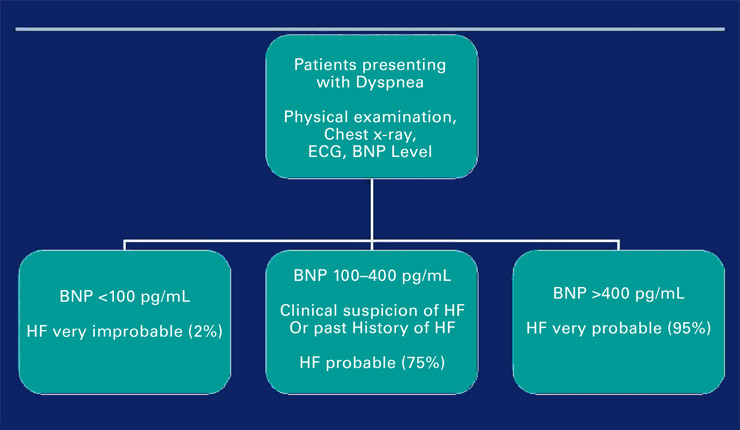
Figure 1
Algorithm of BNP use at presentation for acute dyspnea.
DOI: https://doi.org/10.4414/smw.2010.13031
Acute heart failure (AHF) is defined as a gradual or rapid change in heart failure (HF) signs and symptoms, resulting in the need for urgent therapy. AHF is complex and encompasses multiple diagnoses and etiologies [1].
There are many novelties that were recently published, which might change methods to manage AHF in the near future [2–4]. The first novelty is that AHF is not a single disease but several “syndromes” arising from multiple clinical scenarios. The present review describes those scenarios that are identified by the initial level of systolic blood pressure (SBP) at admission. In addition, each scenario is linked to a primary physiopathologic problem. We think that in the emergency setting, physicians are left alone with symptoms and vital signs of the patients. Herein, initiation of treatments based upon blood pressure is suggested, as patients may not be classified into distinct diagnostic categories of the European Society of Cardiology (ESC) guidelines within minutes of admission, though they may definitely be in need of urgent therapy. The present review insists on the initiation of the appropriate treatment as early as possible. Early treatment is defined as the pre-hospital phase and the first 6–12 hours after presentation [4]. The current paper is not intended to replace existing guidelines, rather implement them for facilitating very early clinical management of patients with AHF syndromes in a practical approach.
The need for immediate treatment is obvious in conditions such as pulmonary edema or cardiogenic shock. Furthermore, a retrospective analysis from ADHERE evaluated the association between clinical outcomes and time to initiation of vasoactive therapy [5]. The authors observed an almost even distribution of patients who received vasoactive agents in the emergency department (ED) (n = 4096) as compared to the inpatient unit (n = 3499). The mean time to vasoactive therapy initiation was 1–2 hours when it was initiated in the ED, compared to 20–22 hours when it was given after admission. Early administration in the ED was associated with a shorter median length of stay in the hospital (4.5 days vs 7 days, p <0.0001) and a lower in-hospital mortality rate (4.3% vs 10.9%, p <0.0001) [5]. These data and others suggest that early initiation of treatment for AHFS is a key factor in improving outcomes among critically ill patients.
ECG and chest X-ray should be performed together with a clinical exam in order to designate perfusion and congestion status in all acute dyspneic patients admitted in the ED [6]. Of note, echocardiography is not needed in the ED for most patients, but should be performed at the earliest appropriate time according to the mechanism of AHF and individual patient need [2]. Biological tests should include sodium, potassium, glucose, blood urea nitrogen or urea, serum creatinine, CK-MB and/or troponin T or I and a complete blood count.

Figure 1
Algorithm of BNP use at presentation for acute dyspnea.
The use of biomarkers to detect HF in the ED is based on the three observations: dyspnea is very frequent in the ED, AHFS is a major cause of acute dyspnea, and clinical signs such as ECG and chest X-ray may not always immediately rule AHFS in or out. Most of the diseases that lead to acute dyspnea need immediate and appropriate treatment: for example delaying antibiotic treatment and giving diuretics for a pulmonary infection may further harm the patient; optimal use of biomarkers can minimize harmful effect of mistreatment [7].
In the ED or in cardiology, natriuretic peptides (NPs; BNP and NT-proBNP as well as midregional proANP and proANP) are now considered as relevant quantitative markers of HF (and/or cardiac stress) that designate the extent of systolic and diastolic left ventricular dysfunction, valvular dysfunction, and right ventricular dysfunction, though they are not perfect [8]. The most recent ESC guidelines state that NPs are particularly useful in excluding HF with a reasonable negative predictive value [2]. In addition, the use of NPs improves medical and economic outcomes in patients with dyspnea [8]. Rational use of NPs in the emergency setting in patients presenting with dyspnea may help avoiding serious adverse events as well [7]. The accepted thresholds to confirm AHF were described recently [8] (fig. 1). However, there are still areas of uncertainty regarding the use of NPs in the emergency room. Indeed, the threshold of plasma NPs to differentiate between AHF and non-AHF is lower in obese patients and higher in chronic renal failure patients than the thresholds described above [9].
Initial biological tests also help to assess organ dysfunction associated with acute dyspnea. AHF may worsen organ function, particularly renal and liver function. Impaired end-organ function should be considered as an alarming sign to intervene, because impaired renal function worsens the prognosis of patients with AHF [10, 11]. Concerning liver function abnormalities, elevated AST, ALT and lactate were shown to influence outcomes in patients with HF [2]. Hence, evidence of poor organ perfusion along with low cardiac output and low SBP may indicate the urgent need for inotropic therapy in these patients [4].
The ESC guidelines were the first to classify patients with AHFS into distinct clinical conditions [2]. These include: i) acute decompensated HF, de novo or decompensated chronic HF; ii) hypertensive AHF; iii) pulmonary edema; iv) cardiogenic shock; v) AHF secondary to ACS; and vi) right HF [2]. However, this classification is a mixture of the clinical phenotype and disease severity on presentation and there is significant overlap among the different conditions. Of note, accurate and timely diagnosis of AHF secondary to ACS is of paramount importance, since timely revascularization could save myocardium, and hence in-fluence prognosis dramatically. However, it is important to remember that troponins are of little benefit in differential diagnosis in this setting, as HF is also associated with an increase in troponins de novo by itself [12]. Hence, symptoms suggestive of ACS should be investigated thoroughly.
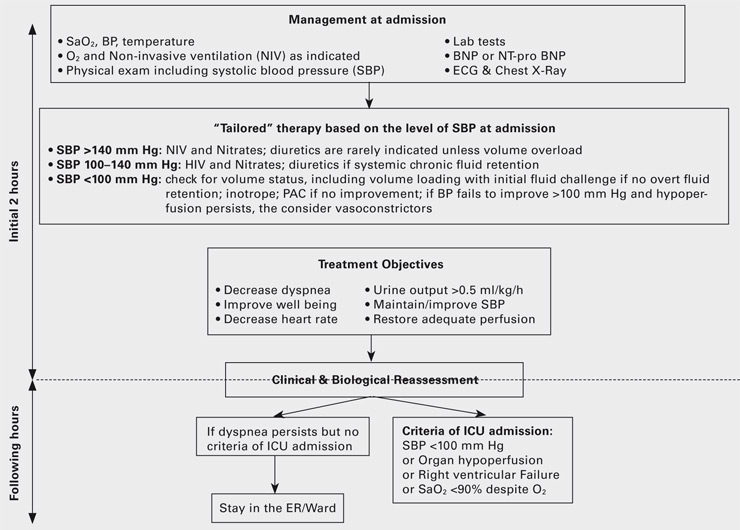
Figure 2
Initial management of acute heart failure syndromes [modified from 4].
Although the above mentioned ESC classification is the optimal approach to treat heart dysfunction, very early ED management of AHFS is primarily based on signs and symptoms. SBP was repeatedly described as the most important predictive factor of morbidity and mortality [4]. Classification by levels of SBP at admission, regardless of other parameters such as previous treatment, can markedly facilitate early risk stratification of AHFS patients. Actually, in the landmark study showing performance of perfusion and congestion based evaluation (clinical judgment classifying patients into one of four categories: dry and warm, dry and cold, wet and warm and wet and cold) in HF, perfusion was mainly based on derivatives of blood pressure (Compromised perfusion was assessed by the presence of a narrow proportional pulse pressure [systolic diastolic blood pressure/systolic blood pressure <25%], pulsus alternans, symptomatic hypotension [without orthostasis],) plus cool extremities, and/or impaired mentation [6].
Monitoring of cardiac output and filling pressures, for instance with a PAC, is suggested in haemodynamically unstable patients who are not responding in a predictable fashion to traditional treatments or who are refractory to initial therapy, who have a combination of congestion and hypoperfusion, whose volume status and cardiac filling pressures are unclear, or who have clinically significant hypotension and worsening renal function during therapy [4].
The SBP cut-offs of 100 and 140 mm Hg were recently proposed by ED, intensive care unit (ICU) and cardiology experts in AHFS and based on published literature (see reference 4 for details). Among patients with dyspnea and/or congestion, at a SBP of >140 mm Hg, left ventricular systolic function is likely preserved, at SBP of 100–140 mm Hg left ventricular systolic function is limited, and some patients with impaired left ventricular systolic function exhibit SBP <100 mm Hg and combination of the two designates the group with poor prognosis [13]. Indeed, clinical judgment is extremely important for the management of all patients with AHFS [6]. SBP guided early therapy upon clinical judgment might provide a modern way to treat AHFS (fig. 2). It is based on “tailored” therapies given as early as possible to the appropriate patient. Further details are given in the following paragraphs.
The three main tools used to treat AHFS with normal or high SBP are non-invasive ventilation, diuretics and vasodilators, and the decision to administer any of three, alone or in combination, is made by congestion status (see fig. 2).
Oxygen is recommended as early as possible to achieve an arterial oxygen saturation =>95% in AHF patients [2]. In chronic obstructive pulmonary disease (COPD) patients, the target is rather an arterial oxygen saturation of 90% in order to avoid hypercapnia. NIV with positive end-expiratory pressure (PEEP) is recommended as early as possible in most AHF patients, especially patients with acute cardiogenic pulmonary oedema and hypertensive AHF. In those patients, early application of NIV reduces both the need for intubation and short-term mortality [2]: This has been recently challenged by the 3CPO, a large randomised controlled trial, which showed that NIV improved clinical parameters but not mortality [16, 17].
Loop diuretics, especially furosemide, are the first line agents around the world for the treatment of patients with AHFS. However, only a few studies have evaluated short and long term clinical outcomes. In addition, AHFS patients with a long lasting history of increased blood pressure and chronic treatment with diuretics, are likely to be systemically euvolemic or hypovolemic. High dose diuretics in these patients may be detrimental. Intravenous diuretics are therefore recommended in AHFS patients in the presence of symptoms secondary to congestion and volume overload. The recommended initial dose is a bolus of furosemide 20–40 mg IV (0.5–1 mg of bumetanide; 10–20 mg of torasemide) at admission [2]. Urine output of patients should be assessed frequently in the initial phase. The dose of diuretics should be repeated once after 45–60 minutes in case of lack of urine. The placement of a bladder catheter is usually desirable in order to monitor urinary output and rapidly assess treatment response. The dose of i.v. furosemide may be increased according to renal function and a history of chronic oral diuretic use. In any case, in order to avoid side effects, the total furosemide dose should remain <100 mg in the first 6 hours and 240 mg during the first 24 hours [2]. In case of diuretic resistance, thiazides (hydrochlorothiazide 25 mg po) and aldosterone antagonists (spironolactone, eplerenone 25–50 mg po) can be used together prior to loop diuretics in order to make sequential nephron blockade. Ultrafiltration may be considered in patients who fail to respond to diuretic therapy, or may be an alternative [14]. However, there is still some room for placing it into routine practice.
Intravenous vasodilators are recommended at an early stage for AHFS patients without symptomatic hypotension, SBP <90 mm Hg or serious obstructive valvular disease [2]. Indeed, intravenous vasodilators (nitroglycerin, isosorbide mononitrate, isosorbide dinitrate, sodium nitroprusside and nesiritide) decrease SBP, decrease left and right heart filling pressures and systemic vascular resistance, and improve dyspnoea while maintaining or increasing coronary blood flow [2]. The initial recommended dose of intravenous nitroglycerin is 10–20 µg/min, increased in increments of 5–10 µg/min every 3 to 5 minutes as needed [2]. Intravenous nesiritide may be initiated with or without a bolus infusion with infusion rates from 0.015–0.03 µg/kg/min. Noninvasive blood pressure measurements are usually adequate. Tachyphylaxis is common after 24–48 hours, necessitating incremental dosing with nitrates. Although intravenous nitrates are strongly recommended in AHFS by several multinational guidelines [2–3], their use is mostly limited to the ED or the coronary care unit (CCU)/ICU, in most western countries, and their intravenous administration is often stopped when patients are transferred to the ward. Whether vasodilators, especially nitrates should be used for a longer period of time (during the entire hospitalization or longer), or in a non-intravenous form, remains unclear, though a recent pilot study showed promising results [15].
Although the aim of NIV is to improve oxygen saturation and intravenous diuretics aim to improve urine output, the clinical target and the length of administration of vasodilator therapy has not been described in recent guidelines. Accordingly, a multicentre Swiss trial, named GALACTIC, conducted by C. Mueller aims to assess the efficacy and safety of 1) non-intravenous forms of vasodilators – namely combining transdermal nitrates and oral hydralazine – in non-ICU AHFS patients, 2) early administration of high dose vasodilators, soon after presentation and 3) maintenance of non-invasive forms of high-dose vasodilators for at least seven days. GALACTIC has already included patients from three Swiss centers (Basel, Aarau, Luzern) and is scheduled to be extended to European centers.
Inotropic agents are still used inappropriately in many European countries and throughout the World. Many surveys show inappropriate high usage of inotropes in AHFS. Inotropic agents should be used in a small number of patients, mainly those with signs of low cardiac output or cardiogenic shock, and vasopressor agents should be used in the presence of low SBP on top of low cardiac output. They are not recommended in patients with high blood pressure. In case of evidence for the use of inotropes, it is advised to administer inotropes as early as possible [4]. Thus, traditional inotropes [dobutamine, milrinone] or the new inodilator levosimendan should be used early in patients with evidence of poor organ perfusion (patient is cold, clammy, or vasoconstricted; or patient has renal impairment, liver dysfunction, or impaired mentation) and low cardiac output, low SBP, and high filling pressures (as detected by physical examination and symptoms), who are not responding to other therapies [2, 4]. Again, these patients account for the minority of AHFS hospitalizations. Inotropes may stabilize patients at risk of progressive haemodynamic collapse or serve as a life-sustaining bridge to more definitive therapy such as mechanical circulatory support, ventricular assist devices, or cardiac transplant. Recent evidence suggests that, in case an inotrope is needed, levosimendan should be the preferred treatment in patients with previous history of heart failure and/or under beta-blockers [18].
In very few cases, norepinephrine is recommended alone or in combination with an inotrope or cardiac enhancer in order to increase SBP in the situation of persistent organ hypoperfusion (e.g., low urine output clearly related to low blood pressure). If no improvement in perfusion is observed, then advanced haemodynamic monitoring should be used. If blood pressure remains low (<100 mm Hg), then a vasoconstrictor should be considered after optimizing preload. The recommended dose for norepinephrine is 0.2 to 1.0 µg/kg/min. It may be started on a peripheral line, but a central line should be placed for its infusion as soon as feasible. Epinephrine is not recommended as a first line therapy. It is used as a rescue therapy in cardiac arrest. There is no evidence of a renal benefit with low-dose dopamine, though preliminary findings from DAD-HF trial using low dose dopamine and low dose diuretic yielded promising results.
An intraaortic balloon pump could be the first line device for patients with AHF syndromes [4, 19]. It can be rapidly placed in the cardiac catheterization laboratory or in the CCU/ICU. It is associated with some risks, including compromised blood flow to the leg, and dissection (particularly in patients with peripheral vascular disease). An intraaortic balloon pump only provides a temporary solution for AHFS. It may be implemented more quickly in patients with suspected ongoing ischemia. In a very recent meta-analysis [20] comparing IABP with percutaneously implanted left ventricular assist devices (LVAD) such Impella and Tandem Heart, it was shown that, though percutaneous LVADs provide superior haemodynamic support in patients with cardiogenic shock compared with IABP, the use of novel devices did not improve early survival. Hence, they can not be suggested in the front line for these patients.
Early mechanical device therapy may be useful in patients who have not responded to other therapies during the first 6–12 hours after presentation. Patients who may be candidates for device therapy include those with severe and persistent hypotension or hypoperfusion despite the use of inotrope, urine output <30 mL/hour, decreasing oxygen saturation, persistent ischemia, or cold or mottled skin. When implemented early, the use of these devices may promote recovery in some patients [2–4, 19].
The majority of patients with AHFS have multiple comorbidities. These conditions may contribute to the development of AHFS, and they should be controlled as soon as possible after presentation. Examples include atrial fibrillation with rapid ventricular response, ventricular arrhythmias, bradycardia, severe anemia, and infection. On the other hand, worsening renal function can negatively influence both in and out of hospital outcomes [11]. However, cardiorenal syndrome is large enough to be a discussion of another paper. In addition, concomitant medications can exacerbate HF and precipitate AHFS. These medications should be stopped immediately after presentation. Examples include non-steroidal anti-inflammatory drugs, COX-2 inhibitors, thiazelinediones, sympathomimetics, tricyclic antidepressants, Class I and III antiarrhythmics (except amiodarone), and non-dihydropyridine calcium channel blockers. By contrast, unless the patient is in cardiogenic shock, beta-blockers can be safely continued during acute decompensations according to a recent study [21].
Patients presenting with AHFS are a complex and heterogenous population at high risk of short term morbidity and mortality. Early classification of patients according to their clinical presentation is a key step in determining the appropriate initial treatment. These categories, based on the initial SBP at presentation along with clinical judgment, identify patients according to the primary pathophysiologic problem, so that early goal directed therapy can be implemented. Early initiation of diagnostic and goal-directed treatment strategies are key factors in improving patient outcomes. Early and frequent reassessment is also imperative so that adjustments in the initial therapeutic approach can be made as clinically indicated.
1 Gheorghiade M, Zannad F, Sopko G, Klein L, Piña IL, Konstam MA, et al. Acute heart failure syndromes: current state and framework for future research. Circulation. 2005;112:3958–68.
2 Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole-Wilson PA, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur Heart J. 2008;29:2388–442.
3 Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, et al. 2009 Focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the International Society for Heart and Lung Transplantation J Am Coll Cardiol. 2009;53:e1-e90.
4 Mebazaa A, Gheorghiade M, Piña IL, Harjola VP, Hollenberg SM, Follath F, et al. Practical recommendations for prehospital and early in-hospital management of patients presenting with acute heart failure syndromes. Crit Care Med. 2008;36(1 Suppl):S129–39.
5 Emerman CL. Treatment of the acute decompensation of heart failure: efficacy and pharmacoeconomics of early initiation of therapy in the emergency department. Rev Cardiovasc Med. 2003;4(Suppl 7):S13–S20.
6 Nohria A, Tsang SW, Fang JC, Lewis EF, Jarcho JA, Mudge GH, et al. Clinical assessment identifies hemodynamic profiles that predict outcomes in patients admitted with heart failure. J Am Coll Cardiol. 2003;41(10):1797–804.
7 Siebert U, Januzzi JL Jr, Beinfeld MT, Cameron R, Gazelle GS. Cost-effectiveness of using N-terminal pro-brain natriuretic peptide to guide the diagnostic assessment and management of dyspneic patients in the emergency department. Am J Cardiol. 2006;98(6):800–5.
8 Maisel A, Mueller C, Adams K Jr, Anker SD, Aspromonte N, Cleland JG, et al. State of the art: using natriuretic peptide levels in clinical practice. Eur J Heart Fail. 2008;10:824–39.
9 Passino C, Poletti R, Fontana M, Vergaro G, Prontera C, Gabutti A, et al. Clinical relevance of non-cardiac determinants of natriuretic peptide levels. Clin Chem Lab Med. 2008;46(11):1515–23.
10 Tarantini L, Cioffi G, Gonzini L, Oliva F, Lucci D, Di Tano G, et al., on behalf of the Italian Acute Heart Failure Survey. Evolution of renal function during and after an episode of cardiac decompensation: results from the Italian survey on acute heart failure. J Cardiovasc Med. (Hagerstown). 2009 Oct 23. [Epub ahead of print]
11 Damman K, Jaarsma T, Voors AA, Navis G, Hillege HL, van Veldhuisen DJ; COACH investigators. Both in- and out-hospital worsening of renal function predict outcome in patients with heart failure: results from the Coordinating Study Evaluating Outcome of Advising and Counseling in Heart Failure (COACH). Eur J Heart Fail. 2009;11(9):847–54.
12 Ilva T, Lassus J, Siirilä-Waris K, Melin J, Peuhkurinen K, Pulkki K, et al. Clinical significance of cardiac troponins I and T in acute heart failure. Eur J Heart Fail. 2008;10(8):772–9.
13 Zannad F, Mebazaa A, Juillière Y, Cohen-Solal A, Guize L, Alla F, et al.; EFICA Investigators. Clinical profile, contemporary management and one-year mortality in patients with severe acute heart failure syndromes: The EFICA study. Eur J Heart Fail. 2006;8(7):697–705.
14 Dahle TG, Sobotka PA, Boyle AJ. A practical guide for ultrafiltration in acute decompensated heart failure. Congest Heart Fail. 2008;14(2):83–8.
15 Breidthardt T, Noveanu M, Potocki M, Reichlin T, Egli P, Hartwiger S, et al. Impact of a high-dose nitrate strategy on cardiac stress in acute heart failure: a pilot study. J Intern Med. 2009 Jun 22. [Epub ahead of print]
16 Gray A, Goodacre S, Newby DE, Masson M, Sampson F, Nicholl J; 3CPO Trialists Noninvasive ventilation in acute cardiogenic pulmonary edema. N Engl J Med. 2008;359(2):142–51.
17 Masip J, Mebazaa A, Filippatos GS. Noninvasive ventilation in acute cardiogenic pulmonary edema. N Engl J Med. 2008;359:2068–9.
18 Mebazaa A, Nieminen MS, Filippatos GS, Cleland JG, Salon JE, Thakkar R, et al. Levosimendan vs. dobutamine: outcomes for acute heart failure patients on {beta}-blockers in SURVIVE. Eur J Heart Fail. 2009;11:304–11.
19 Mebazaa A, Pitsis AA, Rudiger A, Toller W, Longrois D, Ricksten SE, et al. Practical Recommendations on the Management of Perioperative Heart Failure in Cardiac Surgery. Crit Care, Article in press.
20 Cheng JM, den Uil CA, Hoeks SE, van der Ent M, Jewbali LS, van Domburg RT, et al. Percutaneous left ventricular assist devices vs. intra-aortic balloon pump counterpulsation for treatment of cardiogenic shock: a meta-analysis of controlled trials. Eur Heart J. 2009;30(17):2102–8.
21 Jondeau G, Neuder Y, Eicher JC, Jourdain P, Fauveau E, Galinier M, et al.; B-CONVINCED Investigators. B-CONVINCED: Beta-blocker CONtinuation Vs. INterruption in patients with Congestive heart failure hospitalizED for a decompensation episode. Eur Heart J. 2009;30(18):2186–92.
Alexandre Mebazaa received honoraria for lectures by Abbott Laboratories and Inverness.