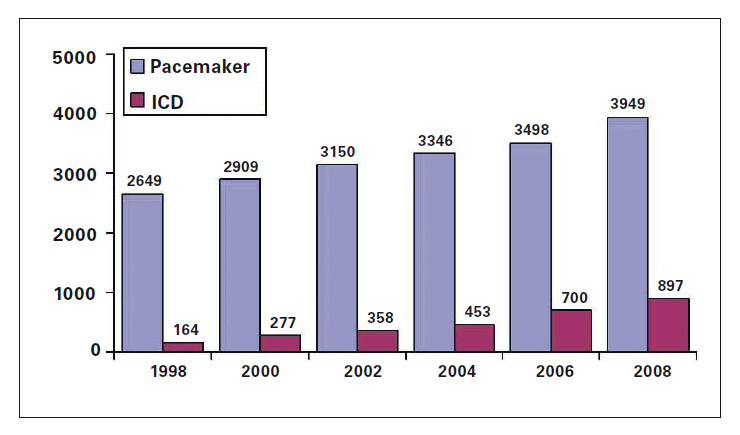
Figure 1
Yearly first pacemaker and ICD implants in Switzerland 1992–2008. From http://www.pacemaker.ch/de/statistik/
DOI: https://doi.org/10.4414/smw.2010.13052
| Abbreviations | |
| AF Atrial fibrillationAT Atrial tachycardiaAV AtrioventricularCARE-HF CArdiac Resynchronization-Heart Failure TrialCIED Cardiovascular implantable electronic devicesCOMPANION Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure TrialCRT Cardiac resynchronisation therapyCV CardiovascularDINAMIT Defibrillator IN Acute Myocardial Infarction TrialEHRA European Heart Rhythm AssociationHF Heart failureICD Implantable cardioverter/defibrillatorILR Insertable loop recorderIRIS Immediate Risk stratification Improves Survival Trial | LV Left ventricularLVEDV Left ventricular end-diastolic volumeLVESV Left ventricular end-systolic volumeMADIT-II Multicenter Automatic Defibrillator Implantation Trial – IIMI Myocardial infarctionNYHA New York Heart AssociationPROSPECT Predictors of Response to CRT TrialREVERSE REsynchronisation reVErses Remodeling in Systolic left vEntricular dysfunction TrialRV Right ventricularSCD Sudden cardiac deathSCD-HeFT Sudden Cardiac Death in Heart Failure TrialVF Ventricular fibrillationVT Ventricular tachycardia |
In the last 50 years, electronic cardiac medicine witnessed an impressive technological and miniaturisation development which resulted in an enormous positive impact on our healthcare. Since the development of the cardiac pacemaker in the early 1950s and then the introduction of the implantable cardioverter-defibrillator (ICD), the spectrum of implantable electronic devices has grown tremendously covering multiple applications in cardiology, neurology, endocrinology, urology and gastroenterology (table 1). Currently, cardiovascular implantable electronic devices (CIEDs) include implantable pacemakers, ICDs, cardiac resynchronisation therapy (CRT), implantable loop recorders (ILRs) and implantable haemodynamic monitoring (IHM). Along with the development of implantable electronic devices, advancement in communication technology has significantly expanded the possibility to exchange key physiological and device information between implanted devices, external home monitors and healthcare providers.
Collectively, implantable electronic devices have already saved and improved millions of lives and have provided more accurate and continuous diagnostic capability, while technologies for remote monitoring have enabled the clinical status of chronically ill patients to be assessed without the need for frequent office visits. There is little doubt that demographic trends will make electronic medicine even more important in the future. The growth of the worldwide population, the general ageing and increasing life expectancy of the population in western countries will lead to an increase in the prevalence of chronic diseases, such as heart failure (HF) and atrial fibrillation (AF), where CIEDs are commonly found. People will expect to remain mobile and maintain their quality of life into advanced age. Furthermore, healthcare systems of the future will only be affordable if we increase the use of home-based care and keep hospital stays to a minimum, not to mention the impact of home care on patients’ quality of life. All this will increase the demand for electronic medicine in the years to come. This review will briefly survey the current status and emerging applications of cardiac device therapies.
| Table 1Applications of electronic medicine. | ||||||
| Therapy | Cardiology | Neurology | Endocrinology | Gastroenterology | Urology | Respiratory |
| CRT | Heart failure | |||||
| ICD | Heart failureArrhythmias | |||||
| Pacemakers | BradycardiaConduction disturbances | |||||
| ILRs | Syncope | |||||
| Vagal nerve stimulation | Heart Failure | Treatment-resistant epilepsyTreatment-resistant depressionAlzheimer’s disease (investigational) | Diabetes (investigational) | |||
| Deep-brain stimulation | Parkinson’s Disease | |||||
| Spinal cord stimulation | Chronic pain | Post-operative ileus | ||||
| Peripheral nervous system stimulation | Gait disorders | |||||
| Sacral nerve stimulation | Faecal incontinence | Urinary incontinence | ||||
| Gastric contractility modulation | Obesity | Gastric contractility alterations | ||||
| Gastric electrical stimulation | Gastroparesis | |||||
| Tibial nerve stimulation | Urinary incontinence | |||||
| Phrenic nerve stimulation | Chronic respiratory insufficiency | |||||
The history of cardiac electronic medicine can be said to have started in earnest in 1958 when Arne Larsson, a Swedish patient suffering from complete heart block with severe Stokes-Adams attacks, received the first implantable pacemaker. By the time he died in 2001, from an unrelated malignancy, the patient had used 22 pulse generators and 5 electrode systems and had outlived both the implanting surgeon, Åke Senning of the Department of Thoracic Surgery, Karolinska Hospital, and the responsible engineer, Rune Elmqvist [1].

Figure 1
Yearly first pacemaker and ICD implants in Switzerland 1992–2008. From http://www.pacemaker.ch/de/statistik/
Pacemaker implants are slowly but constantly increasing each year in developed countries, in part because of the general ageing of the population. In Switzerland, 3949 first pacemaker implantations were recorded in 2008 (fig. 1) and >26 000 patients are living with a pacemaker [2]. The most common reason for first implantation of a pacemaker is an atrioventricular (AV) block accounting for about 40% of all pacemakers, followed by pacing for sinus node disease, mostly sick sinus syndrome (about 13%). Appropriate reasons for pacemaker therapy have been recently reported in guidelines published by the European Society of Cardiology (ESC) [3] and will not be further reviewed here.
Although pacemakers have come closer to commoditisation than any other therapy, developments continue, reflecting not only technological progress but also our increasing understanding of the effects of pacing on cardiac function. There are at least three very active research areas in pacemaker technology: minimisation of frequency of pacing, compatibility with magnetic resonance imaging (MRI) and device miniaturisation (see outlook).
Whereas initially pacemakers were seen as therapy to correct electrical conduction disorders, thus to treat bradycardia and syncope, today there is greater emphasis on the haemodynamic consequences induced by pacing. The improvements in left ventricular (LV) function that can be achieved in HF patients by pacing both ventricles with CRT will be discussed below; in contrast, pacing one ventricle in patients with preserved systolic function can induce ventricular dyssynchrony, which can lead to valvular regurgitation, left atrial enlargement and remodelling, as well as to ventricular remodelling and a predisposition to HF. The risk for arrhythmias from right ventricular (RV) apical pacing has been shown to be directly linked to the length of time being paced [4]. One solution would be to pace both ventricles, but this might create unnecessary risks and complications in patients. However, most patients with symptomatic bradycardia have functional conduction systems for periods of time and thus do not need continuous ventricular pacing. This insight has led to the development of different algorithms that closely monitor for AV conduction failures and changes the pacemaker programming according to the patient’s needs. Usually atrial-based pacing is provided about 90% of the time, but if AV conduction fails for two out of four depolarisation intervals, the device switches programming mode and paces both the atrium and the ventricle [5]. One of the best tested algorithms developed for this purpose is the Managed Ventricular Pacing (MVP), data from the SavePace trial, which followed 1065 patients for a mean of 1.7 ± 1 years and showed that MVP reduced the relative risk of developing persistent AF by 40% (p = 0.009) compared with conventional dual-chamber pacing. The absolute risk reduction was 4.8% and mortality rates were similar in the two groups over this relatively short follow-up time [6].
Lately, other issues have come to the forefront, such as the need for pacemakers to remain unaffected by the magnetic fields generated by MRI scanners. It has been estimated that in 50–75% of all pacemaker patients, an MRI examination is required at least once over the lifetime of their device [7]. Magnetic fields during MRI scans might lead to heating around the pacemaker lead tip, creating arrhythmias, and aberrations in pacemaker performances, such as pacemaker resets due to battery draining, asynchronic or inhibited pacing or over-stimulation [8–11]. In response to these concerns, manufacturers are developing pacemakers that can be used with MRI machines. Most are “MRI conditional”, that is safe in proximity to the MRI provided the conditions for safe operation are defined and observed, rather than “MRI safe” (completely non-magnetic and non-electrically conductive, eliminating all primary potential threats during an MRI procedure). Although the use of MRI with ICDs is generally still contraindicated, it has been shown to be feasible using a 1.5 Tesla MRI provided there is a close cooperation between radiologists and cardiologists, the appropriate device functions are disabled during the procedure and the patient is closely monitored [12]. At the time of writing, one device, the Medtronic EnRhythm MRI pacemaker, is approved with a CE mark by European authorities as safe for MRI use. American authorities have yet to approve MRI-safe device therapies.
Implantable cardioverters/defibrillators have gone from a niche application (implanted abdominally) to primary prevention therapy for sudden cardiac death (SCD) in less than two decades. First approved by the FDA in 1985 for patients who had survived two cardiac arrests, ICDs are now recommended to prevent SCD in HF patients NYHA class II/III with reduced LV function recognised to be at increased risk for SCD, and in patients surviving at least 40 days following a myocardial infarction (MI) [13, 14]. Most recommendations for ICD state that the patients should have a life expectancy of greater than one year, reflecting the implantation risks as well as the costs of the device and procedures. Development has greatly increased battery longevity, memory capability, telemetry function and speed along with significant reductions in the size of the devices from the volume of more than 200 ml to the current ICD which has a volume of less than 40 cc, which is close to the size of the past generation of pacemaker (fig. 2). According to the Swiss ICD registry, 913 first ICD implantations were recorded in 2008 and >4500 patients are living with an ICD but the reason for ICD requirement is unfortunately not reported. In one of the large neighbouring countries, Italy, the most common reason for ICD since 2006 is for primary prevention of SCD accounting, in 2007, for about 60% of all ICDs. Although there has been a stable (ranging between 11% and 18%) growth in the number of first ICD implantations in Switzerland since 2005, the number per million inhabitant is still far below those reported by Austria, Italy and Germany (table 2) indicating a substantial under-penetration of ICD therapy in Switzerland compared to many other European countries.

Figure 2
Reduction in the size of ICDs from 1989 to 2003. Source: Medtronic.
Apart from further reduction in the size of ICDs, areas of intense research span from optimal patient selection to improvement of quality of life by reducing unnecessary shocks. The quest to define patients most likely to benefit from an ICD is a major field of research interest. Although large-scale trials have shown impressive reductions in mortality in overall trial populations [15, 16], patients implanted less than 40 days following an MI do not benefit [17, 18] and it appears as if patients implanted more than six months after MI benefit more than patients implanted earlier [19]. This is not necessarily a reassuring finding, as the high mortality rates immediately after an infarction mean that potentially salvageable patients would be lost in this interval. Not all patients receiving an ICD will experience a life-threatening arrhythmia during the life-time of the device. There ought to be a “Goldilocks” group of patients at high risk for SCD and moderate risk of dying by other means, thus most likely to benefit from ICD therapy. This issue has been extensively reviewed recently in this journal and we will not further address it here [20]. It seems clear that patients post-MI with some, but not too many additional, risk factors benefit greatly from ICDs [21]. However, stratifications based on criteria such as T-wave alternans [22] have been unsuccessful. Recently, risk scores developed on the basis of drug trials such as the Seattle Heart Failure Model [23, 24] were applied to the SCD-HeFT cohort [25]. In the different risk groups, four-year mortality ranged from 12% to 50% and the proportion of SCD of all deaths ranged from 52% in the lowest-risk group to 24% in highest-risk patients. Projected over each patient’s predicted life span, ICD treatment added 6.3, 4.1, 3.0, 1.9, and 0.2 additional years of life in the lowest to highest risk groups, respectively. A different approach is taken in the DISCOVERY trial [26], which is currently enrolling 1300 ICD patients to be followed for an average of two years. All patients are screened for single nucleotide polymorphisms (SNPs) in the genes GNB3, GNAS and GNAQ which have been identified as markers of increased risk for ventricular arrhythmia <400 msec. The primary outcome measures are the positive predictive value of SNPs and the best combination of genetic parameters, baseline data and follow-up data as predictor of primary endpoints: all-cause mortality, cardiac death and atrial arrhythmia. DISCOVERY is scheduled to finish in 2013.
With a therapy, such as ICD, that does not provide symptomatic relief but acts only when an emergency condition manifests itself, there is less patient acceptance of unwanted effects. Hence, much effort is going into minimising the risk for inappropriate shocks (triggered by something other than life-threatening arrhythmias) and unnecessary shocks (terminating arrhythmias that could have been terminated by other means). Published rates of inappropriate and unnecessary shocks from large clinical trials are usually on the high side; SCD-HeFT did not allow the use of any shock-reducing technologies but implanted a simple “shock-box” ICD with default programming. Antitachycardia pacing (ATP) is now standard on most ICDs although the algorithms differ. In the PainFREE Rx II trial [27], ATP reduced the rates of inappropriate shocks in a broad ICD population of 634 patients. Fast ventricular tachycardias (FVTs) are responsible for 76% of all arrhythmias that trigger ICD shocks. In PainFREE Rx II, 73% of FVTs over twelve months were successfully terminated by ATP with no difference in mortality. The reduction was accompanied by a significant improvement in patients’ quality of life compared with patients whose devices had no ATP implemented. There are, however, multiple strategies for reducing the number of unnecessary shocks [28], many of which are currently being tested in a large prospective trial.
| Table 2Selected demographic information and number of device implantations in Switzerland and surrounding countries for the years 2006 and 2007, as recorded by the European Heart Rhythm Association White Book 2008. | |||||
| Switzerland | Austria | Italy | France | Germany | |
| Population (mil) | 7.5 | 8.2 | 58.8 | 63.7 | 82.4 |
| Population aged >65 yrs | 16% | 17% | 20% | 16% | 20% |
| Healthcare expenditure/GDP (2004) | 11.5% | 10.3 | 8.7% | 10.5% | 10.6% |
| PacemakerNo. CentersNo. Implants (2006)No. Implants (2007)Implants/mil (2006)Implants/mil (2007)% Change 2007/2006 | 69504350786726770.7% | 65730675358919183% | 40055000580009359865.5% | 54560049603259439470.4% | 10379690698800117611991.9% |
| Cardiac Resynchronisation TherapyNo. CentersNo. Implants (2006)No. Implants (2007)Implants/mil (2006)Implants/mil (2007)% Change 2007/2006 | 27317412425517% | 65563710698624% | 3006000697210211917% | 12144124912697712% | 132/200*69698196849918% |
| Implantable Cardioverter-DefibrillatorNo. CentersNo. Implants (2006)No. Implants (2007)Implants/mil (2006)Implants/mil (2007)% Change 2007/2006 | 25433531587122% | NA895107410913019% | 400134001540022826115% | NA4189452166718% | 360158741908419323120% |
| * indicates the number of centres implanting CRT-P/CRT-D. | |||||
The life-saving and life-improving benefits of CRT, in HF patients with HF and QRS ≥120 ms who remain symptomatic on optimised drug regimens, are recognised as a standard of care in today’s treatment guidelines [13]. The mortality and morbidity benefits from CRT were shown in several randomised controlled trials, but powerfully in two major trials – COMPANION [29] and CARE-HF [30]. A meta-analysis in 2006 of randomised controlled CRT trials in HF reported a reduction in all-cause mortality by 29% [31]. No major drug trial in HF has showed similar benefits since the aldosterone antagonists in the early 2000s [32]. CRT also improves functional status, LV dimensions and quality of life [33]. The effects can be dramatic and are often noticeable within weeks of an implant, although improvements continue over months [34]. The current guidelines limit recommendations for CRT to NYHA III/IV. The question whether CRT might be beneficial in patients with mild HF has been tentatively answered in the affirmative by two recent trials, REVERSE [35] and MADIT-CRT [36] in NYHA I/II patients. Although none of the trials showed significant mortality benefits, both reported remarkably consistent clinical benefits on the combined endpoint of death and hospitalisation in this patients group (fig. 3). The lack of mortality benefits may be due to a low overall mortality in these patients.
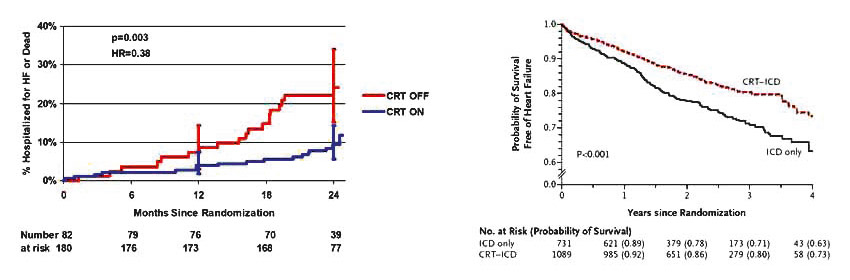
Figure 3
Reduction in all-cause death or HF hospitalisation in patients with mild HF in the REVERSE (left) and MADIT-CRT (right) trials, respectively. Reproduced with permission from references 35 and 36. Left: Reprint with permission from: Daubert C, Gold MR, Abraham WT, Ghio S, Hassager C, Goode G, et al. Prevention of disease progression by cardiac resynchronization therapy in patients with asymptomatic or mildly symptomatic left ventricular dysfunction: insights from the european cohort of the reverse (Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction) trial. J Am Coll Cardiol. 2009;54(20):1837–46. Right: Reprint with permission from: Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361(14):1329–38. Copyright © 2009 Massachusetts Medical Society. All rights reserved.
Major efforts have been made to identify the HF population which would respond best to CRT. Guideline recommendations are based on the inclusion characteristics of patients enrolled in the large-scale outcomes of major trials, but it is frequently reported that a subset of about 30% of patients do not respond to the therapy. In a progressive disease such as HF, the term “response” as defined in trials is not necessarily an appropriate measure of treatment effect, as an attenuation of disease progression may not constitute a measurable response in terms of improvement. Thus, lack of evidence of effect is not evidence of lack of effect. This argument notwithstanding, it appears clear that some patients groups do benefit more than others. It is very likely that multiple reasons account for less response to CRT including pacing site selection, severity of mitral regurgitation, and possibly biological factors. The extent of myocardial scar tissue may influence response [37] and there are indications that patients with narrow QRS respond less to CRT than those with greater QRS width [38]. Use of electrocardiographic and tissue-Doppler-based methods were shown in the PROSPECT study not to be a feasible approach to select CRT responders at today’s state of technology, due to limited reproducibility between different centres and a high percentage of responders also among patients who might have been denied therapy based on the dyssynchrony measures alone [39]. Abnormal QRS duration may not be the best indicator of eligibility for CRT but it is the best we have today. The ongoing Echo-CRT trial [40] in patients with narrow QRS and mechanical dyssynchrony, with a combined primary endpoint of all-cause death and HF hospitalisation will hopefully bring us more solid information.
Without knowing how best to maximise response to CRT in individual patients, the discussion of responder rates is somewhat futile. An important unknown is the best position of the respective ventricular leads. It is recognised that using the best pacing RV site together with the best LV pacing site may not result in the best biventricular pacing [41]. This is painstaking work and far from completion. A more revolutionary approach to optimise pacing is endocardial pacing of the left ventricle. Left ventricular endocardial sites are more centrally located than LV epicardial sites and conceptually, endocardial pacing should provide more homogeneous resynchronisation and better haemodynamic performance than today’s CRT techniques [42, 43]. Implanting an endocardial LV lead with a trans-septal approach carries risks [44] that have limited the interest in endocardial pacing, but the evolution of leadless pacing technologies (see below) may bring about a paradigm shift in this area.
Throughout Europe, the use of CRT-D is increasing with that of CRT-P becoming correspondingly less common [45]. The decision of whether to include a defibrillator in CRT therapy is currently left at the discretion of individual physicians; there is no decisive evidence available from the randomised trials and guidelines do not make specific recommendations. The only direct comparison, in COMPANION, was inconclusive and recent observational studies have reported both added survival benefit [46] and no such benefits [47]. On the related question of whether ICD patients might benefit from CRT-D, the results of REVERSE and MADIT-CRT seem reassuring to physicians who have opted for this therapy in ICD-indicated patients with wide QRS interval, and most likely updates in guideline recommendations will come soon.
Insertable (or implantable) loop recorders are electrocardiogram (ECG) devices implanted under local anaesthesia, usually in the left pectoral region, that provide continuous loop high-fidelity ECG recordings, typically storing 20 minutes of data in a moving time window. ILRs are activated in the case of syncope or an arrhythmia, either by the patient or automatically, and save the latest 20 minutes of readings for interrogation by the physician.
Currently, ILRs are used most frequently to diagnose arrhythmic causes of syncope. The aetiology of around 30% of syncopes remains unexplained after standard diagnostic tests and arrhythmic causes are likely in 20–40% of these patients [48]. The recently updated syncope guidelines from the ESC [49] recommend the use of ILRs early in the diagnostic examination, except for patients who are at high CV risk and need anti-arrhythmic therapies, ICD implantation or other treatment. The change in guideline recommendations reflects the insight that ILRs can document a significant arrhythmia at the time of pre-syncope which has been described as the gold standard criterion for syncope diagnosis [50]. ILRs are also increasingly used to monitor recurrences of AF or atrial tachycardia (AT) after ablation procedures. Even successful cardioversion by ablation is frequently followed by the recurrence of atrial arrhythmias and documented recurrence rates can be as high as 50% in some patients groups [51, 52]. Given the documented association between AT/AF and the risk of major adverse cardiovascular (CV) events, sensitive monitoring would improve management of patients post-ablation. However, recurrence of AT/AF episodes occurs in highly variable patterns which are difficult to monitor on a short-term basis [53] and long-term monitoring with ILRs has been shown to be more accurate than short-term standard ECG or 24 h Holter monitoring in measuring AF recurrence after ablation [54, 55]. The sensitivity of ILRs to detect AT/AF can be over 95% [53]. Patients with ILRs, particularly when diagnosed for syncope, frequently need brain imaging and MRI scans; a potential problem for ILRs. The most frequently used ILR, Reveal (Medtronic), is designated as MRI-conditional and has been tested with magnetic fields up to 3.0 Tesla, but ILRs approved as truly MRI-safe are not yet available.
The benefits of remote monitoring of patients with CIEDs have recently been extensively reviewed in these pages [56]. Remote monitoring has many advantages for patients, for the healthcare system, and ultimately for insurance companies and third-party payers. The patient via his/her device is the central point in a healthcare network represented by a general practitioner, a cardiologist, an electrophysiologist, a HF specialist, nurse practitioners etc.
The first and most immediate benefit to the patient is increased safety due to the ability to monitor the device and lead performance and to warn doctors and patients if a device or a lead is at risk of malfunctioning [57]. Manufacturers have responded to concerns by developing algorithms that provide lead integrity alerts and the safety aspect of remote monitoring was recently defined by the Heart Rhythm Society and the European Heart Rhythm Association (EHRA) as the primary goal of remote monitoring with CIEDs [58]. There is no doubt that remote monitoring with CIEDs has the additional benefit of potentially reducing the burden of clinic visits. The follow-up burden for physicians is growing concomitantly with the rapid growth in device implants and the need for patient management may become overwhelming unless efficient ways can be found to reduce the need for scheduled office visits. Remote monitoring can reduce the number of unscheduled office visits (usually the most costly follow-up visit) due to AF episodes, VT or change in haemodynamic status, notably increased pulmonary fluid levels that might lead to cardiac decompensation [59]. Clinical data are scarce at present, but there are reports that such early identification of patients at risk can lead to corrective actions that reduce hospitalisations [60]. For syncope patients, remote monitoring with ILRs would enable the definition of cardiac syncope at the time of an event and also the recurrence of AF after ablation, as described above.
Implementation of remote monitoring has, however, important implications for hospitals and private practices because it not only requires a closer collaboration between stakeholders, enhancement in data sharing and communication capability but also in the requirement to provide way more homecare rather than hospital care to the patient. The implementation of current remote monitoring technologies into the broader communication network is also expected in the near future. This novel trend is already signalised by a strategic partnership between device manufactures and large telecommunication companies. Multiple applications can be expected; one of the goals of this upcoming development is to have patients directly informed of abnormal heart and haemodynamic conditions requiring behavioural and medication changes, or alarming the patient to contact his/her family doctor or hospital for further diagnostic procedures.
Taking this further, data from remotely monitored CIEDs may revolutionise our CV risk models. Indeed, current risk assessment models are all built on static analyses in which the time between measurement of a qualifying parameter in relation to some other parameters or outcomes was maintained fixed. Perhaps the most common example in cardiology is the use of LVEF as an indicator of risk for SCD. A risk model closer to biological reality needs to take into account the dynamic process of disease where risk markers and their importance do not remain stable over time [61]. For the first time in cardiology, the information CIEDs provide is dynamic and parameters can be measured continually over time. A first stab at creating such a dynamic model, where data provided over time by devices for heart rate variability, mean heart rate, and patient’s physical activity were combined to create a risk score predictive of mortality, was reported recently [62]. Emerging technologies to measure RV pressure [63] would add to the power of such models.
The possibility to obtain device diagnostic variables through remote monitoring highlights the potential to use the wealth of data available to refine risk models continually and predict not only clinical deterioration but also acute events such as acute MI or SCD which today often strike without advance warning. Prevention and patient profiling would be the greatest benefit from remote monitoring in the near future.
Despite the documented success of CIEDs, device therapies remain substantially underused in Europe. As healthcare systems differ widely, it is difficult to obtain reliable comparable data on implantation rates across the continent. A recent survey by van Veldhuisen et al. [64] used sales data from the device industry between 2004 and 2008 as a proxy for implant rates. Such data are approximations and provide no information on medical history of patients, conditions treated, or what percentage of the sold devices were actually implanted for example. Nevertheless, a clear trend is visible towards increased use of CIEDs during the period surveyed. This trend is consistent with the slow adoption, over many years, of new therapies, which has been observed for other therapies following the publications of large-scale positive clinical trials [65]. Also notable are the wide and often unexplained disparities between different European countries in the use of device therapies [45]. This argues against some of the explanations proffered for the relatively low use of device therapies, such as cost. Device therapies are costly, with most expenses occurring initially. However, when calculated over the life-time of a device, costs are not dramatically different from many other therapies that reduce mortality and morbidity [66]. Some therapies, such as ILRs, even have the potential to save costs for diagnosis of syncope compared with diagnosis without ILRs [67]. If cost were the limiting factor, the richest European countries ought to show the highest rates of use of device therapies. Yet in Europe, Switzerland implants less than half as many ICDs yearly as Germany, and rates in Norway are still lower [45]. Sweden has the highest rates of CRT-P implants but rates of CRT-D vary much less between countries. Clearly, other factors influence implant rates, possibly differences in reimbursement systems, attitudes between countries, or differences in medical cultures and in the way new medical information is communicated between specialities. However according to a recent survey [68], in Switzerland, a country with universal healthcare insurance coverage and little incentive to develop new healthcare strategies, chronic disease management programmes are scarce. The authors of the survey called for appropriate evaluations of existing programmes and stressed the need to involve all healthcare stakeholders, as well as the need for strong leadership and, not least, of political will. In this sense, patients with CIEDs represent an ideal group of patients because the management of the disease affecting them (most commonly HF, AF, syncope) requires close collaboration between experts in a range of fields, from general practitioners, to cardiologists, electrophysiologists, neurologists and others as well [69].
Future developments in electronic medicine will be driven by several considerations: greater understanding of the treated diseases themselves, advances in technology and the application of therapies to new indications. As has already happened with pacemakers, knowing more about the biological effects of CRT will help refine the therapy. Improved mapping technologies are teaching us more about ventricular sequential activation in HF [70]. Together with developments in lead technology that allow for pacing at several different sites, this will help to improve responder rates [71]. Similarly, a better stratification of ICD patients is highly desirable in order to lower the number needed to treat. Dynamic models developed with the use of device diagnostics will surely help here [62]. Such developments, coupled with improvements in the technology to terminate arrhythmias, may transform ICDs from “shock boxes” to “antiarrhythmic therapies” with options to shock as a last resort. Improved technology and increased physician experience with the procedures will continually increase safety for patients.
Devices, in particular ILRs, can be expected to miniaturise further, towards a size where they will be inserted subcutaneously or even reach the size to be injectable. These improvements will improve patient safety and reduce implant times. Once this stage is reached, we can expect an explosion in use as ILRs are transformed into standard, rather than specialised diagnostic tools. Together with the development of dynamic risk models discussed above, subcutaneous diagnostic monitors would refine the selection of appropriate therapies for individual patients, saving a number of time-consuming and expensive steps in the process. An example is the application of ILRs to detect AF in patients with cryptogenic stroke. It is thought that undiagnosed arrhythmias can be the cause of as much as 20% of strokes of unknown origin [72]. Studies in these patients are underway to provide a greater understanding of the prevalence of AF, which if treated would reduce the risk of recurrent stroke. Such use of ILRs will require a successful collaboration between cardiologists and neurologists, another example of the need for greater interaction and expansion of the networking between medical specialties in the era of electronic medicine.
The need for closer collaboration between different medical disciplines in the future cannot be overstated. As noted initially, implantable electrical devices are expanding their therapeutic use into almost any conceivable therapeutic field and are finding applications in such diverse conditions as Parkinson’s Disease, chronic pain and urinary incontinence (table 1). With all newly implantable devices expected to carry remote monitoring capabilities, the possibility is tantalising to integrate all these data in the future into a single system and provide a holistic assessment of patients’ status that can be downloaded into electronic medical records.
Doing away with the need for a lead inserted in the heart would be a substantial improvement to cardiac device therapies. Among the benefits would be greatly reduced risks to patients from implantation procedures, lead replacements [73] and lead failure; greater access to CRT in patients with unfavourable coronary sinus anatomy; greater efficiency of LV stimulation by stimulating LV endocardium [74]; and the ability to pace children, in whom venous leads cannot be applied because of the risk of venous thrombosis and expected growth [75]. The most advanced current attempts use an ultrasound transmitter delivering energy from the chest wall to a receiver-electrode in contact with the myocardium that then converts the ultrasound energy into sufficient electrical energy to pace [76]. An alternative technology in development is based on electromagnetic induction to provide the energy, rather than ultrasound [77].
A small number of feasibility studies have been published [76–78]. The ability to pace, including multisite pacing, at a variety of sites in the heart has been demonstrated in animal studies. The first attempts in humans (only one of which had HF) were published in 2007 [78] and a very recent study in 10 HF patients (NYHA III) indicates that leadless LV pacing is indeed feasible. However, the study population did not have electrical dyssynchrony and thus were not representative of patients indicated for CRT. Furthermore, the patients presented with no abnormal thoracic or venous anatomies and we do not know how the technique would work in those patients who would need it the most.
Leadless pacing has enormous potential, if early results can be replicated and long-term studies show safety concerns to be overblown. None of today’s systems would be able to provide the energy required to defibrillate however. Although a lot of work has gone into the development of leadless subcutaneous ICDs, such devices would need higher defibrillation energies and correspondingly larger canisters than the traditional kinds. Moreover, they also lack pacing options which is particularly important not only for regular bradycadia pacing and/or CRT, but more importantly for antitachycardia pacing [79]. Although integration of leadless pacing and defibrillation technology is highly desirable, it is still one of the most challenging technological future developments in electronic cardiac medicine.
The therapeutic importance of reducing neurohormonal activation in HF was demonstrated, perhaps most dramatically, by the effects of β blockers. Elevated noradrenaline levels are a well known factor in the progress of HF and increased release of noradrenaline by myocardial ischaemia is linked to a greater risk of SCD [80]. Vagal activation can, to a certain extent, attenuate the cardiac effects of noradrenaline with antifibrillatory effects [81–83]. Conversely, reduced baroreflex sensitivity (BRS), a marker of vagal activity, is associated with increased risk for VF during myocardial ischaemia [84]. In HF patients, levels of BRS can be used to predict outcomes [85] and vagal withdrawal has been shown to precede acute decompensation [86].
Vagal stimulation therapy uses low current electrical pulses delivered by an implantable neuro-stimulator, which is similar in size to a defibrillator, to stimulate the vagus nerve. Experiments in animal models have been promising. The first widely reported study by Li et al in 2004 used rats with induced MI leading to HF. In this model, vagal stimulation over 140 days significantly improved LV haemodynamics and reduced mortality from 50% to 14% [87]. In dogs with pacing-induced HF, significant beneficial effects of vagal stimulation were seen on both LV end-diastolic volumes (LVEDV) and LV end-systolic volumes (LVESV) over eight weeks. These effects were accompanied by improvements in heart rate variability and baroreflex sensitivity [88]. The first experiences in humans, in 2008 [89], were carried out in eight HF patients, seven in NYHA class III and one in NYHA class II. The safety and tolerability were acceptable and at least the short-term results were promising: NYHA class improved in the seven NYHA III patients at 3 months and there were sustained improvements in quality of life throughout the 6-month follow-up. When two diabetic patients were excluded from the analysis, improvements in LVESV and LVEDV were significant at six months. These results are intriguing but need to be replicated in larger patient populations and ultimately in large-scale morbidity and mortality trials. If it passes these tests, vagal nerve stimulation may become the preferred electronic therapy on top of optimal pharmacological regimens in patients who do not have documented dyssynchrony and are thus not recommended for CRT.
If the 20th century was mostly dominated by important advancements in pharmacotherapy, the 21st century might bring a similar revolution from electronic medicine due to prolongation of life expectancy and the importance to maintain significant mobility even in aged populations, but also due to miniaturisation processes and increased connectivity of devices. However, such an outlook is quite possibly too simplistic. There is no real border between the two areas. We expect that the benefits from device therapies are due to their effects on patients’ biology and our understanding of what determines responses to device therapies is based on biology and genetics.
Innovation is more than bright ideas. It takes collaboration across a range of specialties: medical, technical, financial, managerial and much more. Successful collaboration between disciplines and between academics and business is the only way to make sure tomorrow’s generations will have access to therapies as revolutionary as current therapies have proved themselves to be in our lifetimes.
1 Rydén L, Schüller H, Larsson B. Biography of Arne Larsson. http://www.hrsonline.org/News/ep-history/notable-figures/arnelarsson.cfm
2 http://www.pacemaker.ch/de/statistik/
3 Vardas PE, Auricchio A, Blanc JJ, et al. for the Task Force for Cardiac Pacing and Cardiac Resynchronization Therapy. Guidelines for cardiac pacing and cardiac resynchronization therapy. Europace. 2007;10:959–98.
4 Sweeney MO, Hellkamp AS, Ellenbogen KA, et al., for Mode Selection Investigators. Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation. 2003;107:2932–7.
5 Gillis AM, Pürerfellner H, Israel CW, et al. Reducing Unnecessary Right Ventricular Pacing with the Managed Ventricular Pacing Mode in Patients with Sinus Node Disease and AV Block Pacing Clin Electrophysiol. 2006;29:697–705.
6 Sweeney MO, Bank AJ, Nsah E, et al. Minimizing ventricular pacing to reduce atrial fibrillation in sinus-node disease. N Engl J Med. 2007;357:1000–8.
7 Kalin R, Stanton MS. Current clinical issues for mri scanning of pacemaker and defibrillator patients. Pacing Clin Electrophysiol. 2005;28:326–8.
8 Duru F, Luechinger R, Scheidegger MB, Luscher TF, Boesiger P, Candinas R. Pacing in magnetic resonance imaging environment: Clinical and technical considerations on compatibility. Eur Heart J. 2001;22:113–24.
9 Levine GN, Gomes AS, Arai AE, et al. Safety of magnetic resonance imaging in patients with cardiovascular devices: An american heart association scientific statement from the committee on diagnostic and interventional cardiac catheterization, council on clinical cardiology, and the council on cardiovascular radiology and intervention: Endorsed by the American College of Cardiology Foundation, the North American Society for Cardiac Imaging, and the Society for Cardiovascular Magnetic Resonance. Circulation. 2007;116:2878–91.
10 Luechinger R, Zeijlemaker VA, Pedersen EM, Mortensen P, Falk E, Duru F, et al. In vivo heating of pacemaker leads during magnetic resonance imaging. Eur Heart J. 2005;26:376–83.
11 Roguin A, Schwitter J, Vahlhaus C, Lombardi M, Brugada J, Vardas P, et al. Magnetic resonance imaging in individuals with cardiovascularimplantable electronic devices. Europace. 2008;10:336–46.
12 Rougin A, Schwitter J, Vahlhaus C, Lombardi M, Brugada J, Vardas P, et al. Magnetic resonance imaging in individuals with cardiovascular implantable electronic devices. A Position Paper from European Heart Rhythm Association and Working Group on Cardiovascular Magnetic Resonance of the European Society of Cardiology (EuroCMR). Europace. 2008;10:336–46.
13 Dickstein K, Cohen-Solal A, Filippatos G, et al. Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008. Eur J Heart Fail. 2008;10:933–89.
14 Connolly SJ, Hallstrom AP, Cappato R, Schron EB, Kuck KH, Zipes DP, et al. on behalf of the investigators of the AVID, CASH and CIDS studies Meta-analysis of the implantable cardioverter defibrillator secondary prevention trials. Eur Heart J. 2000;21:2071–8.
15 Moss AJ, Zareba W, Hall WJ, et al., for the Multicenter Automatic Defibrillator Implantation Trial (MADIT) II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–83.
16 Bardy GH, Lee KL, Mark DB, et al. Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Investigators. Amiodarone or implantable cardioverter-defirbrillator for congestive heart failure. N Engl J Med. 2005;352:225–37.
17 Hohnloser SH, Kuck KH, Dorian P, Roberts RS, Hampton JR, Hatala R, et al.; DINAMIT Investigators. Prophylactic use of an implantable cardioverter-defibrillator after acute myocardial infarction. N Engl J Med. 2004;351:2481–8.
18 Steinbeck G, Andresen D, Seidl K, Brachmann J, Hoffmann E, Wojciechowski D, et al.; IRIS Investigators. Defibrillator implantation early after myocardial infarction. N Engl J Med. 2009;361:1427–36.
19 Goldenberg I, Moss AJ, McNitt S, Zareba W, Hall WJ, Andrews ML, et al., for the MADIT-II Investigators. Time dependence of defibrillator benefit after coronary revascularization in the Multicenter Automatic Defibrillator Implantation Trial (MADIT)-II. J Am Coll Cardiol. 2006;47:1811–7.
20 Schaer B, Kühne M, Koller MT, Sticherling C, Osswald S. Therapy with an implantable cardioverter defibrillator (ICD) in patients with coronary artery disease and dilated cardiomyopathy: benefits and disadvantages. Swiss Med Wkly. 2009;139:647–53.
21 Goldenberg I, Vyas AK, Hall WJ, et al., for the MADIT-II Investigators. Risk stratification for primary implantation of a cardioverter defibrillator in patients with ischemic left ventricular dysfunction. J Am Coll Cardiol. 2008;51:288–96.
22 Gold MR, Ip JH, Costantini O, Poole JE, McNulty S, Mark DB, et al. Role of microvolt T-Wave alternans in assessment of arrhythmia vulnerability among patients with heart failure and systolic dysfunction: primary results from the T-Wave Alternans Sudden Cardiac Death in Heart Failure Trial Substudy. Circulation. 2008;118:2022–8.
23 Levy WC, Mozaffarian D, Linker DT, Sutradhar SC, Anker SD, Cropp AB, Anand I, Maggioni A, Burton P, Sullivan MD, Pitt B, Poole-Wilson PA, Mann DL, Packer M. The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation. 2006;113:1424–33.
24 Mozaffarian D, Anker SD, Anand I, Linker DT, Sullivan MD, Cleland JG, et al. Prediction of mode of death in heart failure: the Seattle Heart Failure Model. Circulation. 2007;116:392–8.
25 Wayne C, Levy WC, Lee KL, Hellkamp AS, Poole JE, Mozaffarian D, et al. Maximizing Survival Benefit With Primary Prevention Implantable Cardioverter-Defibrillator Therapy in a Heart Failure Population. Circulation. 2009;120:835–42.
26 http://clinicaltrials.gov/ct2/show/NCT00478933
27 Wathen MS, DeGroot PJ, Sweeney MO. Prospective Randomized Multicenter Trial of Empirical Antitachycardia Pacing Versus Shocks for Spontaneous Rapid Ventricular Tachycardia in Patients With Implantable Cardioverter-Defibrillators. Circulation. 2004;110:2591–6.
28 Wilkoff BL, Williamson BD, Stern RS. Strategic Programming of Detection and Therapy Parameters in Implantable Cardioverter-Defibrillators Reduces Shocks in Primary Prevention Patients. J Am Coll Cardiol. 2008;52:541–50.
29 Bristow MR, Saxon LA, Boehmer J, et al.Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–50.
30 Cleland JGF, Daubert J-C, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L. The effect of cardiac resynchronization on morbidity and mortality in heart failure. New Engl J Med. 2005;352:1539–49.
31 Rivero-Ayerza M, Theuns DA, Garcia-Garcia HM, Boersma E, Simoons M, Jordaens LJ. Effects of cardiac resynchronisation therapy on overall mortality and mode of death: a meta-analysis of randomized controlled trials. Eur Heart J. 2006;27:2682–8.
32 Pitt B, Remme W, Zannad F, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators. N Engl J Med. 2003;348:1309–21.
33 Cleland JG, Calvert MJ, Verboven Y, Freemantle N. Effects of cardiac resynchronization therapy on long-term quality of life: an analysis from the CArdiac Resynchronisation-Heart Failure (CARE-HF) study. Am Heart J. 2009;157:457–66.
34 Ghio S, Freemantle N, Scelsi L, Serio A, Magrini G, Pasotti M, et al. Long-term left ventricular reverse remodelling with cardiac resynchronization therapy: results from the CARE-HF trial. Eur J Heart Fail. 2009;11:480–8.
35 Daubert C, Gold MR, Abraham WT, Ghio S, Hassager C, Goode G, et al. Prevention of disease progression by cardiac resynchronization therapy in patients with asymptomatic or mildly symptomatic left ventricular dysfunction: insights from the european cohort of the reverse (Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction) trial. J Am Coll Cardiol. 2009;54:1837–46.
36 Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361:1329–38.
37 Wikstrom G, Blomström-Lundqvist C, Andren B, et al. The effects of aetiology on outcome in patients treated with cardiac resynchronization therapy in the CARE-HF trial. Eur Heart J. 2009;30:782–8.
38 Beshai JF, Grimm RA, Sherif DO, Nagueh F, Baker II JH, Beau, Greenberg SM Pires LA, Tchou PJ, for the RethinQ Study Investigators. Cardiac-Resynchronization Therapy in Heart Failure with Narrow QRS Complexes. N Engl J Med. 2007;357:2461–71.
39 Chung ES, Leon AR, Tavazzi L, et al. Results of the Predictors of Response to CRT (PROSPECT) Trial. Circulation. 2008;117:2608–16.
40 http://clinicaltrials.gov/ct2/show/NCT00683696
41 Barold SS, Herwerg B. Pacing in heart failure: how many leads and where? Heart. 2008;94:10–2.
42 Rademakers LM, van Hunnik A, Lampert A, Kuiper M, Auricchio A, Echt DS, et al. Electrical and hemodynamic benefits of endocardial CRT with chronic infarction and LBBB. Heart Rhythm. 2009;6:S237.
43 van Deursen C, van Geldrop I, van Hunnik A, Auricchio A, Echt D, Prinzen FW. Improved myocardial repolarization and left ventricular systolic and diastolic function during endocardial cardiac resynchronization therapy. Heart Rhythm. 2008;5:S188.
44 van Gelder BM, Scheffer MG, Meijer A, Bracke FA. Transseptal endocardial left ventricular pacing: an alternative technique for coronary sinus lead placement in cardiac resynchronization therapy. Heart Rhythm. 2007;4:454–60.
45 Van Veldhuisen DJ, Charlesworth A, Crijns HJGM, Lie KI, Hampton JR. Differences in drug treatment of chronic heart failure between European countries. Eur Heart J. 1999;20:666–72.
46 Abraham WT, Young JB, Wheelan K, Johnson WB, Smith AL, Brinkman P, Chang Y. Comparison of sudden cardiac death in heart failure patients with cardiac resynchronization and defibrillator therapy (CRT-D) versus CRT alone (CRT-P). (Abstract). J Card Fail. 2008;14(Suppl. 1):S71.
47 Stabile G, Solimene F, Bertaglia E, La Rocca V, Accogli M, Scaccia A, et al. Long-term outcomes of CRT-PM versus CRT-D recipients. Pacing Clin Electrophysiol. 2009;32:S141–5.
48 Farwell DJ, Sulke AN. Does the use of a syncope diagnostic protocol improve the investigation and management of syncope? Heart. 2004;90:52–8.
49 The Task Force for the Diagnosis and Management of Syncope of the European Society of Cardiology (ESC). Guidelines for the diagnosis and management of syncope (version 2009). Eur Heart J. 2009;30:2631–71.
50 Brignole M, Vardas P, Hoffman E, et al. Indications for the use of diagnostic implantable and external ECG loop recorders. Europace. 2009;11:671–87.
51 Roy D, Talajic M, Dorian P, Connolly S, Eisenberg MJ, Green M, et al., Canadian Trial of Atrial Fibrillation Investigators Amiodarone to prevent recurrence of atrial fibrillation. N Engl J Med. 2000;342:913–20.
52 Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EB, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825–33.
53 Ziegler PD, Koehler JL, Mehra R. Comparison of continuous versus intermittent monitoring of atrial arrhythmias. Heart Rhythm. 2006;3(12):1445–52.
54 Senatore G, Stabile G, Bertaglia E, Donnici G, De Simone A, Zoppo F, et al. Role of transtelephonic electrocardiographic monitoring in detecting short-term arrhythmia recurrences after radiofrequency ablation in patients with atrial fibrillation. J Am Coll Cardiol. 2005;45:873–6.
55 Kottkamp H, Tanner H, Kobza R, Schirdewahn P, Dorszewski A, Gerds-Li JH, et al. Time courses and quantitative analysis of atrial fibrillation episode number and duration after circular plus linear left atrial lesions: trigger elimination or substrate modification: early or delayed cure? J Am Coll Cardiol. 2004;44:869–77.
56 Sticherling C, Kühne M, Schaer B, Altmann D, Osswald S. Remote monitoring of cardiovascular implantable electronic devices. Prerequisite or luxury? Swiss Med Wkly. 2009;139:596–601.
57 Swerdlow CD, Gunderson BD, Ousdigian KT, et al. Downloadable algorithm to reduce inappropriate shocks caused by fractures of implantable cardioverter-defibrillator leads, Circulation. 2008;118:2122–9.
58 Wilkoff BL, Auricchio A, Brugada J, Cowie M, Ellenbogen KA, Gillis AM, et al. HRS/EHRA expert consensus on the monitoring of cardiovascular implantable electronic devices (CIEDs): description of techniques, indications, personnel, frequency and ethical considerations. Heart Rhythm. 2008;5:907–25.
59 Yu CM, Wang L, Chau E, Chan RH, Kong S, Tang M, et al. Intrathoracic impedance monitoring in patients with heart failure: correlation with fluid status and feasibility of early warning preceding hospitalization. Circulation. 2005;112:841e8.
60 Catanzariti D, Lunati M, Landolina M, Zanotto G, Lonardi G, Iacopino S, et al. Monitoring intrathoracic impedance with an implantable defibrillator reduces hospitalizations in patients with heart failure. Pacing Clin Electrophysiol. 2009;32:363–70.
61 Auricchio A. Is it time to start with device-based prognosticators? Europace. 2009;12:7–8.
62 Singh JP, Rosenthal LS, Hranitzky PM, Berg KC, Mullin CM, Thackeray L, Kaplan A. Device diagnostics and long-term clinical outcome in patients receiving cardiac resynchronization therapy. Europace. 2009;11:1647–53.
63 Husain S, Pamboukian SV, Tallaj JA, McGiffin DC, Bourge RC. Invasive monitoring in patients with heart failure. Curr Cardiol Rep. 2009;11:159–66.
64 van Veldhuisen DJ, Maass AH, Priori SG, Stolt P, van Gelder IC, Dickstein K, Swedberg K. Implementation of device therapy (cardiac resynchronization therapy and implantable cardioverter defibrillator) for patients with heart failure in Europe: changes from 2004 to 2008. Eur J Heart Fail. 2009;11:1143–51.
65 Komajda M, Follath F, Swedberg K, Cleland J, Aguilar JC, Cohen-Solal A, et al. The EuroHeart Failure Survey Programme – a survey on the quality of care among patients with heart failure in Europe. Part 2: treatment. Eur Heart J. 2003;24:464–74.
66 Cowie MR, Marshall D, Drummond M, Ferko N, Maschio M, Ekman M, et al. Lifetime cost effectiveness of prophylactic implantation of a cardioverter defibrillator in patients with reduced left ventricular systolic function: results of Markov modelling in a European population. Europace. 2009;11:716–26.
67 Farwell DJ, Freemantle N, Sulke AN. Use of implantable loop recorders in the diagnosis and management of syncope. Eur Heart J. 2004;25:1257–63.
68 Peytremann-Bridevaux I, Burnand B. Inventory and perspectives of chronic disease management programs in Switzerland: an exploratory survey. Int J Integr Care. 2009;9:e93
69 Swedberg K, Cleland J, Cowie MR, Nieminen M, Priori SG, Tavazzi L, et al. Successful treatment of heart failure with devices requires collaboration. Eur J Heart Fail. 2008;10:1229–35.
70 Regoli F, Auricchio A. The role of invasive mapping in the electrophysiology laboratory. Europace. 2009;11:v40-5.
71 Leclercq C, Gadler F, Kranig W, Ellery S, Gras D, Lazarus A, et al. A randomized comparison of triple-site versus dual-site ventricular stimulation in patients with congestive heart failure. J Am Coll Cardiol. 2008;51:1455–62.
72 Jørgensen HS, Nakayama H, Reith J, et al. Acute stroke with atrial brillation. The Copenhagen stroke study. Stroke. 1996;27:1765–9.
73 Verma A, Wilkoff BL. Intravascular pacemaker and defibrillator lead extraction: a state-of-the-art review. Heart Rhythm. 2004;1:739–45.
74 Peschar M, de Swart H, Michels KJ, Reneman RS, Prinzen FW. Left ventricular septal and apex pacing for optimal pump function in canine hearts. J Am Coll Cardiol. 2003;41:1218–26.
75 Cohen MI, Bush DM, Vetter VL, Tanel RE, Wieand TS, Gaynor JW, Rhodes LA. Permanent epicardial pacing in pediatric patients: Seventeen years of experience and 1200 outpatient visits. Circulation. 2001;103:2585–90.
76 Echt DS, Cowan MW, Riley RE, Brisken AF. Feasibility and safety of a novel technology for pacing without leads. Heart Rhythm. 2006;3:1202–6.
77 Wieneke H, Konorza T, Erbel R, Kisker E. Leadless pacing of the heart using induction technology: a feasibility study. Pacing Clin Electrophysiol. 2009;32:177–83.
78 Lee KL, Lau CP, Tse HF, Echt DS, Heaven D, Smith W, Hood M. First human demonstration of cardiac stimulation with transcutaneous ultrasound energy delivery: Implications for wireless pacing with implantable devices. J Am Coll Cardiol. 2007;50:877–83.
79 Santini M, Cappato R, Andresen D et al. Current state of knowledge and experts’ perspective on the subcutaneous implantable cardioverter-defibrillator. J Interv Card Electrophysiol. 2009;25(1):83–8.
80 Malliani A, Schwartz PJ, Zanchetti A. A sympathetic reflex elicited by experimental coronary occlusion. Am J Physiol. 1969;217:703–9.
81 Schwartz PJ, Brown AM, Malliani A, Zanchetti A, editors. Neural Mechanisms in Cardiac Arrhythmias. New York: Raven Press, 1978:442.
82 Schwartz PJ, Priori SG. Sympathetic nervous system and cardiac arrhythmias. In: Zipes DP, Jalife J, editors. Cardiac Electrophysiology: From Cell to Bedside. Third Edition. Philadelphia: WB Saunders, 2000:330–43.
83 Schwartz PJ, Zipes DP. Autonomic modulation of cardiac arrhythmias. In: Zipes DP, Jalife J, editors. Cardiac Electrophysiology: From Cell to Bedside. Third Edition. Philadelphia: WB Saunders, 2000:300–14.
84 Schwartz PJ, Vanoli E, Stramba-Badiale M, De Ferrari GM, Billman GE, Foreman RD. Autonomic mechanisms and sudden death. New insights from analysis of baroreceptor reflexes in conscious dogs with and without a myocardial infarction. Circulation. 1988;78:969–79.
85 La Rovere MT, Pinna GD, Maestri R, et al. Prognostic implications of baroreflex sensitivity in heart failure patients in the beta-blocking era. J Am Coll Cardiol. 2009;53:193–9.
86 Adamson PB, Smith AL, AbrahamWT, et al. InSync IIIModel 8042 and Attain OTW Lead Model 4193 clinical trial investigators. Continuous autonomic assessment in patients with symptomatic heart failure: prognostic value of heart rate variability measured by an implanted cardiac resynchronization device. Circulation. 2004;110:2389–94.
87 Li M, Zheng C, Sato T, Kawada T, Sugimachi M, Sunagawa K. Vagal nerve stimulation markedly improves long-term survival after chronic heart failure in rats. Circulation. 2004;109:120–4.
88 Zhang Y, Popovic ZB, Bibevski S, et al. Chronic vagus nerve stimulation improves autonomic control and attenuates systemic inflammation and heart failure progression in a canine high rate pacing model. Circ Heart Fail 2009;2:642–9.
89 Schwartz PJ, De Ferrari GM, Sanzo A, et al. Long term vagal stimulation in patients with advanced heart failure. First experience in man. Eur J Heart Fail. 2008;10:884–91.
Professor Auricchio is consultant to: Sorin, Medtronic, Biotronik, EBR, Merck; institutional research agreement with Sorin, Medtronic, Boston Scientific, Biotronik; speaker fee: Sorin, Medtronic, Biotronik.