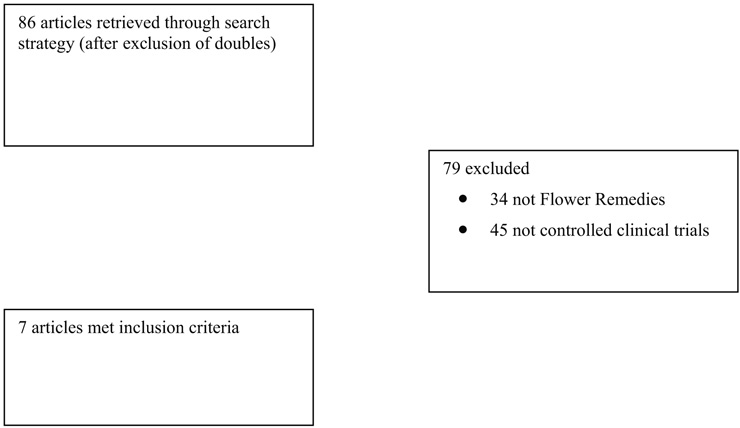
Figure 1
Flow chart of publications.
DOI: https://doi.org/10.4414/smw.2010.13079
“Bach Flower Remedies” (also sometimes called “Flower Essences” or “Flower Remedies”) were invented about 80 years ago by the British physician and microbiologist Dr Edward Bach (1886–1936). Dr Bach became convinced that most human illnesses are caused by negative states of mind (e.g., fear, jealousy, despair). He identified 38 remedies – each based on one native flower which, according to his conviction, would alleviate such negative feelings and thus restore health [1]. Further flower remedies have since been added by Bach’s followers. Dr Bach claimed to treat the whole person in an individualised fashion; two patients afflicted by the same mainstream diagnosis might therefore be treated with two different remedies.
Flower remedies are produced by dropping fresh flowers into water; this yields the “mother tincture” to which brandy is subsequently added as a preservative. Thus they do not contain pharmacologically relevant amounts of constituents of the flowers they originate from. Flower remedies thus have similarities to homeopathic medicines, yet there are clear distinctions between the two systems [2]. According to proponents of flower remedies, their mode of action does not depend on molecular or pharmacological mechanisms but on the subtle “energy” that is transmitted from the flowers to this remedy [3]. This “energy” has so far defied quantification, and critics therefore argue that flower remedies are pure placebos [4].
Flower remedies have become a thriving business. They are readily available from a wide range of outlets and many consumers strongly believe in their effectiveness. A previously published systematic review of flower remedies by the current author [5] is now outdated. A more recent review was focussed specifically on pain and psychological problems and included only four controlled trials [6]. Another review also only included four randomised clinical trials (RCTs) [7]. It is therefore timely and relevant to conduct an update.
Systematic searches were carried out on the Medline, Embase, Biosis, Cochrane Library and AMED databases. The search terms used were Bach Flower Remedies, Flower Remedies, Flower Essence and Rescue Remedy. Each database was searched from its inception until January 2002. For the current update, these searches were repeated in May 2010. Manufacturers of such preparations and experts in the field were asked for published or unpublished trials and the bibliographies of all papers were searched for further studies. Finally, several specialised journals and the departmental files were searched by hand for further relevant articles. No language restrictions were applied.
Contrary to previous reviews [5, 6], this update was restricted to RCTs. Non-randomised trials, uncontrolled studies, case reports and case series were excluded [8–13]. RCTs with human subjects were considered regardless of the disease or illness they related to and regardless of the outcome measures or the type of control intervention employed. Studies which were not aimed at testing efficacy but at other issues (e.g., expectancy) were excluded [14, 15].
Data were validated and extracted by the author according to predefined criteria (table 1). When information was insufficient, the authors of the study in question were approached to obtain more details. Methodological quality was assessed using the Jadad scale [16], which quantifies the likelihood of bias inherent in trials on the basis of their description of randomisation, blinding, and withdrawals; this score ranges from a minimum of 0 to a maximum of 5 points. The validity of the primary trials was estimated on a score ranging from 0 to 3 points (one point was given for a positive answer for each of the three questions: Was the study sample relevant? Was the intervention appropriate? Was the outcome measure suitable?). Due to the statistical and clinical heterogeneity of the primary data, no statistical pooling was performed.
| Table 1Randomised clinical trials of Flower Remedies (Flower Remedies). | ||||||||
| Reference | Study design | Jadad score | Validity score | Sample | Interventions | Main outcome measures | Main result | Comment |
| von Rühle(1995)[17] | RCT, 3 parallel groups(not placebo controlled, not double-blinded) | 2 | 3 | 24 pregnant women with overdue births | Individualised flower remedies daily up to date of birth,attention control group (no flower remedies),no such therapies,(all groups had standard care in addition) | Time to birth,type of birth,use of medication during birth,anxiety during birth,well-being | Significantly less medication was used in group A (p = 0.032) | Birth was delayed in group A by 5.1 days, in groups B by 6.6 and in group C by 4.4 days |
| Armstrong(1999)[18] | RCT, double-blind, 2 parallel arms | 5 | 3 | 100 healthy University students sitting exams | ‘Rescue Remedy’ (1–4 doses during 7 days before and during exams)placebo (same treatment schedule) | Anxiety measured with Spielberger State-Trait-Anxiety Inventory(SSTAI) | No significant differences between groups | Study suffered from high drop-out rate |
| Walach(2001)[19] | RCT, double-blind, cross-over | 5 | 3 | 51 healthy students sitting exams | Rescue Remedy (4 drops daily for 2 weeks or more if necessary)placebo (same treatment schedule) | Anxiety measured with Text-Anxiety Inventory | No significant differences between groups | Primary authors conclude that flower remedies are “an effective placebo” |
| Pintov(2005)[20] | RCT, double-blind, 2 parallel arms | 4 | 3 | 40 school children with ADHD | A) Rescue Remedy (4 drops 4x per day for 3 months)B) placebo (same treatment schedule) | Performance evaluated by teacher (Conner’s questionnaire) | No significant differences between groups | 17 drop outs |
| Toyota(2006)[21] | RCT, double-blind, 2 parallel groups | 3 | 3 | 40 surgical patients | A) Rescue Remedy in drinking waterB) drinking water without Rescue Remedy | Anxiety and tension (VAS) | No significant differences between groups | Write-up is unclear in several aspects |
| Halberstein(2007)[22] | RCT, double-blind, 2 parallel groups | 2 | 3 | 111 students under stressful exam situa- tion | A) Rescue Remedy (5 doses during a 3 hour class)B) placebo | Anxiety measured with SSTAI | No significant differences between groups | A subanalysis of high anxiety students favoured A over B |
| Forshaw(2009)[23] | RCT, single-blind, 3 parallel groups | 4 | 3 | 62 students under experimental stress | Rescue Remedy (4 drops in water)Placebo (pure water + participants were told that it contained Rescue Remedy)Placebo (pure water + participants were told that it was water) | Stress (VAS) | No significant differences between groups | All groups reported stress reduction regardless of treatment |
ADHD = attention deficit hyperactivity disorder
VAS = visual analogue scale
Seven RCTs met the inclusion criteria [17–23]. Three of them [17–19] had already been available for the previous systematic review [5] and four had been included in the reviews by Thaler et al. [6] or Halberstein et al. [7]. Figure 1 provides a flow chart of the articles located and included. Key data from the included RCTs are summarised in table 1. The methodological quality of the studies was variable but two scored the maximum of five points on the Jadad scale [18, 19]. All but one RCT [17] were placebo-controlled. The validity score was three in all cases. All but one RCT [17], the one that lacked a placebo group, failed to show significant differences between verum and control groups.

Figure 1
Flow chart of publications.
The trial by von Rühle [17] was described by the author as a pilot study. It lacked a placebo group and had a small sample size. Thus its results are not reliable.
The trial by the current author [18] was a randomised, placebo-controlled, double-blind study with a formal sample size calculation. Unfortunately, the drop-out rate turned out to be high. Its results showed no significant effect for flower remedies.
The study by Walach et al. [19] was also rigorously designed with several features similar to our own trial [18]. The main difference was that it had a cross-over design. Its results were almost identical to those of the author’s study [18].
Pintov et al. randomised 40 children with attention deficit hyperactivity disorder (ADHD) to receive either Rescue Remedy (4 drops 4 times per day) or placebo for 3 months [20]. The teacher, who was blinded to treatment allocation, evaluated performance with Conner’s questionnaire. The results show no significant inter-group differences.
Toyota randomised 40 surgical patients to receive either diluted Rescue Remedies or placebo as premedication before surgery [21]. Anxiety, tension, heart rate and blood pressure showed no difference between the groups. Unfortunately the report is unclear in several respects. The author draws a positive conclusion which is not borne out by the data.
Halberstein et al. randomised 111 student nurses who were exposed to a stressful exam situation into two groups [22]. During a three-hour class, they received five doses of Rescue Remedy or placebo. Anxiety levels did not differ between groups, but a subgroup of exceedingly anxious nurses generated findings that seemed to favour verum over placebo.
Forshaw and Jones randomised 62 students who were submitted to experimental stress into three groups. Group A received four drops of Rescue Remedy in mineral water. Group B received pure mineral water and were told it contained Rescue Remedy and group C drank pure mineral water and were told it was pure water. Stress levels decreased in all groups in a similar manner, and there were no significant treatment effects related to Rescue Remedy [23].
The data summarised above confirm that RCTs of flower remedies are possible and demonstrate that several such studies have recently become available. While the previous systematic reviews included three and four RCTs respectively [5–7], the present one is based on seven RCTs. Collectively they fail to produce convincing evidence to suggest that flower remedies are associated with clinical effects that differ from those of placebo.
This systematic review has a number of limitations. Even though the search strategy was comprehensive, there is no certainty that all studies were located. Trials of flower remedies may have been published in journals not listed in electronic databases. Moreover, negative publication bias may have distorted the overall result. It is known that there is a tendency for negative trials to remain unpublished, and journals of alternative medicine publish very few negative results [24]. The paucity of the available data renders the final verdict about the efficacy of flower remedies problematic. Data extraction was only done by one person; the author of this paper. This increases the risk of error and bias in interpreting the findings.
In most countries, flower remedies are marketed not as medicines but as food supplements. Therefore there is no legal requirement to demonstrate efficacy and no health claims are permitted. Yet there is an abundance of literature on flower remedies which does make such claims. Customers are thus attracted to flower remedies with certain expectations, and the question arises whether this has the potential for causing harm to patients. Due to their highly dilute nature, flower remedies are devoid of toxicology. However, flower remedies may be used in cases of severe illness as an “alternative” to effective therapy. In such a scenario, the use of flower remedies could become life-threatening [25].
When professional flower remedy organisations were asked for which indications they would recommend flower remedies [26], they named the following conditions: anxiety/stress, depression, general mental stress, lack of confidence, trauma (emotional and physical), cancer and HIV/AIDS. This systematic review shows that these claims are not based on evidence. Others have stressed that “the basic principles of Bach’s theory are settled on ungrounded, deeply intuitive hypotheses, belong to magical thinking, and do promote philosophical approaches that weaken patients-consumers, particularly with regard to sectarian trends” [27]. The implication from the negative clinical evidence and the lack of biological plausibility might be that further research in this area is not warranted.
In conclusion, the most reliable clinical trials of flower remedies available to date fail to show efficacy.
1 Barnard J. Bach E. (1931). The twelve Healers. Republished in: The collected writings of Edward Bach. Bath: Ashgrove Press. 1998.
2 van Haselen RA. The relationship between homeopathy and the Dr Bach system of flower remedies: a critical appraisal. Br Homeopath J. 1999;88(3):121–7.
3 NN. The work of Dr Edward Bach. An introduction and guide to the 38 flower remedies. London: Wigmore Pub Ltd. 1995.
4 Fricke U. Die Tops und Flops der Naturmedizin. Bild der Wissenschaf. 1999;11:52–7.
5 Ernst E. “Flower remedies”: a systematic review of the clinical evidence. Wien Klin Wochenschr. 2002;114(23–24):963–96.
6 Thaler K, Kaminski A, Chapman A, Langley T, Gartlehner G. Bach Flower Remedies for psychological problems and pain: a systematic review. BMC Complement Altern Med. 2009;26(9):16.
7 Halberstein RA, Sirkin A, Ojeda-Vaz MM. When less is better: a comparison of Bach Flower Remedies and homeopathy. Ann Epidemiol. 2010;20(4):298–307.
8 Kaminski P. Flowers that heal. Dublin: Gill MacMillan. 1998.
9 Balinski AA. Use of Western Australian flower essences in the management of pain and stress in the hospital setting. Complement Ther in Nursing & Midwifery 1998;4(4):111–7.
10 Scheffer VM. Die Bach-Blütentherapie. Eine sanfte Heilmethode in der täglichen Praxis. Erfahrungsheilkunde. 1991;40: 390–401.
11 Cram JR. Flower essence in the treatment of major depression. Cam 2002;8–15.
12 Howard J. Do Bach flower remedies have a role to play in pain control? A critical analysis investigating therapeutic value beyond the placebo effect, and the potential of Bach flower remedies as a psychological method of pain relief. Complement Ther Clin Pract. 2007;13(3):174–83.
13 Oliva i Segura M. Emotional support and Bach Flower Therapy. [In Spanish]. Rev Enferm. 2009;32(10):16–9.
14 Hyland ME, Geraghty AW, Joy OE, Turner SI. Spirituality predicts outcome independently of expectancy following flower essence self-treatment. J Psychosom Res. 2006;60(1):53–8.
15 Hyland ME, Whalley B, Geraghty AW. Dispositional predictors of placebo responding: a motivational interpretation of flower essence and gratitude therapy. J Psychosom Res. 2007;62(3): 331–40.
16 Jadad AR, Moore A, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ ea. Assessing the quality of reports of randomized clinical trials: Is blinding necessary. Controlled Clin Trials. 1996; 17(1):1–12.
17 Von Rühle G. Pilotstudie zur Anwendung von Bach-Blütenessenzen bei Erstgebärenden mit verlängerter Tragzeit. Erfahrungsheilkunde. 1995;44:854–60.
18 Armstrong NC, Ernst E. A randomised; double-blind; placebo-controlled trial of Bach Flower Remedy. Perfusion. 1999;11: 440–6.
19 Walach H, Rilling C, Engelke U. Efficacy of Bach-flower remedies in test anxiety: A double-blind, placebo-controlled, randomized trial with partial crossover. J Anxiety Disord. 2001;15(4): 359–66.
20 Pintov S, Hochman M, Livne A, Heyman E, Lahat E. Bach flower remedies used for attention deficit hyperactivity disorder in children – a prospective double blind controlled study. Eur J Paediatr Neurol. 2005;9(6):385–98.
21 Toyota S. The study of Bach flower remedies as premedication. J Intl Soc Life Info Sci. 2006;24(2):455–60.
22 Halberstein R, DeSantis L, Sirkin A, Padron-Fajardo V, Ojeda-Vaz M. Healing with Bach® flower essences: testing a complementary therapy. Complement Health Pract Rev. 2007;12(1):3–14.
23 Forshaw MJ, Jones SJ. A test of the properties of a proprietary complementary remedy in conditions of temporary, induced, cognitive stress. Perfusion. 2010;22(4):7.
24 Ernst E, Pittler MH. Alternative therapy bias. Nature. 1997; 385:480.
25 Ernst E, Pittler MH, Wider B, Boddy K. The Desktop Guide to Complementary and Alternative Medicine. 2nd edition. Edinburgh: Elsevier Mosby. 2006.
26 Long L, Huntley A, Ernst E. Which complementary and alternative therapies benefit which conditions? A survey of the opinions of 223 professional organisations. Complementar Ther Med. 2001;9(3):178–85.
27 Monvoisin R. Bach flower remedies: a critic of the pseudoscientific, pseudomedicinal concepts and philosophical postures inducted by Dr Bach theory. Ann Pharm Fr. 2010;63(6):416–28.
No funding; no competing interests.