in larger polymer spheres, are used for in vitro separation of biomolecules and cells. Other interesting applications such as molecular imaging enabling early detection of cancer or other diseases or the more sophisticated transport of drug and genes have shown their feasibility, but are still far from clinical applications. The main problem, which slows down the transfer from research to clinic, is insufficient knowledge on the stability of the particles after injection, long-term biocompatibility, and even more important, the need of complex coated particles produced in a reproducible and cost-efficient manner. The colloidal stability is strongly influenced by the unspecific adsorption of proteins during circulation in the blood stream. The investigation of the adsorption is very difficult, because equilibrium or steady-state situations in complex systems like blood are only achieved after long residence times of more than several hours. This means during the first period where adsorption is observed, a dynamic change of the protein composition at the particle surface also occurs. Therefore the method explained in section four is a very useful tool to investigate the protein adsorption in a quantitative manner and to compare these results with the observed behaviour of the particles. As the interaction of a functionalised particle with a certain biofluid depends strongly on the composition of the particle surface, including targeting peptides and the actual composition of the human biofluids, a very complex situation occurs in which the reaction particle-fluid is very difficult to predict. The key to further success and also to a personalised medicine is the development of particles and investigation methods in welldefined environmental requirements with a focused application. A general coating and functionalisation concept seems, at least today, not to be possible. Therefore close co-operation between material science, cell and molecular biology, pharmaceutical and clinical research is the only way to enable a clinical application of SPION in the next years, also including more challenging applications such as molecular imaging and biomarker detection.
Correspondence:
Dr.-Ing. Margarethe Hofmann-Amtenbrink,
Mat Search
Consuting
Ch. Jean Pavillard 14
1009 Pully
Imhofmann@matsearch.ch
References
1 Neumaier CE, Baio G, Ferrini S, Corte G, Daga A. MR and iron magnetic nanoparticles. Imaging opportunities in preclinical and translational research. Tumori. 2008;94(2):226–33.
2 Budde MD, Frank JA. Magnetic tagging of therapeutic cells for MRI. J Nucl Med. 2009;50(2):171–4.
3 Dousset V, Tourdias T, Brochet B, Boiziau C, Petry KG. How to trace stem cells for MRI evaluation? J Neurol Sci. 2008; 265(1–2):122–6. Epub 2007 Oct 25.
4 Pankhurst QA, Thanh NKT, Jones SK, Dobson J. Progress in applications of magnetic nanoparticles in biomedicine. J Phys D: Appl Phys. 2009; 42:224001.
5 Jordan A, Scholz R, Maier-Hauff K, Johanssen M, Wust P, Nadobny J, et al. Presentation of a new magnetic field therapy system for the treatment of human solid tumors with magnetic fluid hyperthermia. J Magn Magn Mater. 2001;225(1–2), 2001:118–26.
6 Le Renard PE, Jordan O, Faes A, Petri-Fink A, Hofmann H, Rüfenacht D, et al. The in vivo performance of magnetic particle-loaded injectable, in situ gelling, carriers for the delivery of local hyperthermia. Biomaterials. 2010;31(4):691–705. Epub 2009 Oct 29.
7 Pankhurst QA, Connolly J, Jones SK, Dobson J. Applications of magnetic nanoparticles in biomedicine. J Phys D: Appl Phys. 2003;36:R167–81.
8 Walling MA, Novak JA, Shepard JR. Quantum dots for live cell and in vivo imaging. Int J Mol Sci. 2009;10(2):441–91. Epub 2009 Feb 3.
9 Wyss C, Schaefer SC, Juillerat-Jeanneret L, Lagopoulos L, Lehr HA, Becker CD, et al. Molecular imaging by micro-CT: specific E-selectin imaging. Eur Radiol. 2009;19(10):2487–94.
10 Montet X, Pastor CM, Vallée JP, Becker CD, Geissbuhler A, Morel DR, et al. Improved visualization of vessels and hepatic tumors by micro-computed tomography (CT) using iodinated liposomes. Invest Radiol. 2007;42(9):652–8.
11 Gilmore JL, Yi X, Quan L, Kabanov AV. Novel Nanomaterials for Clinical Neuroscience. J Neuroimmune Pharmacol. 2008;3(2):38–94. Epub 2008 Jan.
12 LaConte L, Nitin N, Bao G. Magnetic nanoparticle probes. NanoToday. 2005; 8(5):32–8.
13 Son SJ, Bai X, Lee SB. Inorganic hollow nanoparticles and nanotubes in nanomedicine Part 1. Drug/gene delivery applications. Drug Discov Today. 2007;12(15-16):650–6.
14 Deerinck TJ. The application of fluorescent quantum dots to confocal, multiphoton, and electron microscopic imaging. Toxicol Pathol. 2008;36(1):112–6.
15 Fang C, Zhang MQ. Multifunctional magnetic nanoparticles for medical imaging applications. J Mater Chem. 2009;19:6258–66.
16 Duncan R, Spreafico F. Polymer conjugates. Pharmacokinetic considerations for design and development. Clin Pharmacokinet. 1994;27(4):290–306.
17 Boyd BJ. Past and future evolution in colloidal drug delivery systems”, Expert Opin Drug Deliv. 2008;5(1):69–85.
18 Duncan R. Designing polymer conjugates as lysosomotropic nanomedicines. Biochem Soc Trans. 2007;35(1):56–60.
19 Widder KJ, Senyel AE, Scarpelli GD. Magnetic microspheres: a model system of site specific drug delivery in vivo. Proc Soc Exp Biol Med. 1978:158;141–6.
20 Widder KJ, Senyel AE, Ranney DF. Magnetically responsive microspheres and other carriers for the biophysical targeting of antitumor agents. Adv Pharmacol Chemother. 1979;16: 213–71.
21 Gupta AS, Curtis G. Lactoferrin and ceruloplasmin derivatized superparamagnetic iron oxide nanoparticles for targeting cell surface receptors. Biomaterials. 2004;25(15):3029–40.
22 Babicˇ M, Horák D, Trchová M, Jendelová P, Glogarová K, Lesný P, et al. Poly(l-lysine)-Modified Iron Oxide Nanoparticles for Stem Cell Labeling. Bioconjug Chem. 2008;19(3):740–50. Epub 2008 Feb 21.
23 Euliss LE, Grancharov SG, O’Brien S, Deming TJ, Stucky GD, Murray CB, et al. Cooperative Assembly of Magnetic Nanoparticles and Block Copolypeptides in Aqueous Media. Nano Lett. 2003;3(11):1489–93.
24 Bowen CV, Zhang X, Saab G, et al. Application of the static dephasing regime theory to superparamagnetic iron-oxide loaded cells. Magn Reson Med. 2002;48:52–61.
25 Mornet S, Portier J, Duguet E. A method for synthesis and functionalisation of ultra small superparamagnetic covalent carriers based on maghemite and dextran. J Magn Magn Mater. 2005;293:127–34.
26 Roch A, Gossuin Y, Muller RN, et al. Superparamagnetic colloid suspensions: Water magnetic relaxation and clustering. J Magn Magn Mater. 2005;293:532–9.
27 Corot C, Robert P, Idée JM, Port M. Recent advances in iron oxide nanocrystal technology for medical imaging. Adv Drug Deliv Rev. 2006;58:1471–504.
28 Thorek DLJ, Cehn AK, Czupryna J, Tsourkas A. Superparamagnetic Iron Oxide Nanoparticle Probes for Molecular Imaging. Ann Biomed Engineering. 2006;34(1):23–38.
29 Neuberger T, Schöpf B, Hofmann H, Hofmann M, von Rechenberg B. Superparamagnetic nanoparticles for biomedical applications: Possibilities and limitations of a new drug delivery system. J Magn Magn Mater. 2005;1:483–96.
30 Mc Bain CS, Yiu HHP, Dobson J. Magnetic nanoparticles for gene and drug delivery. Intern J Nanomed. 2008;3(2):169–8031 Gupta AK, Gupta M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials. 2005;26(18):3995–4021.
32 Rosensweig RE. Heating magnetic fluid with alternating magnetic field. J Magn Magn Mater. 2002;252:370–4.
33 Montet-Abou K, Montet X, Weissleder R, Josephson L. Cell internalization of magnetic nanoparticles using transfection agents. Mol Imaging. 2007;6(1):1–9.
34 Montet-Abou K, Montet X, Weissleder R, Josephson L. Transfection agent induced nanoparticles cell laoding. Mol Imaging. 2005;4(3):165–71.
35 Stella B, Arpicco S, Peracchia MT, Desmaële D, Hoebeke J, Renoir M, et al. Design of folic acid-conjugated nanoparticles for drug targeting. J Pharm Sci. 2002;89:1452–64. Epub 2000 Sep 15.
36 Grüttner C, Teller J, Schütt W, Westphal F, Schümichen C, Paulke BR. Preparation and characterization of magnetic nanospheres for in vivo application. In: Häfeli U, Schütt W, Teller J, Zborowski M, editors. Scientific and clinical applications of magnetic carriers. New York: Plenum Press; 1997, p. 53–68.
37 Petri-Fink A, Chastellain M, Juillerat-Jeanneret L, Ferrari A, Hofmann H. Development of functionalized superparamagnetic iron oxide nanoparticles for interaction with human cancer cells. Biomaterials. 2005;26(15):2685–94.
38 Chastellain M, Petri-Fink A, Hofmann H. Particle size investigations of a multistep synthesis of PVA coated superparamagnetic nanoparticles. Journal of Colloid and Interface Science. 2004;278(2):353–60.
39 Alexiou C, Schmid RJ, Jurgons R, Kremer M, Wanner G, Bergemann C, et al. Targeting cancer cells: magnetic nanoparticles as drug carriers. Eur Biophys J. 2006;35(5):446–50.
40 Bruce IJ, Taylor J, Todd M, Davies MJ, Borioni E, Sangregorio C et al. Synthesis, characterisation and application of silica-magnetite nanocomposites. J Magn Magn Mater. 2004;284:145-60.
41 Mikhaylova M, Kim DK, Bobrysheva N, Osmolowsky M, Semenov V, Tsakalakos T, et al. Superparamagnetism of magnetite nanoparticles: dependence on surface modification. Langmuir. 2004;20(6):2472–7.
42 Levin M, Carlesso N, Tung CH, Tang XW, Cory D, Scadden DT, et al. Tat peptide derivatized magnetic nanoparticles allow in vivo tracking and recovery of progenitor cells. Nature Biotechnology. 2000;18:410–4.
43 Gupta AK. Recent advances on surface engineering of magnetic iron oxide nanoparticles and their biomedical applications. Nanomed. 2007;2(1):23–3.
44 Di Marco M. Physicochemical characterization of ultra small superparamagnetic iron oxide particles (USPIO) for biomedical application as MRI contrast agents. Int J Nanomedicine. 2007;2(4):609–22.
45 Kim YR, Yudina A, Figueiredo JL, Reichardt W, Hu-Lowe D, Petrovsky A, et al. Detection of early antiangiogenic effects in human colon adenocarcinoma xenografts: in vivo changes of tumour blood volume in response to experimental VEGFR tyrosine kinase inhibitor. Cancer Res. 2005; 65(20):9253–60.
46 Montet X, Lazeyras F, Howarth N, Mentha G, Rubbia-Brandt L, Becker CD, et al. Specificity of SPIO particles for characterization of liver hemangiomas using MRI. Abdom Imaging. 2004;29(1):60–70.
47 Sun C, Lee JSH, Zhang M. Magnetic Nanoparticles in MR Imaging and Drug Delivery. Adv Drug Deliv Rev. 2008;60(11):1252–65.
48 Wang YXJ, Hussain SM, Krestin GP. Superparamagnetic iron oxide contrast agents: Physicochemical characteristics and applications in MR imaging. Eur Radiol. 2001;11:2319–31.
49 Harisinghani MG, Barentsz J, Hahn PF, Deserno WM, Tabatabaei S, van de Kaa CH, et al. Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. N Engl J Med. 2003;348:2491–9.
50 Bonnemain B. Superparamagnetic agents in magnetic resonance imaging: Physicochemical characteristics and clinical applications – A review. J Drug Target. 1998;6:167–74.
51 Okuhata Y. Delivery of diagnostic agents for magnetic resonance imaging. Adv Drug Deliv Rev. 1999;37:121–37.
52 Ogushi M, Nagayama K, Wada A. Dextrane-magnetite: A new relaxation reagent and its application to T2 measurements in gel systems. J Magn Res. 1978;29;599–601.
53 Tanimoto A, Yuasa Y, Shinmoto H, Kurata T, Yamashita T, Okuda S, et al. Investigation of in vivo contrast mechanism of superparamagnetic iron oxide (SPIO) particles. Proc Intl Soc Mag Reson Med. 2001;9:2034.
54 Stoll G, and Bendszus M. Imaging of inflammation in the peripheral and central nervous system by magnet resonance imaging. Neuroscience. 2009;158:1151–60.
55 Beckmann N, Falk R, Zurbrügg S, Dawson J, Engelhardt P. Macrophage infiltration into the rat knee detected by MRI in a model of Antigen induced arthritis. Magn Reson Med. 2003;49:1047–55.
56 Weinmann HJ, Ebert W, Misselwitz B, Schmitt-Willich H. Tissue-specific MR contrast agents. Eur J Radiol. 2003;46:33–44.
57 Saebo KB. Degradation, metabolism and relaxation properties of iron oxide particles for magnetic resonance imaging [dissertation]. University of Uppsala; 2004.
58 Lange N, Becker CD, Montet X. Molecular imaging in a (pre-)clinical context. Acta Gastroenterol Belg. 2008;71(3):308–17.
59 Reiner CS, Lutz AM, Tschirch F, Fröhlich JM, Gaillard S, Marincek B, et al. USPIO-enhanced magnetic resonance imaging oft he knee in asymptotic volunteers. Eur Radiol. 2009;19:1715–22.
60 Hill JM, Dick AJ, Raman VK, Thompson RB, Yu ZX, Hinds KA, et al. Serial cardiac magnetic resonance imaging of injected mesenchymal stem cells. Circulation. 2003;108:1009–14. Epub 2003 August 11.
61 von zur Mühlen C, von Elverfeldt D, Bassler N, Neudorfer I, Steitz B, Petri-Fink A, et al. Superparamagnetic iron oxide binding and uptake as imaged by magnetic resonance is mediated by the integrin receptor Mac-1 (CD11b/CD18): Implications on imaging of atherosclerotic plaques. Atherosclerosis. 2007;193:102–11.
62 Steitz B, Hofmann H, Kamau SW, Hassa PO, Hottiger MO, von Rechenberg B, et al. Characterization of PEI-coated superparamagnetic iron oxide nanoparticles for transfection: Size distribution, colloidal properties and DNA interaction. J Magn Magn Mater. 2007;311:300–5.
63 Montet-Abou K, Daire JL, Hyacinthe JN, Jorge-Costa M, Grosdemange K, Mach F, et al. In vivo labeling of resting monocytes in the reticuloendothelial system with fluorescent iron oxide nanoparticles prior to injury reveals that they are mobilized to infarcted myocardium. Eur Heart J. 2010 Jun;31(11):1410–20.
64 Herschman HR. Molecular Imaging: Looking at problems, seeing solutions. Science. 2003;302:605–8.
65 Wickline SA, Lanza, GM. Nanotechnology for molecular Imaging and targeted therapy. Circulation. 2003;107:1092–5.
66 Wickline SA, Neubauer AM, Winter PM, Shelton DC, Lanza GM. Molecular Imaging and Therapy of Artherosclerosis with targeted nanoparticles. J Mag Res Imaging. 2007;25:667–80.
67 Montet X, Figueiredo JL, Alencar H, Ntziachristos V, Mahmood U, Weissleder R. Tomographic fluorescence imaging of tumour vascular volume in mice. Radiology. 2007;242(3):751–8.
68 Zhang Z, van den Bos EJ, Wielopolski PA, de Jong-Popijus M, Bernsen MR, Duncker DJ, Krestin GP. In vitro imaging of single living human umilical vein endothelial cells with a clinical 3.0-T MRI scanner. 2005;18(4):175–85.
69 Montet X, Weissleder R, Josephson L. Imaging pancreatic cancer with a peptide-nanoparticle conjugate targeted to normal pancreas. Bioconjug Chem. 2006;17(4):905–11.
70 Salaklang J, Steitz B, Finka A, O’Neil CP, Moniatte M, van der Vlies AJ, et al. Superparamagnetic Nanoparticles as a Powerful Systems Biology Characterization Tool in the Physiological Context. Angewandte Chemie International Edition. 2008;47(41):7857–60.


