
Box 1
Screening Definitions.
DOI: https://doi.org/10.4414/smw.2010.13061
"All screening programmes do harm; some do good as well, and of these, some do more good than harm at reasonable cost"JAM Gray in BMJ 2008 [1]
In March 2009, two large randomised trials reported conflicting results on the effect of testing for prostate specific antigen (PSA) in reducing deaths from prostate cancer in men aged over 50 [2, 3]. Two earlier randomised trials conducted in Canada and Sweden [4–6] had been inconclusive [7]. In Switzerland, Kwiatkowski, Huber and Recker commented [8] on the results of the European study [2]. They highlighted the 20% reduction in prostate cancer-specific mortality over a median follow-up time of nine years, and their claim that PSA testing should now become widespread elicited a wide range of responses [9–11]. In contrast, the American study [3], nested in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial [12], did not show a reduction in prostate cancer-specific mortality. Discussions to determine the reasons for the difference in results are ongoing [13, 14].
The case of PSA testing for early detection of prostate cancer provides a good example of how difficult it is to decide whether population-wide screening should be recommended or not [13–17]. The Swiss Society of Urologists initially endorsed PSA testing but later revised its position to state that the available evidence does not allow routine PSA testing in men to be recommended ("Aufgrund der vorliegenden Datenlage kann ein systematisches Testen der männlichen Bevölkerung mit dem PSA-Test nicht befürwortet werden.” ( http://www.urologie.ch/upload/Prostatafrueherkennung09.pdf ). However, the Society goes on to state that PSA testing could be performed in men aged 50–70 years with a life expectancy of at least ten years, after careful briefing of subjects on PSA testing. The role of specialist medical societies in making recommendations on a complex public health intervention for the general population should be discussed.
In this article we cite examples from cancer and genetic screening to explain the issues involved in assessing the evidence for and against screening. We focus on screening as a population-based intervention involving administration of the screening test together with follow-up examination and treatment, all of which has benefits, harms and costs. We argue that, in Switzerland, these factors, together with plans for implementation and ongoing monitoring, should be considered before deciding whether or not to start a new screening programme. We describe the processes established in a number of other countries where an independent committee conducts this assessment, and suggest that a similar body should be established in Switzerland.
Screening has been defined in many different ways (Box 1). Common to all definitions is that something is done to people who seem healthy. This sets screening apart from most healthcare interventions. When people actively present with a health problem that requires treatment, they accept that the diagnostic process or treatment carry some risk of inflicting harm. When the same pro-cesses are applied to seemingly healthy people, the acceptable level of risk is much lower.

Box 1
Screening Definitions.
What should a screening intervention in healthy people achieve? With regard to cancer, the people who profit from screening are those who a) would have died from the cancer but are cured, owing to earlier detection; b) would have been successfully treated for their cancer but whose quality of life is improved owing to earlier detection and less debilitating treatment; and c) do not have cancer and are reassured by the results of a screening test that correctly shows they do not have the disease. However, screening can also be harmful. The people who do not benefit, and might be harmed by screening, are those who a) die from a screen-detected cancer but whose clinical course was not improved by treatment; b) have cancer but would have survived even without screening; c) have a screening-detected cancer that would have not surfaced clinically during their lifetime, resulting in overdiagnosis and unnecessary treatment; d) have cancer but have a false negative screening test result; e) have a false positive result, which results in anxiety or unnecessary further investigation and treatment.
More than 40 years ago, Wilson and Jungner [18] proposed a framework for evaluating the appropriateness of screening to rationalise the increasing use of tests for early disease detection. Several countries have since introduced permanent bodies, independent of government, whose purpose is to assess new and existing screening technologies and to make recommendations to healthcare providers and funding authorities. Examples include the U.S. Preventive Services Task Force (USPSTF), the New Zealand National Health Committee and the United Kingdom National Screening Committee (UKNSC). The UKNSC has updated the Wilson and Jungner criteria to develop a 22-item list of criteria concerning the condition, the screening test, the treatment and the screening programme, all of which are to be met before introducing a screening programme (see http://www.screening.nhs.uk/criteria ). Here we highlight a range of issues that need to be evaluated.
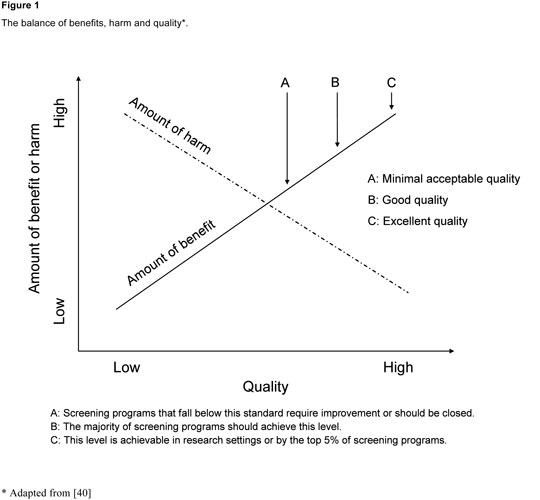
Figure 1
The balance of benefits, harm and quality (Adapted from [40]).
For cancer screening, the randomised controlled trial with mortality as the outcome is the only study design that allows unbiased comparison of outcomes in screened and unscreened groups [12, 19]. The UKNSC requires "evidence from high quality randomised controlled trials that the screening programme is effective in reducing mortality or morbidity”.
In observational studies comparing screened and unscreened people, those whose cancer was diagnosed through screening often appear to survive longer than those who presented with symptoms, even if there is no benefit from screening. This is due to "lead time bias”. In addition, tumours that are detected as a result of screening are more likely to be indolent, slow-growing or less aggressive than those that present with symptoms spontaneously or in the interval between two scheduled rounds of screening (interval cancers). This phenomenon is referred to as "length bias” [20–22].
Overdiagnosis is an extreme case of length bias: it refers to the detection of cancers by a screening test that would never have caused overt disease. These cancers result in unnecessary treatment and, at the very least, cause anxiety. Whether a screening-detected cancer is an overdiagnosis cannot be determined in the individual case. However, the extent of overdiagnosis in cancer can be shown from randomised trials where an elevated cancer incidence persists in the screened group in comparison with age-specific national cancer incidence data [23–26]. Furthermore, for several cancers many more cancers are found in autopsy studies than will ultimately matter. The most prominent case is prostate cancer, for which overdiagnosis is estimated at 50 to 70%, meaning that cancer diagnosis in the target group for screening will increase by a factor of 1.5 to 1.7 when PSA testing is introduced [2, 27, 28].
There is considerable debate about whether trials of cancer screening should use a reduction in total mortality or a reduction in cancer-specific mortality as the outcome [29–31]. In general, it depends largely on the proportion of deaths attributable to the disease. If this proportion is small, an impact on overall mortality is unlikely to be observed. For PSA testing, however, uncertainty remains as to whether prostate cancer-specific mortality is appropriate, as overdiagnosis leads to overtreatment and may lead to an increased mortality risk for other causes of death.
Evidence from randomised trials of a beneficial effect of screening on cancer-specific mortality is limited to mammography for breast cancer [32, 33] and faecal occult blood testing (FOBT) for colorectal cancer [34]. Data from randomised trials of the effectiveness of colonoscopy alone or in combination with FOBT are still awaited. Whilst screening for cervical cancer is well-established, there are no randomised trials to demonstrate its effectiveness. The observational evidence is, however, widely accepted as showing that regular cervical cytological screening lowers cervical cancer morbidity and mortality [35]. Technological advances in cervical cancer screening, following the discovery of human papillomavirus (HPV) as the causative agent, are now being evaluated in randomised trials. A cluster randomised trial in 52 villages in India suggests that testing for carcinogenic HPV types may improve the clinical utility and cost-effectiveness of cervical cancer screening by prolonging the screening interval in women with a negative test. A single round of HPV testing reduced the numbers of advanced cervical cancers and deaths from cervical cancer [36]. Additional randomised studies on the benefit of cervical cancer screening by HPV testing are currently ongoing in Canada, Finland, Italy, the Netherlands, Sweden and the UK [35].
Policymakers, practitioners and the public also need solid evidence on the extent of the harmful effects of screening. Only then is it possible to judge whether the benefits outweigh the harms. In the case of PSA testing we now have weak evidence for a beneficial effect with regard to prostate-specific mortality [2, 3]. However, the two trials make it clear that the harm from overdiagnosis is a major problem. A better picture of the spectrum of harmful effects (including psychological, physical and financial costs of false positive results) should emerge from further evaluations of these large randomised trials and additional research [14, 16]. The European Urological Association endorsed this view in April 2009 [37].
Resources spent on screening need to be compared with other population-based measures in primary and secondary prevention (opportunity cost) [38]. The UKNSC requires that the costs "should be economically balanced in relation to expenditure on medical care as a whole (i.e., value for money)”. Cost-effectiveness evaluations needed for rational decisions on the introduction of population-based screening are always conditional on the quality of evidence regarding the effectiveness and harms of the screening procedure and assumptions about the natural history of the disease. They need to be continuously revised in the light of medical and technical progress, such as for example increases in the cost of novel and targeted chemotherapies [39].
The best available evidence usually stems from well conducted studies in dedicated settings. But screening is a programme, a whole chain of activities, and not a test alone [19]. Hence it is not guaranteed that the same balance of benefits and harms will be achieved when scaling up screening to a whole country and to all healthcare providers. Nationwide implementation requires a system to train staff involved in the screening and follow-up activities, continuous evaluation and quality control [1, 19, 40, 41]. The ultimate goal of all these activities is to maximise benefit and minimise harm (fig. 1).Whether screening should be implemented as a systematic programme or in an opportunistic way is an important decision. In systematic programmes eligible individuals are invited for screening at regular agreed intervals. All those in the target population are included, screening coverage can be monitored and the quality of screening ensured. But such systems are difficult to implement in the absence of population registries or in highly mobile target populations. In opportunistic screening, healthcare providers offer screening when people attend health care settings for unrelated reasons. There are advantages to using existing infrastructure, but people who do not use health services or use them rarely will not have the opportunity to be screened regularly. Healthcare professionals may forget to offer the screening test regularly if consultation times are limited. It is also more difficult to monitor the coverage and quality of opportunistic screening, especially if the population is not well-defined. In the case of mammography screening for early detection of breast cancer [42], a whole set of indicators have been established which should be monitored regularly [43]. In Switzerland this type of monitoring and evaluation has, for example, been implemented in the systematic mammography screening programme in the canton of Vaud [44–46]. Obtaining some of the monitoring information was only possible because the canton of Vaud was already operating a population-based cancer registry.
Irrespective of whether screening is offered in a systematic or opportunistic manner, people need to be properly informed of the benefits and harms of the screening programme, from both the personal and population viewpoints, and the information must be offered in an understandable fashion [47–49]. It is thought to be challenging to achieve high participation rates in screening programmes while informing target groups in a balanced, transparent and comprehensible way [50]. Several studies have shown marked public overestimation of the benefits of screening for breast and prostate cancer, including in Switzerland [51–53]. Furthermore, it is important to understand the reasons why persons participate in screening programmes. A study in Norway surveyed women after an invitation for a first round of mammography screening [54]. Trust, gratitude and convenience were more important in the women’s decision to participate than information on benefits and harms. However, it is possible to improve the presentation of statistical information by using clear reference classes and natural frequencies [55, 56], so that persons or patients can take better-informed decisions.
The availability of genetic tests is expected to grow more rapidly than that of other screening tests [57–59]. The hope of health improvement through genomics ranges from susceptibility testing to prevention of chronic diseases through targeted chemoprevention or behavioural interventions, testing for susceptibility genes in early detection, and testing for gene variants or expression profiles for targeted treatment [57]. However, evaluation of genetic tests for public health practice remains poorly structured. Since the benefits and harms in genetic and non-genetic population screening do not differ in substantial ways, the same criteria established for non-genetic screening apply [58, 60, 61]. But additional ethical, legal, and social aspects and possible harms need to be considered [62]. For example, a positive test result for BRCA1 mutations and breast cancer risk in grandmother and daughter automatically implies the presence of a BRCA1 mutation in the mother, whether she agreed to testing or not [63].
In presymptomatic genetic screening additional safeguards against discrimination by insurance companies and employers, as well as against social stigmatisation, may become necessary. For example, a national targeted population carrier screening programme for severe and frequent genetic diseases in Israel has been implemented. It is targeted at Jewish and non-Jewish communities with a high degree of consanguinity and therefore prevalent genetic syndromes [64]. To avoid stigmatisation, genetic testing in these communities is offered to all couples in their reproductive period, irrespective of their family history. While genetic testing in this programme is not mandatory, the Jewish ultraorthodox community requires genetic screening before marriage and the test result is one of the factors considered in the decision-making process for prearranged marriages. Premarital testing for thalassaemia is mandatory in some Middle-Eastern countries such as Iran and Saudi Arabia, albeit with no implications for the provision of marriage certificates by the government. To lower the incidence of thalassaemia a law has been enacted in Iran allowing termination of pregnancies before and up to the 120th day of pregnancy in cases of severe foetal disease [64].
The example of cystic fibrosis (CF) exemplifies the challenges in implementing DNA tests in neonatal screening. According to data from randomised trials and observational studies, newborn screening for cystic fibrosis is associated with better growth and other nutritional indicators, lower morbidity, lower early mortality and improved lung function [65]. It has also been associated with economic benefits in some studies [66], improved quality of life in family members, and improved reproductive decision-making regarding additional children [67]. Potential harms of neonatal screening for cystic fibrosis include false-positive results leading to unnecessary follow-up tests and associated risk of acquisition of Pseudomonas aeruginosa infections in cystic fibrosis clinics, premature diagnosis of mild or atypical cases, identification of asymptomatic mutation carriers, and the risk of not recognising the presence of specific CFTR mutations [67]. The types of screening test and how to use them in cascade remains controversial and needs adaptation to the population-specific genotype distribution and health system. Over 1600 mutations have been identified in the CFTR gene. In many cases their penetrance remains unclear and positive tests for homozygous or compound heterozygous mutation status are of unclear predictive value. Accordingly, two-step testing models for CF newborn screening are generally applied. Given the large number of mutations in the CFTR gene, the first step is the analysis of immunoreactive trypsin (IRT) levels in dried blood spots. As IRT testing is associated with poor specificity and positive predictive values, a second test is essential, often a second IRT, a DNA test or a combination of all these. The implementation of CF newborn screening as well as the screening protocols adopted vary widely across Europe [68, 69].
Neonatal screening for alpha-1-antitrypsin deficiency (AATD) provides insight into the risks and benefits of genetic testing for late onset disorders [70]. AATD is an autosomal co-dominant genetic disorder. Various mutations of the SERPINA 1 gene can in part cause liver disease or emphysema, smokers being more prone to develop the latter [71]. Early screening and detection of severe AAT deficiency in the AATD-screening programme for newborns in Sweden was found to prevent uptake of smoking among AATD adolescents, but did not affect parents’ smoking behaviour [72]. In contrast, the α Coded Testing (ACT) study reported persistent smoking among AATD individuals after a positive test result [70]. As most AATD subjects have a normal childhood and adulthood, and often have normal life expectancy in the absence of inhaled irritants such as smoking [71], ethical considerations on screening of newborns for AATD nevertheless arise.
Many novel genes with common low-penetrance variants and small relative and population-attributable risks for chronic adult-onset disorders are currently being identified. Their net benefit for population screening remains largely unresolved. Hereditary haemochromatosis, a prevalent inherited condition chiefly caused by a single mutation in the HFE gene, was long viewed as the "poster child” for population genetic screening [59, 73]. Clinical symptoms in haemochromatosis (i.e., fatigue, arthritis, impotence, cirrhosis, diabetes, cardiomyopathy) are the result of iron overload and can be prevented efficiently and at low cost by venesection. Initially it was believed that most subjects homozygous for the HFE mutation would ultimately develop haemochromatosis. Results from longitudinal studies now suggest much lower penetrance of the mutations, especially among women due to their monthly blood loss. This probably changes the cost-benefit balance of population HFE screening. If considered at all, screening should be target to men only or to specific age groups.
Despite a large number of recent studies linking various genetic variants to cardiovascular disease or type 2 diabetes, it is difficult to derive a net benefit from this new information. Adding information about novel genetic variants associated with the risk of developing these diseases to establish risk scores failed to improve risk prediction beyond that of obesity, smoking, cholesterol levels and family history [74–77]. Independent studies and evaluations are currently underway to assess the clinical utility of these tests ( http://www.egappreviews.org/ ).
To promote the integration of validated genomic knowledge into medical and public health practice it is imperative to perform state of the art evidence synthesis [57]. Consensus guidelines to assess the credibility of genetic associations have been defined and use three criteria: a) amount of evidence, b) replication of associations, and c) protection of observed associations from bias [78]. RCTs to assess the clinical utility of genomic testing are very few in number, since assessing the effect of a genetic test on disease-specific morbidity and mortality may be related to ethical concerns. Randomised trials could, however, be beneficial in answering key questions of relevance to population screening [57], such as assessment of the differences in the effectiveness of lifestyle or therapeutic interventions between genetic subgroups. In assessing the clinical utility of a genetic test, non-medical benefits and harms are also relevant. Test information can be of individual use ("personal utility”) in the absence of effective medical intervention. Genetic testing to identify people at elevated risk of Alzheimer is not paralleled by effective interventions to prevent the disease. Yet in the Risk Evaluation and Education for Alzheimer disease study (REVEAL) some subjects testing positive found the information helpful in preparing themselves and their families for potential development of the disease at a later age [79].
Future decisions about the allocation of sparse health care resources will require structured economic assessments [63]. The economic burden will even increase if testing is left to the free market. Subjects opting to supply their DNA to companies offering direct-to-consumer genetic testing (i.e., 23andme ( https://www.23andme.com/ ) or Navigenics ( http://www.navigenics.com ) are likely to seek medical advice and, potentially, further testing and screening. For this and other reasons the expert committee on genetic testing in humans has published a warning against direct-to-consumer genetic testing which does not comply with legal standards in Switzerland ( http://www.bag.admin.ch/themen/medizin/00683/02724/04638/07332/index.html?lang=de ).
A central, multidisciplinary screening commission in Switzerland would help ensure that the introduction of population-based screening is evi-dence-based and safeguard against medical and non-medical harms. An expert panel for screening would provide essential skills in evaluating the existing evidence in a structured way, determining the structure of screening programmes and the provision of balanced information, and deciding on additional data needs before and after implementation of a screening programme.

Box 2
Characteristics of HTA.
A screening commission in Switzerland would have international and national roles. International multi-centre trials are often needed to generate the study sizes needed to obtain definitive evidence about the efficacy and effectiveness of screening. The complexity of screening-related issues might also necessitate collaboration with other international commissions. At the national level, data are also required for policy development and implementation including estimating prevalence of disease and risk factors (genetic and non-genetic), investigating cultural acceptance of screening and informed consent procedures, evaluating the psychosocial impact, availability of adequate health care services for screening and follow-up interventions and economic evaluation. A screening commission will therefore also have a central role in stimulating and directing research topics and infrastructure. National registration of diagnosis, large, internationally harmonised clinical and population cohorts and biobanks, as well as research on screening-related aspects of communication, behavioural and social aspects are fundamental to obtaining data for policy decisions in the area of population screening.
There is no specific advisory body on screening in Switzerland at present. Two federal commissions deal with some screening-related issues: The Federal Commission for Medical Services (Eidg. Leistungs- und Grundsatzkommission) advises the Federal Department of Home Affairs on the reimbursement of specific procedures and services, including screening tests, in the context of compulsory health insurance; and the Federal Commission on Genetic Tests will be confronted with various issues surrounding predictive genetic tests. Neither commission, however, evaluates the screening interventions themselves. Screening is not the only complex medical intervention for which comprehensive evaluation is needed to advise healthcare professionals, health policy decision makers and the public. There are at least three other specialised federal commissions in Switzerland giving advice to professionals and the authorities on the adoption of new interventions after in-depth evaluation: immunisation; AIDS-related questions; and tobacco, alcohol and drug use. These commissions have their own budgets for communication and the conduct of evaluations and assessments.
Health technology assessment (HTA) provides an established methodological framework for evaluation that is well suited to the assessment of a complex population-based intervention such as screening (Box 2: HTA definitions [80]). HTA summarises available information on clinical and cost effectiveness as well as on societal aspects of health technologies; the results are directed mainly to decision makers at the institutional, administrative and political levels in assisting decision-making on implementation, financing or reimbursement. The following example illustrates the application of HTA to screening. In 1998 the British Columbia, Canada Advisory Council on Women’s Health asked the British Columbia Office of Health Technology Assessment (BCOHTA) to: review current practice on triple marker screening (TMS) in pregnancy for early diagnosis of Down’s syndrome; assess the performance of the tests; and critically examine the broader social, ethical and economic implications of establishing a TMS programme in British Columbia. BCOHTA used a variety of methods to address the research questions. Quantitative methods included a systematic literature review, analysis of routinely collected data, and economic modelling. Qualitative methods included focus group interviews with parents and caregivers of children with Down’s syndrome or spina bifida, genetic counsellors, and primary care providers. The authors then formulated several policy options on how to offer and organise TMS in this Canadian province [81].
There is increasing pressure from politicians, healthcare providers and the public to make new screening tests available even in the absence of RCT evidence. Opportunities for screening are bound to increase in view of the increasing prevalence of degenerative diseases and due to technological advances in diagnostic and therapeutic procedures. There are good examples showing that effective screening may have a profound impact on the population’s health, e.g., in cardiovascular disease (screening for hypertension or for dyslipidaemia) or on congenital malformation (e.g., aneuploidias). However, providing a new screening test on its own does not automatically result in a health benefit for the population screened.
The examples of PSA, breast cancer and genetic screening show that, in Switzerland, there is a deficit in the structure of scientific advice to the population, healthcare providers and the authorities. It is therefore time for Switzerland to follow the example of other countries. The Swiss healthcare system needs a national screening commission that is not influenced by vested interests and is mandated to conduct HTA on specific screening-related questions, to give advice to the public, clinicians, and decision makers, to issue recommendations and to supervise the performance of the screening programmes that are introduced. Without such an explicit effort, there is a danger in Switzerland that some beneficial screening programmes will be neglected and other ineffective, inefficient and potentially harmful screening procedures introduced.
1 Gray JAM, Patnick J, Blanks RG. Maximising benefit and minimising harm of screening. BMJ. 2008;336:480–3.
2 Schroder FH, Hugosson J, Roobol MJ, et al. Screening and Prostate-Cancer Mortality in a Randomized European Study. N Engl J Med. 2009;360:1320–8.
3 Andriole GL, Grubb RL, III, Buys SS, et al. Mortality Results from a Randomized Prostate-Cancer Screening Trial. N Engl J Med. 2009;360:1310–9.
4 Labrie F, Candas B, Dupont A, et al. Screening decreases prostate cancer death: first analysis of the 1988 Quebec prospective randomized controlled trial. Prostate. 1999;38:83–91.
5 Labrie F, Candas B, Cusan L, et al. Screening decreases prostate cancer mortality: 11-year follow-up of the 1988 Quebec prospective randomized controlled trial. Prostate. 2004;59:311–8.
6 Sandblom G, Varenhorst E, Lofman O, et al. Clinical consequences of screening for prostate cancer: 15 years follow-up of a randomised controlled trial in Sweden. Eur Urol. 2004;46:717–23.
7 Ilic D, O’Connor D, Green S, et al. Screening for prostate cancer. Cochrane.Database.Syst.Rev 2006;3:CD004720.
8 Kwiatkowski M, Huber A, Recker F. PSA-Screening verringert Sterblichkeit um 20%. Schweiz Ärztezeitung. 2009;90:514.
9 Strebel U. Kommentar zum Artikel «PSA-Screening verringert Sterblichkeit um 20%». Schweiz Ärztezeitung. 2009;90:751.
10 Bumbacher H. Entgegnung zum Artikel «PSA-Screening verringert Sterblichkeit um 20%». Schweiz Ärztezeitung. 2009;90:752.
11 Paky A. Männer über 55: hopp, hopp zum PSA-Test… sonst sterbt ihr. Schweiz Ärztezeitung. 2009;90:753–4.
12 Prorok PC, Andriole GL, Bresalier RS, et al. Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Control Clin Trials. 2000;21:273S–309S.
13 Boyle P, Brawley OW. Prostate Cancer: Current Evidence Weighs Against Population Screening. CA: A Cancer Journal for Clinicians 2009;59:220–4.
14 Holmberg L. Prostate Cancer Screening: The Need For Problem-Solving that Puts Men’s Interests First. Eur Urol. 2009;56:34–7.
15 Barry MJ. Screening for Prostate Cancer – The Controversy That Refuses to Die. N Engl J Med. 2009;360:1351–4.
16 Stark JR, Mucci L, Rothman KJ, et al. Prostate cancer screening: the controversy continues. BMJ. 2009;339:784–6.
17 Brawley OW. Prostate Cancer Screening; Is This a Teachable Moment? J Natl Cancer Inst. 2009;101:1295–7.
18 Wilson JMG, Jungner AG. Principles and practice of screening for disease. World Health Organization. 34, 1-163. 1968. Geneva. Public Health Papers.
19 Raffle AE, Gray JAM. Screening: evidence and practice. New York: Oxford University Press, 2007.
20 ‚t Veer LJ, Esserman LJ, Linn S, et al. Evaluation of the effect of screening on the detection of good and poor prognosis breast cancers. ASCO Meeting Abstracts 2009;27:1525.
21 Ikeda DM, Andersson I, Wattsgard C, et al. Interval carcinomas in the Malmo Mammographic Screening Trial: radiographic appearance and prognostic considerations. AJR Am J Roentgenol. 1992;159:287–94.
22 Ciatto S, Visioli C, Paci E, et al. Breast density as a determinant of interval cancer at mammographic screening. Br J Cancer. 2004;90:393–6.
23 Esserman L, Shieh Y, Thompson I. Rethinking Screening for Breast Cancer and Prostate Cancer. JAMA. 2009;302:1685–92.
24 Zahl PH, Strand BH, Maehlen J. Incidence of breast cancer in Norway and Sweden during introduction of nationwide screening: prospective cohort study. BMJ. 2004;328:921–4.
25 Zahl PH, Maehlen J, Welch HG. The Natural History of Invasive Breast Cancers Detected by Screening Mammography. Arch Intern Med. 2008;168:2311–6.
26 Zahl PH, Jorgensen KJ, Maehlen J et al. Biases in estimates of overdetection due to mammography screening. Lancet Oncol. 2008;9:199–201.
27 Barry MJ, Mulley AJ Jr. Why Are a High Overdiagnosis Probability and a Long Lead Time for Prostate Cancer Screening So Important? J Natl Cancer Inst. 2009;101:362–3.
28 Etzioni R, Penson DF, Legler JM, et al. Overdiagnosis due to prostate-specific antigen screening: lessons from U.S. prostate cancer incidence trends. J Natl Cancer Inst. 2002;94:981–90.
29 Juffs HG, Tannock IF. Screening trials are even more difficult than we thought they were. J Natl Cancer Inst. 2002;94:156–7.
30 Black WC, Haggstrom DA, Welch HG. All-cause mortality in randomized trials of cancer screening. J Natl Cancer Inst. 2002;94:167–73.
31 Olsen O, Gotzsche PC. Cochrane review on screening for breast cancer with mammography. Lancet. 2001;358:1340–2.
32 Gotzsche PC, Nielsen M. Screening for breast cancer with mammography. Cochrane.Database.Syst.Rev 2009;CD001877.
33 Nelson HD, Tyne K, Naik A et al. Screening for Breast Cancer: An Update for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;151:727–37.
34 Hewitson P, Glasziou P, Watson E, et al. Cochrane systematic review of colorectal cancer screening using the fecal occult blood test (hemoccult): an update. Am J Gastroenterol. 2008;103:1541–9.
35 Koliopoulos G, Arbyn M, Martin-Hirsch P, et al. Diagnostic accuracy of human papillomavirus testing in primary cervical screening: a systematic review and meta-analysis of non-randomized studies. Gynecol Oncol. 2007;104:232–46.
36 Franceschi S, Cuzick J, Herrero R et al. EUROGIN 2008 roadmap on cervical cancer prevention. Int J Cancer. 2009;125:2246–55.
37 Abrahamsson PA, Artibani W, Chapple CR, et al. European Association of Urology position statement on screening for prostate cancer. Eur Urol. 2009;56:270–1.
38 Coffield AB, Maciosek MV, McGinnis JM, et al. Priorities among recommended clinical preventive services. Am J Prev Med. 2001;21:1–9.
39 Lansdorp-Vogelaar I, van Ballegooijen M, Zauber AG, et al. Effect of rising chemotherapy costs on the cost savings of colorectal cancer screening. JNCI Cancer Spectrum 2009;101:1412–22.
40 Gray JA. New concepts in screening. Br J Gen Pract. 2004;54:292–8.
41 Gray M. Problems of evidence-based patient choice in screening programmes. Z Arztl Fortbild Qualitatssich. 2006;100:494–9.
42 Shapiro S, Coleman EA, Broeders M, et al. Breast cancer screening programmes in 22 countries: current policies, administration and guidelines. International Breast Cancer Screening Network (IBSN) and the European Network of Pilot Projects for Breast Cancer Screening. Int J Epidemiol. 1998;27:735–42.
43 European Commission. European guidelines for quality assurance in mammography screening – Third edition. Perry, N, Broeders, M., de Wolf, C., Törnberg, S., and Schouten, J. 1-366. 2001. Brussels, Luxembourg, Office for Official Publications of the European Communities.
44 Bulliard JL, De Landtsheer JP, Levi F. Results from the Swiss mammography screening pilot programme. Eur J Cancer. 2003;39:1761–9.
45 Bulliard JL, De Landtsheer JP, Levi F. Reattendance in the Swiss mammography screening pilot programme. J Med Screen. 2004;11:59–64.
46 Bulliard JL, De Landtsheer JP, Levi F. Profile of women not attending in the Swiss Mammography Screening Pilot Programme. Breast. 2004;13:284–9.
47 Lipkus IM, Hollands JG. The visual communication of risk. J Natl Cancer Inst Monogr. 1999;149–63.
48 Thornton H, Edwards A, Baum M. Women need better information about routine mammography. BMJ. 2003;327:101–3.
49 Fletcher SW, Elmore JG. Clinical practice. Mammographic screening for breast cancer. N Engl J Med. 2003;348:1672–80.
50 Weller DP, Patnick J, McIntosh HM, et al. Uptake in cancer screening programmes. Lancet Oncol. 2009;10:693–9.
51 Slaytor EK, Ward JE. How risks of breast cancer and benefits of screening are communicated to women: analysis of 58 pamphlets. BMJ. 1998;317:263–4.
52 Chamot E, Perneger TV. Misconceptions about efficacy of mammography screening: a public health dilemma. J Epidemiol Community Health. 2001;55:799–803.
53 Domenighetti G, D’Avanzo B, Egger M, et al. Women’s perception of the benefits of mammography screening: Population based survey in four countries. Intl J Epidemiol. 2003;32:816–21.
54 Osterlie W, Solbjor M, Skolbekken JA, et al. Challenges of informed choice in organised screening. J Med Ethics. 2008;34:e5.
55 Gigerenzer G, Edwards A. Simple tools for understanding risks: from innumeracy to insight. BMJ. 2003;327:741–4.
56 Gigerenzer G, Gaissmaier W, Kurz-Milcke E, et al. Helping Doctors and Patients Make Sense of Health Statistics. Psychological Science In The Public Interest. 2008;8:53–96.
57 Khoury MJ, McBride CM, Schully SD, et al. The Scientific Foundation for personal genomics: recommendations from a National Institutes of Health-Centers for Disease Control and Prevention multidisciplinary workshop. Genet Med. 2009;11:559–67.
58 Burke W, Coughlin SS, Lee NC, et al. Application of population screening principles to genetic screening for adult-onset conditions. Genet Test. 2001;5:201–11.
59 Khoury MJ, McCabe LL, McCabe ER. Population screening in the age of genomic medicine. N Engl J Med. 2003;348:50–8.
60 Rogowski WH, Grosse SD, Khoury MJ. Challenges of translating genetic tests into clinical and public health practice. Nat Rev Genet. 2009;10:489–95.
61 Khoury MJ, Feero WG, Reyes M, et al. The genomic applications in practice and prevention network. Genet Med. 2009;11:488–94.
62 Khoury MJ, Berg A, Coates R, et al. The evidence dilemma in genomic medicine. Health Aff. (Millwood.) 2008;27:1600–11.
63 Rogowski WH, Grosse SD, Khoury MJ. Challenges of translating genetic tests into clinical and public health practice. Nat Rev Genet. 2009;10:489–95.
64 Zlotogora J, Carmi R, Lev B, et al. A targeted population carrier screening program for severe and frequent genetic diseases in Israel. Eur J Hum Genet. 2009;17:591–7.
65 Wilcken B. Cystic fibrosis: refining the approach to newborn screening. J Pediatr. 2009;155:605–6.
66 Sims EJ, Mugford M, Clark A, et al. Economic implications of newborn screening for cystic fibrosis: a cost of illness retrospective cohort study. Lancet. 2007;369:1187–95.
67 Balfour-Lynn IM. Newborn screening for cystic fibrosis: evidence for benefit. Arch Dis Child. 2008;93:7–10.
68 Castellani C, Southern KW, Brownlee K, et al. European best practice guidelines for cystic fibrosis neonatal screening. J Cyst Fibros. 2009;8:153–73.
69 Bodamer OA, Hoffmann GF, Lindner M. Expanded newborn screening in Europe 2007. J Inherit Metab Dis. 2007;30:439–44.
70 Hogarth DK, Rachelefsky G. Screening and familial testing of patients for alpha 1-antitrypsin deficiency. Chest. 2008;133:981–8.
71 Senn O, Russi EW, Imboden M, et al. alpha1-Antitrypsin deficiency and lung disease: risk modification by occupational and environmental inhalants. Eur Resp J. 2005;26:909–17.
72 Thelin T, Sveger T, McNeil TF. Primary prevention in a high-risk group: smoking habits in adolescents with homozygous alpha-1-antitrypsin deficiency (ATD). Acta Paediatr. 1996;85:1207–12.
73 Allen KJ. Population genetic screening for hereditary haemochromatosis: are we a step closer? Med J Aust. 2008;189:300–1.
74 Paynter NP, Chasman DI, Buring JE, et al. Cardiovascular Disease Risk Prediction With and Without Knowledge of Genetic Variation at Chromosome 9p21.3. Ann Intern Med. 2009;150:65–72.
75 Lango H, Palmer CN, Morris AD, et al. Assessing the combined impact of 18 common genetic variants of modest effect sizes on type 2 diabetes risk. Diabetes. 2008;57:3129–35.
76 van Hoek M, Dehghan A, Witteman JC, et al. Predicting type 2 diabetes based on polymorphisms from genome-wide association studies: a population-based study. Diabetes. 2008;57:3122–8.
77 Lyssenko V, Jonsson A, Almgren P, et al. Clinical risk factors, DNA variants, and the development of type 2 diabetes. N Engl J Med. 2008;359:2220–32.
78 Ioannidis JP, Boffetta P, Little J, et al. Assessment of cumulative evidence on genetic associations: interim guidelines. Int J Epidemiol. 2008;37:120–32.
79 Chao S, Roberts JS, Marteau TM, et al. Health behavior changes after genetic risk assessment for Alzheimer disease: The REVEAL Study. Alzheimer Dis Assoc Disord. 2008;22:94–7.
80 Jonsson E, Banta HD, Henshall C, et al. Summary report of the ECHTA/ECAHI project. European Collaboration for Health Technology Assessment/Assessment of Health Interventions. Int J Technol Assess Health Care. 2002;18:218–37.
81 Bassett K, Lee PM, Green CJ, et al. Triple-marker screening in British Columbia: current practice, future options. Fletcher, G. 2000. Vancouver, British Columbia., Office of Health Technology Assessment, Centre for Health Services and Policy Research, University of British Columbia (BCOHTA).
No funding; no competing interests.