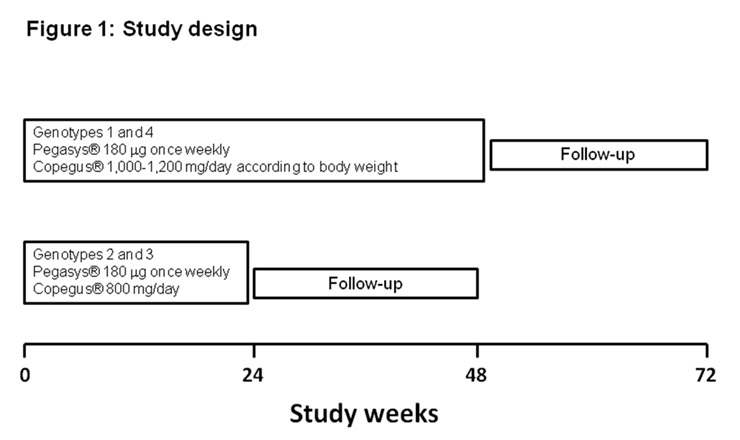
Figure 1
Study design.
DOI: https://doi.org/10.4414/smw.2010.13055
In Switzerland, approximately 30% of patients with HIV infection are co-infected with HCV [1]. Among HIV-infected intravenous drug users, this figure rises to 90% [1]. Life expectancy of HIV-positive patients has increased due to the introduction of highly active antiretroviral treatment (HAART) [2], but this gain in life expectancy has been associated with increased mortality due to liver disease, and end-stage liver disease has become the leading cause of non-AIDS-related death in these patients [3]. A recent study suggests that the achievement of a sustained virological response (SVR) in patients co-infected with HIV-HCV reduces liver-related complications and mortality [4].
Several randomised trials [5–8] have evaluated anti-HCV treatment combining pegylated interferon (PEG-IFN) or standard interferon and ribavirin (RBV) for HIV-HCV co-infected patients with controlled HIV infection. These studies showed that combination therapy with PEG-IFN and RBV is associated with a higher response rate compared to standard interferon plus RBV or PEG-IFN monotherapy. Overall, SVR was lower in these patients than in the non-HIV-infected population, particularly in those with HCV genotype 1.
The likelihood of achieving an SVR in HCV mono-infected patients can be predicted based upon the change in viral load during the course of treatment [9–11]. Lack of an early virological response (EVR), defined as at least a 2 log10 reduction in HCV-RNA, or HCV-RNA negativity, by week 12, suggests that a sustained virological response will be very unlikely (negative predictive value approaching 100%). This result allows for the early discontinuation of treatment in those who do not respond and the avoidance of side-effects and expense for these patients. The absence of a viral load response at 12 weeks has also been shown to be a powerful negative predictor of SVR in HIV-HCV co-infected patients [5, 6, 8].
A rapid viral response (RVR), defined as undetectable serum HCV-RNA at 4 weeks of therapy, is increasingly recognised as an important independent predictor of SVR. It has recently been shown in a retrospective analysis of 1,383 patients that achieving RVR correlates with a high probability (86–100%) of SVR to a combination treatment of PEG-IFN alpha 2a and RBV in HCV mono-infected patients regardless of genotype [12]. Rapid viral response has also been studied in HIV-HCV co-infected patients. An undetectable HCV-RNA level at 4 weeks has recently been shown to have a positive predictive value of SVR of 98% [13].
The primary objectives of this open-labelled multicentre comparison study were to evaluate the antiviral efficacy of PEG-IFN alpha 2a plus RBV in patients with unique HCV-infection and in patients with HCV-HIV co-infection with and without HAART, and to examine whether 6 months of therapy would have the same efficacy in HIV patients with favourable genotypes 2 and 3 as in mono-infected patients. Secondary objectives were to evaluate predictors of sustained virological response (baseline HCV-RNA, rapidity of HCV viral load decline in response to treatment, and liver histology), frequency and development of side-effects as well as tolerability of treatment and health-related quality of life (HRQOL) during treatment.
This open-labelled study was prospectively conducted at 16 Swiss centres (see footnote) between September 2003 and December 2006. The study protocol was approved by the Ethical Committees of all centres taking part in the study and all participating patients provided written informed consent. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. The patient had the right to discontinue the treatment without prejudice to him/her and without being obliged to give a reason for his/her withdrawal.

Figure 1
Study design.
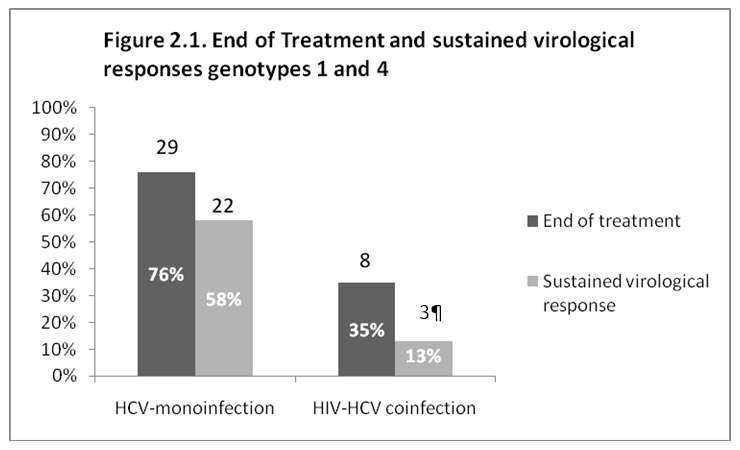
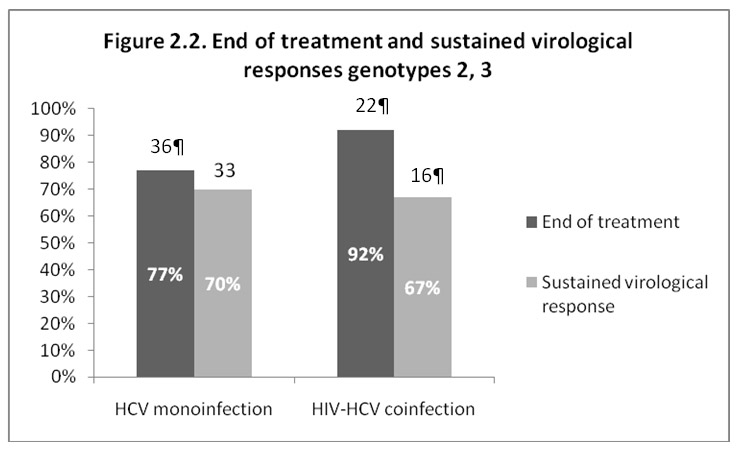
Figure 2
End of treatment and sustained virological responses. a: types 1 and 4; b: types 2 and 3.
All patients received PEG-IFN alpha 2a (Pegasys®, Roche) 180 µg once weekly plus RBV (Copegus®, Roche), given at doses of 1000 mg/day if body weight was <75 kg and of 1200 mg/day if >75 kg (genotypes 1 and 4) or 800mg/day (genotypes 2 and 3). The duration of treatment was 48 weeks for patients with genotypes 1 and 4 and 24 weeks for patients with genotypes 2 and 3. As recommended in HCV mono-infected patients, virological stopping rules were applied: thus patients with genotypes 1 and 4 who did not achieve a reduction of >2 log10in serum HCV-RNA at week 12 of therapy were considered to represent treatment failures and discontinued HCV therapy prematurely [14–16]. Likewise, patients with detectable serum HCV-RNA at week 24 were also considered to represent treatment failures and stopped HCV treatment. Sustained virological response (SVR) was defined as an undetectable HCV-RNA 6 months after the end of treatment.
Safety was assessed at weeks 1, 2, 4, 8, 12 and thereafter every 6 weeks, and 12 and 24 weeks after the discontinuation of the study drug. Each visit consisted of a physical examination, reporting of any adverse events, full blood-count and blood chemistry. Additional unscheduled visits could take place if required for assessment of laboratory parameters or clinical safety. All adverse events, defined as any untoward medical occurrence, which did not necessarily need to have a causal relationship to the study treatment, had to be recorded in the patient’s medical records and on the study case report form. The severity of the adverse events and their relationship to study drugs had to be assessed using specific grading guidelines. Safety analysis was performed in all patients, who were assessed at least once after the start of the treatment. Specific dose-reduction tables for PEG-IFN and RBV for subjective symptom and laboratory parameters were provided for all investigators.
The study design is described in figure 1.
Patients between 18 and 65 years, treatment-naïve for chronic hepatitis C, with quantifiable HCV-RNA via Roche Cobas Amplicor 2.0 (lower limit of detection 600 IU/ml) and elevated serum alanine aminotransferase activity in serum, as well as HIV-positive patients with or without highly active antiretroviral therapy (HAART), were eligible for enrolment in the study. Eligibility criteria included a CD4+ T cell count>350 cells/mm3for HIV patients naïve for HAART and a CD4 T cell count >200 cells/mm3, stable antiretroviral therapy (HAART) for at least 6 months and a controlled viraemia with plasma levels of no more than 5.000 copies/ml for patients on HAART.
Eligibility criteria also included liver biopsy findings consistent with the diagnosis of chronic hepatitis C (when liver biopsy was not contra-indicated), compensated liver disease (Child-Pugh A), haemoglobin in males and females ≥110 g/l, absolute neutrophil count >1500 cell/mm3, platelet count >75.000/mm3, a negative HbsAg, ANA ≤1:320 and no evidence of autoimmune hepatitis, TSH within normal limits or adequately controlled thyroid dysfunction, and negative urine or blood pregnancy test for women of childbearing age potentially documented within the 2–3-week period prior to the first dose of the study drug. Additionally, all fertile males and females were required to use effective contraception during treatment and for 6 months after treatment end.
Patients with any of the following were not eligible to participate: significant psychiatric illness or significant coexisting condition other than HIV infection, active seizure disorders, history of chronic liver disease other than HCV (for example, alcoholic liver disease), history or evidence of decompensated liver disease (Child-Pugh B/C), histologically absent or minimal liver disease (Metavir score A0F0 or A1F0), hepatocellular carcinoma or alpha-foetoprotein >50 µg/l, haemoglobinopathy or any cause of or tendency to haemolysis, significant cardiovascular dysfunction, renal dysfunction, evidence of severe retinopathy, positive test at screening for anti-HAV IgM, HbsAg, anti-HbcIgM Ab, Hbe Ag. Women with ongoing pregnancy or breastfeeding were also excluded, as were patients who participated in any other clinical trial within 30 days of entry into the protocol.
HCV-RNA quantification: HCV-RNA was determined by Roche Cobas Amplicor 2.0 (lower limit of detection 600 IU/ml, Roche, Penzberg, Germany). The primary efficacy endpoint was SVR defined as undetectable serum HCV-RNA (<600 IU/ml) 6 months after the end of treatment.
HCV genotype determination: HCV genotypes were determined by Inno-LiPA HCV Innogenetics, Gent, Belgium.
Health-related quality of life was calculated using the MOS-SF36 questionnaire [17]. The SF36 is a 36-item general HRQOL measure that contains eight subscales that are scored independently. The subscales cover the following eight domains of health-related quality of life: general health perception, physical functioning, social functioning, role functioning (physical), role functioning (emotional), emotional wellbeing, pain and vitality. Moreover, physical and mental summary scores can be calculated [18]. The SF36 has been used extensively and validated in a variety of populations such as HIV patients and HCV patients [19–24] and is available in several languages (including German, French and Italian). The self-administered SF36 questionnaire takes an average of 10–15 minutes to complete. It had to be completed by patients at baseline, week 12, week 24 (= end of treatment for patients with genotypes 2 and 3), week 48 (= end of treatment for patients with genotypes 1 and 4) and at end of follow-up. HCV mono-infected patients were compared to HIV-HCV co-infected patients.
Continuous variables were expressed as mean and standard deviation or median and range. Means were compared using Student’s t-test, Mann-Whitney U-test or Wilcoxon signed rank test for paired samples. Categorical variables were compared by chi-square or Fisher’s exact test. Response rates were calculated on an intention-to-treat basis: all patients who received at least one dose of study medication were included in the analysis. Analysis of covariance (ANCOVA) with the response at the end of follow-up as a dependant variable, response at week 4 as a continuous variable and the group as factor was performed to estimate the effect of the group on the response.
All P values are two-sided and differences were considered significant when P <0.05. Data analyses were performed using Stata 10, Data analysis and statistical software (Stata Corp, College Station, Texas, USA).
Box-plots (or box and whisker diagrams) were used to present the SF36 results graphically. Box-plots summarise the following statistical measures: median, upper and lower quartile, and minimum and maximum values. The box contains 50% of the data. The vertical lines or whiskers indicate the minimum and maximum data unless outliers are present.
One hundred and thirty-two patients were enrolled in the study between September 2003 and December 2006. Forty-seven (36%) were HCV-HIV co-infected patients, representing between about 5% and 30% of the HCV-HIV co-infected patients followed up by the different centres participating in the study (Lausanne 5% and 15%, Lugano 10% and Basle 30%). Patient characteristics at baseline are expressed in table 1. Mean body mass index was higher in the HCV mono-infected group. There were no significant differences in the Metavir scores between mono and co-infected patients. Mean CD4 T-cell count was not different in HCV-HIV co-infected patients on HAART as compared to HCV-HIV co-infected patients without HAART (561 ± 336 cells/mm3 vs 536 ± 172 cells/mm3). There were also no differences in HCV viral load at baseline between mono- and co-infected patients. Eighteen mono-infected patients and 9 HIV-HCV co-infected patients had an HCV-RNA <500000 IU/ml at baseline.
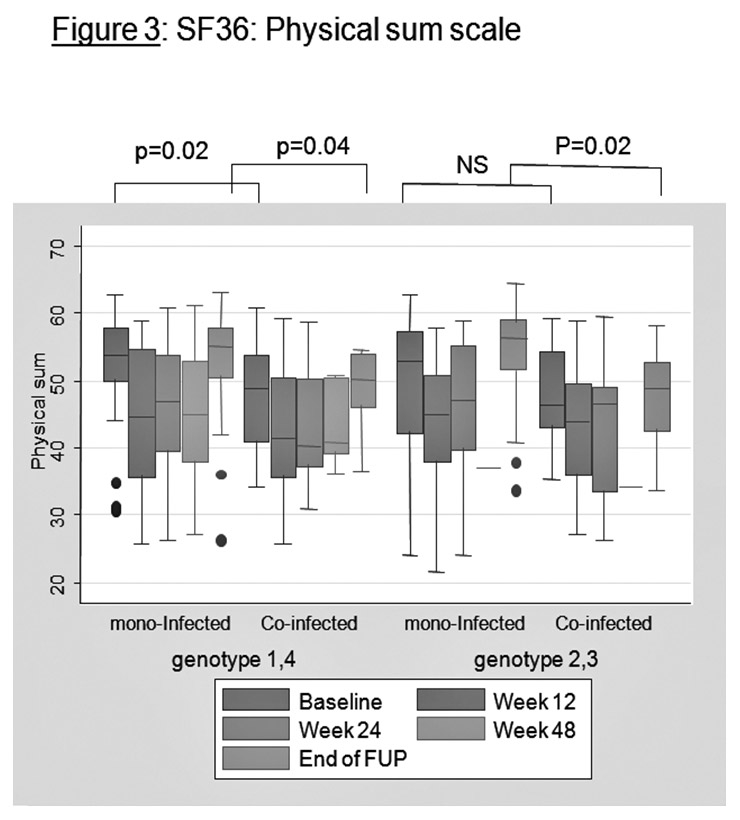
Figure 3
SF36: physical sum scale.
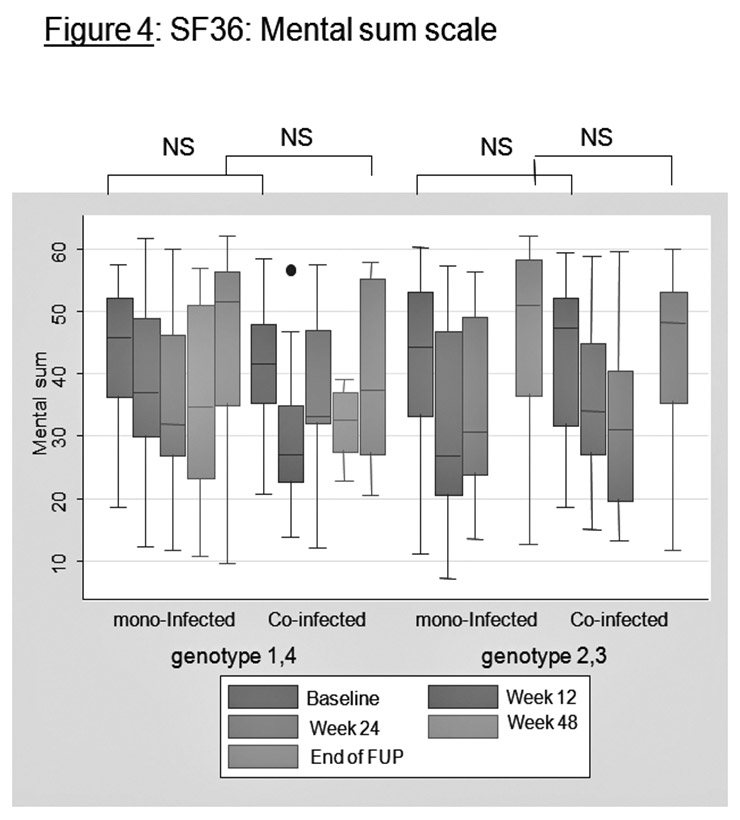
Figure 4
SF36: mental sum scale.
Modes of infection for HCV and for HIV are expressed in table 2. For co-infected patients, two modes of infection were considered in 12 patients.
Figures 2.1 and 2.2 illustrate the virological response at the end of treatment and the sustained virological response (SVR) 6 months after cessation of treatment. For genotypes 1 and 4, SVR was observed in 22 out of 38 (58%) of the mono-infected patients and in only 3 out of 23 (13%) of the HIV-HCV co-infected patients (P = 0.001). For genotypes 2 and 3, 33 patients out of 47 (70%) of the mono-infected and 16 out of 24 (67%) of the HIV-HCV co-infected patients attained an SVR (P = 0.973). The low SVR observed in co-infected patients with genotypes 1 and 4 is due partially to the high drop-out rate; 9 out of the 23 HIV-HCV patients (39%) with genotypes 1 and 4 who completed the treatment had an SVR.
In our study, 11 of the 47 HIV-HCV co-infected patients were not on highly active anti-retroviral treatment (HAART) and 8 of them had an SVR (73%); 36 patients were on HAART and 11 of them had an SVR (31%). The difference in SVR between the two groups is significant (P <0.02). This is due to the fact that there are more patients with genotypes 1 and 4 in the group with HAART (55%) than in the group without HAART (27%). After correction, the difference is no longer significant.
A higher drop-out rate was also observed in co-infected patients on HAART (36%; 13 out of 36) versus co-infected patients not on HAART (10%; 1 out of 10). For patients with genotypes 2 and 3, an SVR was attained in 88% (7 out of 8 patients) without HAART and in 56% (9 out of 16) of the patients on HAART (NS).
The positive predictive value of an SVR to treatment was calculated according to undetectable HCV-RNA at week 4 and week 12 (table 3). For genotype 1 and 4 mono-infected patients, the positive predictive value of negative HCV-RNA at week 4 was 0.78 (CI95%: 0.54–0.93). The positive predictive value was not calculated for HIV-HCV co-infected patients as there was only one patient with a negative HCV-RNA at week 4. For patients with genotypes 2 and 3, the positive predictive value of negative HCV-RNA at week 4 was 0.81 (CI 95%: 0.64–0.92) for mono-infected patients and 0.76 (CI 95%: 0.50–0.93) for HIV-HCV co-infected patients.
The positive predictive value of undetectable HCV-RNA at week 12 was 0.66 for genotype 1 and 4 mono-infected patients (CI 95%: 0.47–0.83), 0.89 (95% CI: 0.74–0.97) for genotype 2 and 3 mono-infected patients and 0.66 (95% CI: 0.43–0.85) for genotype 2 and 3 co-infected patients.
The frequency and percentage of adverse events and reasons for not completing the study for patients with genotypes 1 and 4 are described in table 4. Eight of the 38 mono-infected patients (21%) did not complete the study compared to 14 of the 23 HIV-HCV co-infected patients (61%). This difference is significant (P = 0.003).
In the group of mono-infected patients, the following adverse events leading to treatment stop were observed: depression (judged to be incompatible with continuation of the treatment by a psychiatrist) (2x) and severe fatigue. Non-response at 12 weeks was observed in 3 patients and the treatment was stopped. In the group of co-infected patients, one patient died from a myocardial infarction. Four patients, mostly complaining of poor tolerance, withdrew their consent and 3 patients had one or more adverse events (neutropenia, severe depression, abscess, cachexia). Non-response at 12 weeks was observed in 5 patients and the treatment was stopped in all of them.
The frequency and percentage of adverse events and reasons for not completing the study for patients with genotypes 2 and 3 are described in table 5. Eleven of the 47 mono-infected patients with genotypes 2 and 3 did not terminate the study (23%) compared to 1 (4%) in the co-infected group. Adverse events leading to treatment stop were observed in 7 patients: severe depression: 3 patients, liver decompensation: 1 patient, intractable migraine: 1 patient, loss of libido and depression: 1 patient, severe itching: 1 patient, toxidermia: 1 patient.
The frequency and variety of adverse events reported throughout the study period, but which did not lead to treatment stop, are shown in table 6. The most common was depression followed by haematological events (neutropenia, thrombopenia, anaemia).
Figures 3 and 4 present the SF36 results graphically for the physical sum scale and for the mental sum scale between mono-infected patients and HIV-HCV co-infected patients. For physical sum scale, co-infected patients tend to have lower values at baseline (genotypes 1 and 4) and lower values at the end of follow-up (all genotypes). For physical functioning and general health, co-infected patients also have lower values at baseline and at end of follow-up (not represented).
For mental sum scale, no significant differences were observed.
| Table 1Patient characteristics at baseline. | |||
| Genotypes 1 and 4 | 38 (46%) | 23 (50%) | 61 (47%) |
| Genotypes 2 and 3 | 47 (54%) | 24 (50%) | 71 (53%) |
| Total | 85 | 47 | 132 (100%) |
| Genotypes 1 and 4 | |||
| Patients (N) | 38 | 23 | |
| Male/Female | 26/12 | 16/7 | |
| Mean Age ± SD | 41 ± 10 yrs | 43.8 ± 4 yrs | |
| Genotype 1 | 33 | 20 | |
| Genotype 4 | 5 | 3 | |
| BMI | 25.2* | 22.0* | |
| Metavir A0-A1/A2-A3 | 26/12 | 14/9 | |
| Metavir F1-F2/F3-F4 | 28/10 | 13/10 | |
| Receiving HAART (N) | 20 | ||
| Mean baseline HCV-RNA | 2.4x106 IU/ml | 4.9x106IU/ml | |
| HCV-RNA <5x105 IU/ml (N) | 8 | 4 | |
| Genotypes 2 and 3 | |||
| Patients (N) | 47 | 24 | |
| Male/Female | 33/14 | 12/12 | |
| Mean Age ± SD | 41.9 ± 9yrs | 39.3 ± 7 yrs | |
| Genotype 2 | 8 | 2 | |
| Genotype 3 | 39 | 22 | |
| BMI | 25.5** | 21.4** | |
| Metavir A0-A1/A2-A3 | 20/25 | 9/15 | |
| Metavir F1-F2/F3-F4 | 31/14 | 15/9 | |
| Receiving HAART (N) | 16 | ||
| Mean baseline HCV-RNA | 3x106IU/ml | 1.8x106IU/ml | |
| HCV-RNA <5x105 IU/ml (N) | 10 | 5 | |
| *P = 0.025**P = 0.003 | |||
| Table 2Modes of infection. | ||||
| Genotypes 1 and 4 | Genotypes 2 and 3 | |||
| Hepatitis C | ||||
| Mono-infected(n = 38) | Co-infected(n = 23) | Mono-infected(n = 47) | Co-infected(n = 24) | |
| Transfusion/blood product | 11 | 5 | ||
| Intravenous drug use | 20 | 20 | 26 | 20 |
| Tattoo | 2 | 1 | 1 | |
| Other way/ unknown | 5 | 2 | 15 | 4 |
| HIV | ||||
| Intravenous drug use | – | 20* | – | 20* |
| Sexual contact | – | 4* | – | 8* |
| Other way/ unknown | – | – | ||
| *Two modes of infection considered | ||||
| Table 3Positive predictive value of an SVR with undetectable HCV-RNA at week 4 and week 12. | ||
| Genotypes 1 and 4 | ||
| HCV-RNA negative at week 4 | 0.78(CI 95%: 0.54–0.93) | – |
| HCV-RNA negative at week 12 | 0.66(CI 95%: 0.47–0.83) | 0.37(CI 95%: 0.08–0.75) |
| Genotypes 2 and 3 | ||
| HCV-RNA negative at week 4 | 0.81(CI 95%: 0.64–0.92) | 0.76(CI95%: 0.50–0.93) |
| HCV-RNA negative at week 12 | 0.89(CI 95%: 0.74–0.97) | 0.66(CI 95%: 0.43–0.85) |
| Table 4Frequency and percentage of adverse events and reasons for not completing the study (genotypes 1 and 4). | |||
| HCV(N = 38) | HIV-HCV(N = 23) | All(N = 61) | |
| Study not completed | 8 (21%)* | 14(61%)* | 22(36%) |
| – death | – | 1a | 1 |
| – adverse events | 3b | 3c | 6 |
| – consent withdrawal | – | 4 | 4 |
| – no response at week 12 | 3 | 5 | 8 |
| – lost to follow-up | 1 | – | 1 |
| – other | 1 | 1 | 2 |
| *P = 0.003a myocardial infarctionb severe depression, fatiguec neutropenia, depression, abscess, cachexia | |||
| Table 5Frequency and percentage of adverse events and reasons for not completing the study (genotypes 2 and 3). | |||
| HCV(N = 47) | HIV-HCV(N = 24) | All(N = 71) | |
| Study not completed | 11(23%)* | 1(4%)* | 12(17%) |
| – death | – | – | – |
| – adverse events | 7a | – | 7 |
| – consent withdrawal | 2 | 1 | 3 |
| – lost for follow-up | 1 | – | 1 |
| – poor compliance | 1 | – | 1 |
| *P = NS a) depression (3x), toxidermia, intractable migraine, loss of libido and depression, severe itching, liver decompensation | |||
| Table 6Adverse events observed during treatment without leading to treatment stop. | |||
| Depression | 7 patients | Depression | 2 patients |
| Pneumonia | 1 patient | Pneumonia | 1 patient |
| Fever | 3 patients | Fever | 1 patient |
| Chest pain | 1 patient | Nausea | 1 patient |
| Itching | 2 patients | Surgical arterial bypass right leg | 1 patient |
| Pulmonary sarcoidosis | 1 patient | ||
| Cutaneous porphyria | 1 patient | ||
| Neutropenia | 5 patients | ||
| Anaemia | 1 patient | ||
| Depression | 4 patients | Depression | 5 patients |
| Vomiting | 2 patients | Pneumonia | 2 patients |
| Itching | 2 patients | Herpes | 2 patients |
| Dyspnea | 1 patient | Appendicitis | 1 patient |
| Eczema | 1 patient | Haemolytic anaemia | 1 patient |
| Cough | 1 patient | Neutropenia | 5 patients |
| Biliary colic | 1 patient | ||
| Neutropenia | 2 patients | ||
| Thrombopenia | 1 patient | ||
In HIV-HCV co-infected patients, the treatment of chronic HCV infection has become a priority given the faster progression to end-stage liver disease. PEG-IFN in combination with RBV represents the current gold standard of care for both mono- and co-infected patients with HCV. The primary role of treatment is to achieve a sustained virological response (SVR), which is defined as an undetectable HCV-RNA 6 months after the end of treatment. Clearance of HCV-RNA is generally interpreted as a cure of infection and is associated with improved clinical outcome [4, 25, 26]. In this study, efficacy and safety of PEG-IFN alpha 2a in combination with RBV was assessed in HCV mono-infected and in HIV-HCV co-infected patients.
An SVR was achieved in 58% of the HCV mono-infected patients with genotypes 1 and 4, and in 70% of the mono-infected patients with genotypes 2 and 3. These results are comparable to the results of the 3 major studies on PEG-IFN in combination with RBV [10, 11, 27]. An SVR was achieved in only 13% of the HIV-HCV co-infected patients with genotypes 1 and 4 and in 67% of the co-infected patients with genotypes 2 and 3. The low rate of SVR in patients with genotypes 1 and 4 could be due in part to the high percentage of patients who did not complete the study (61%; 14 patients out of 23. Lower rates of drop-outs of between 16% and 39% have been described in the literature for this group of patients [5, 8, 28, 29]. Indeed, the proportion of patients with genotypes 1 and 4 withdrawing from the treatment strongly differed between HCV mono-infected and HIV-HCV co-infected patients. Five mono-infected patients did not complete the study for reasons other than non-response at week 12: adverse events: 3 patients, lost for follow-up: 1 patient, other reason: 1 patient. Eight co-infected patients did not complete the study for reasons other than non-response at week 12: consent withdrawal: 4 patients, adverse events: 3 patients, other reason: 1 patient. Poor tolerance was the main reason for withdrawal of consent. Many premature discontinuations reported in clinical trials on the treatment of HIV-HCV co-infection were related to neuro-psychiatric disorders [5, 6]. Neuro-psychiatric disorders are common in HIV-infected patients and IFN can exacerbate depression, insomnia, mood swings and generalised irritability. The high rate of drop-outs in co-infected patients with genotypes 1 and 4 does not entirely explain the lower rate of SVR in this group in comparison to mono-infected patients. The viral kinetic response to anti-HCV therapy is slower in patients infected with HCV and HIV [30] and although our study population was relatively immunocompetent, as reflected by their absolute CD4 counts, some yet unrecognised qualitative defects in immune function might also have affected their ability to eradicate HCV.
There are few data on whether a shorter course of therapy of 24 weeks versus the usual 48 weeks used in the pivotal studies may have the same efficacy in HIV-HCV co-infected patients with genotypes 2 and 3. This is particularly important because a shorter duration of treatment could reduce therapy-related toxicity and subsequently the rate of drop-out. On an intention-to-treat basis, no difference in SVR was observed in this study between HCV genotype 2 and 3 mono-infected and co-infected patients, both groups being treated for 24 weeks. One might argue that this absence of difference could be due to the fact that, surprisingly, there were more mono-infected patients who did not complete the study (23%) than co-infected patients (4%) and that if one considers only the patients who did complete the study, 33 out of the 36 mono-infected (92%) attained an SVR compared to 16 out of 23 (70%) of the co-infected patients (P <0.05). We do not have any explanation for this unexpected difference. On the other hand, the SVR rate observed in our genotype 2 and 3 HIV-HCV co-infected patients treated for 24 weeks is within the range of what was observed in the four pivotal studies listed in table 7.
HCV genotype is the most important baseline predictor of treatment response for PEG-IFN alpha-based therapy. In this study as in other studies, mono-infected patients infected with genotypes 1 and 4 are less likely to experience an SVR than those infected with other genotypes [10, 11, 27, 31–33] This is also true for HIV-HCV co-infected patients [5, 6, 8, 28].
HCV viral load decline after the initiation of therapy gives prognostic information about the patient’s probability of responding to treatment. A rapid viral response defined as undetectable serum HCV-RNA at 4 weeks of therapy is increasingly recognised as an important independent predictor of SVR. It has recently been shown, in a retrospective analysis of 1383 patients, that achieving RVR correlates to a high probability (86–100%) of SVR to PEG IFN/RBV combination therapy in HCV mono-infected patients regardless of genotype [12]. The HCV response at 4 weeks has also been studied in HIV-HCV co-infected patients. Among 289 patients with genotype 1 infection in the APRICOT trial, 22 (13%) had a rapid viral response; 18 of these 22 patients (82%) achieved an SVR(34).
In our study, a positive predictive value of SVR of negative HCV-RNA at week 4 was 78% for mono-infected patients with genotypes 1 and 4, 81% for mono-infected patients with genotypes 2 or 3 and 76% for HIV-HCV co-infected patients with genotypes 2 or 3. Thus our findings support the concept that patients with rapid virological HCV-RNA clearance have the likelihood of being cured by therapy.
Another small study demonstrated that 15 of 21 (71%) co-infected patients with genotypes 2 or 3 who attained a RVR at 4 weeks attained an SVR after only 6 months of therapy [35]. Although these data are preliminary, due to the small number of patients studied, they suggest that the virological response at 4 weeks may be a helpful parameter in shortening treatment duration in patients with favourable genotypes, particularly in those who are having difficulty tolerating therapy. This has been endorsed by the recent European Aids Clinical Society Guidelines which suggest a 24-week treatment for genotype 2 or 3 [36].
In our study, co-infected patients not on HAART had an SVR in 73% while 31% of patients on HAART had an SVR. This difference is due to the higher percentage of patients with genotypes 1 and 4 in the group of patients with HAART (55%) as compared to the patients without HAART (27%). After correction, this difference is not significant. In the PRESCO trial [7], concomitant use of HAART was found to be a negative predictor of an SVR in a univariate analysis, but this was not confirmed by a multivariate analysis. Interestingly, treatment with protease inhibitors in the RIBAVIC trial was also associated with a lower SVR [5]. According to these authors, this effect may be related to drug hepatotoxicity, increased HCV replication or cytochrome P450-mediated drug interactions.
The baseline CD4 T-cell-count has not clearly been associated with virological outcome of HIV-HCV co-infected patients to date. In the APRICOT, ACTG, RIBAVIC trials CD4 T-cell-count at baseline was not predictive of SVR [5, 6, 8]. In our study, the rate of SVR did not vary according to baseline CD4 T-cell-count.
HCV baseline viral load is also an important tool for the prediction of treatment outcome as a low viral load (<600000–800000 IU/ml or less) was shown to be an independent predictor of SVR regardless of genotype in numerous studies in HCV mono-infected patients [11, 31–33, 37–41] as well as in HCV-HIV co-infected patients [5, 6, 8, 42]. In our study, the difference in the rate of SVR is not statistically significant for low (<500000 IU/ml) versus high(>500000 IU/ml) viral load (P = 0.3). The fact that there were only 21% and 19% of mono-infected or co-infected patients with a baseline HCV-RNA <500000 IU/ml could explain the absence of difference.
Health-related quality of life (HRQOL) is increasingly recognised as an important measure for assessing the burden of chronic diseases. Studies suggest that HCV infection significantly reduces HRQOL, even in the absence of cirrhosis, and certain studies suggest that successful treatment of HCV is associated with an improvement in HRQOL [19, 24, 43–49]. Results of initial studies involving HIV-infected patients suggest that HIV infection is also associated with reduced HRQOL, with evidence that concurrent psychiatric conditions and illicit drug use significantly impair HRQOL in these patients [50–52]. The impact of HCV/HIV co-infection on HRQOL is not well known. In a cross-sectional study comprising patients enrolled in a prospective natural history cohort study of chronic HCV and HIV infection, Fleming et al. [21] enrolled three groups of patients: 136 HIV-HCV co-infected patients, 110 patients with HCV infection only and 53 patients with HIV infection only. Patients with HIV infection, HCV infection and HIV-HCV co-infection had similar perception of their HRQOL and such perception was significantly lower than that of the general population. In our study, HIV-HCV co-infected patients tended to have lower values than the HCV mono-infected patients at baseline or at end of follow-up for physical functioning, general health and physical sum scale. We were unable to identify host- or virus-related factors that might explain these differences.
Twenty-two percent of HCV mono-infected patients did not complete the study. Withdrawal rates of 14% to 22% have been described in the literature [10, 11, 27]. Overall, 32% of HIV-HCV co-infected patients did not complete the study. Withdrawal rates of 12% to 39% were observed in the literature [6, 8, 35]. The proportion of patients who withdrew from treatment because of adverse events differed between treatment groups (tables 3 and 4) and was highest for genotype 1 and 4 HIV-HCV co-infected patients. Most patients usually reported transient mild to moderate flu-like symptoms (headache, malaise, fever, myalgia).
Management of underlying hepatitis C in patients with HIV infection is of great importance in preventing liver disease-associated morbidity and mortality. In this study, a significant problem with the treatment of HIV-HCV co-infected patients has been the difficulty in identifying candidates for treatment, given the number of comorbidities and underlying psychiatric and substance-abuse issues that can complicate delivery of care to this complex population. It has been estimated in this study that only between 5% and 30% of co-infected patients could be considered for a combination therapy of PEG-IFN plus RBV.
| Table 7SVR in four pivotal studies on treatment of HCV in HIV-HCV co-infected patients; comparison with the Swiss study. | ||||||
| Apricot(8) | ACTG(6) | Ribavic(5) | Presco(7)Short arm | Presco(7)Extended arm | Swissstudy | |
| N enrolled | 868 | 133 | 412 | 288 | 101 | 47 |
| – given HAART | 84% | 85% | 83% | 76% | 67% | 76% |
| Metavir F3/F4 | 16% | 10% | 40% | 27% | 27% | 32% |
| Peginterferon | 2a | 2a | 2b | 2a | 2a | 2a |
| Genotype 1 | 60% | 77% | 48% | 49% | 49% | 50% |
| SVR by genotype: | ||||||
| – genotypes 1, 4 | 29% | 14% | 17% | 31% | 53% | 13% |
| – genotypes 2, 3 | 62% | 73% | 44% | 67% | 82% | 67% |
Being infected by HCV genotypes 2 and 3 predicts the likelihood of SVR in HCV mono-infected and HCV-HIV co-infected patients. A 6-month treatment with PEG-IFN alpha plus RBV has the same efficacy in HIV-HCV co-infected patients with genotypes 2 and 3 as in mono-infected patients, thus minimising HCV-therapy-related toxicities. HCV-RNA negativity at 4 weeks has a good predictive value for SVR. Aggressive treatment of adverse effects to avoid dose reduction, withdrawal of consent or drop-out is crucial, especially when the duration of treatment is 48 weeks.
1 Rauch A, Rickenbach M, Weber R, et al. Unsafe sex and increased incidence of hepatitis C virus infection among HIV-infected men who have sex with men: the Swiss HIV Cohort Study. Clin Infect Dis. 2005;41:395–402.
2 Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008;372:293–9.
3 Weber R, Sabin CA, Friis-Moller N, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med. 2006;166:1632–41.
4 Berenguer J, Alvarez-Pellicer J, Martin PM, et al. Sustained virological response to interferon plus ribavirin reduces liver-related complications and mortality in patients coinfected with human immunodeficiency virus and hepatitis C virus. Hepatology. 2009;50:407–13.
5 Carrat F, Bani-Sadr F, Pol S, et al. Pegylated interferon alfa-2b vs standard interferon alfa-2b, plus ribavirin, for chronic hepatitis C in HIV-infected patients: a randomized controlled trial. JAMA. 2004;292:2839–48.
6 Chung RT, Andersen J, Volberding P, et al. Peginterferon Alfa-2a plus ribavirin versus interferon alfa-2a plus ribavirin for chronic hepatitis C in HIV-coinfected persons. N Engl J Med. 2004;351:451–9.
7 Nunez M, Marino A, Miralles C, et al. Baseline serum hepatitis C virus (HCV) RNA level and response at week 4 are the best predictors of relapse after treatment with pegylated interferon plus ribavirin in HIV/HCV-coinfected patients. J Acquir Immune Defic Syndr. 2007;45:439–44.
8 Torriani FJ, Rodriguez-Torres M, Rockstroh JK, et al. Peginterferon Alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N Engl J Med. 2004;351:438–50.
9 Davis GL, Wong JB, McHutchison JG, et al. Early virologic response to treatment with peginterferon alfa-2b plus ribavirin in patients with chronic hepatitis C. Hepatology. 2003;38:645–52.
10 Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–82.
11 Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–65.
12 Fried MW, Hadziyannis SJ, Shiffman M, et al. Rapid viral response is a more important predictor of sustained virological response (SVR) than genotype in patients with chronic hepatitis C virus infection. J Hepatol. 2008;48(Suppl.2):5A.
13 Payan C, Pivert A, Morand P, et al. Rapid and early virological response to chronic hepatitis C treatment with IFN alpha2b or PEG-IFN alpha2b plus ribavirin in HIV/HCV co-infected patients. Gut. 2007;56:1111–6.
14 Alberti A, Clumeck N, Collins S, et al. Short statement of the first European Consensus Conference on the treatment of chronic hepatitis B and C in HIV co-infected patients. J Hepatol. 2005;42:615–24.
15 Dhumeaux D, Marcellin P, Lerebours E. Treatment of hepatitis C. The 2002 French consensus. Gut. 2003;52:1784–7.
16 Strader DB, Wright T, Thomas DL, et al. Diagnosis, management, and treatment of hepatitis C. Hepatology. 2004;39:1147–71.
17 Ware JE Jr, Snow K, Kosinski M, et al. SF-36 Health Survey: Manual and Interpretation Guide. The Health Institute, New England Medical Center, Boston, Massachusetts 1993.
18 Ware JE Jr, Kosinski M, Keller S. Physical and Mental Health Summary Scores: A User’s Manual. The Health Institute, New England Medical Center, Boston, Massachusetts 1994.
19 Bonkovsky HL, Woolley JM. Reduction of health-related quality of life in chronic hepatitis C and improvement with interferon therapy.The Consensus Interferon Study Group. Hepatology. 1999;29:264–70.
20 Call SA, Klapow JC, Stewart KE, et al. Health-related quality of life and virologic outcomes in an HIV clinic. Qual Life Res. 2000;9:977–85.
21 Fleming CA, Christiansen D, Nunes D, et al. Health-related quality of life of patients with HIV disease: impact of hepatitis C coinfection. Clin Infect Dis. 2004;38:572–8.
22 Foster GR. Hepatitis C virus infection: quality of life and side effects of treatment. J Hepatol. 1999;31(Suppl 1):250–4.
23 Spiegel BM, Younossi ZM, Hays RD, et al. Impact of hepatitis C on health related quality of life: a systematic review and quantitative assessment. Hepatology. 2005;41:790–800.
24 Ware JE Jr, Bayliss MS, Mannocchia M, et al. Health-related quality of life in chronic hepatitis C: impact of disease and treatment response. The Interventional Therapy Group. Hepatology. 1999;30:550–5.
25 Soriano V, Maida I, Nunez M, et al. Long-term follow-up of HIV-infected patients with chronic hepatitis C virus infection treated with interferon-based therapies. Antivir Ther. 2004;9:987–92.
26 Veldt BJ, Heathcote EJ, Wedemeyer H, et al. Sustained virologic response and clinical outcomes in patients with chronic hepatitis C and advanced fibrosis. Ann Intern Med. 2007;147:677–84.
27 Hadziyannis SJ, Sette H Jr, Morgan TR, et al. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004;140:346–55.
28 Nunez M, Miralles C, Berdun MA, et al. Role of weight-based ribavirin dosing and extended duration of therapy in chronic hepatitis C in HIV-infected patients: the PRESCO trial. AIDS Res Hum Retroviruses. 2007;23:972–82.
29 Perez-Olmeda M, Nunez M, Romero M, et al. Pegylated IFN-alpha2b plus ribavirin as therapy for chronic hepatitis C in HIV-infected patients. Aids. 2003;17:1023–8.
30 Torriani FJ, Ribeiro RM, Gilbert TL, et al. Hepatitis C virus (HCV) and human immunodeficiency virus (HIV) dynamics during HCV treatment in HCV/HIV coinfection. J Infect Dis. 2003;188:1498–507.
31 Jacobson IM, Brown RS, Jr., Freilich B, et al. Peginterferon alfa-2b and weight-based or flat-dose ribavirin in chronic hepatitis C patients: a randomized trial. Hepatology. 2007;46:971–81.
32 Poynard T, McHutchison J, Goodman Z, et al. Is an “a la carte” combination interferon alfa-2b plus ribavirin regimen possible for the first line treatment in patients with chronic hepatitis C? The ALGOVIRC Project Group. Hepatology. 2000;31:211–8.
33 Shiffman ML, Suter F, Bacon BR, et al. Peginterferon alfa-2a and ribavirin for 16 or 24 weeks in HCV genotype 2 or 3. N Engl J Med. 2007;357:124–34.
34 Dieterich DT, Duff F, Sulkowski M, et al. Sustained virologic response (SVR) in HIV/HCV coinfected patients with HCV genotype 1 infection who have a rapid virologic response at week four of treatment with peginterferon alfa-2a plus ribavirin. Presented at the 13th annual Conference on Retroviruses and Opportunistic Infections, Denver, CO, February 5–8, 2006; abstract #856. 2006.
35 Crespo M, Sauleda S, Esteban JI, et al. Peginterferon alpha-2b plus ribavirin vs interferon alpha-2b plus ribavirin for chronic hepatitis C in HIV-coinfected patients. J Viral Hepat. 2007;14:228–38.
36 Rockstroh JK, Bhagani S, Benhamou Y, et al. European AIDS Clinical Society (EACS) guidelines for the clinical management and treatment of chronic hepatitis B and C coinfection in HIV-infected adults. HIV Med. 2008;9:82–8.
37 Berg T, Sarrazin C, Herrmann E, et al. Prediction of treatment outcome in patients with chronic hepatitis C: significance of baseline parameters and viral dynamics during therapy. Hepatology. 2003;37:600–9.
38 Berg T, von Wagner M, Nasser S, et al. Extended treatment duration for hepatitis C virus type 1: comparing 48 versus 72 weeks of peginterferon-alfa-2a plus ribavirin. Gastroenterology. 2006;130:1086–97.
39 Poynard T, Marcellin P, Lee SS, et al. Randomised trial of interferon alpha2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alpha2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. International Hepatitis Interventional Therapy Group (IHIT). Lancet. 1998;352:1426–32.
40 Zeuzem S, Buti M, Ferenci P, et al. Efficacy of 24 weeks treatment with peginterferon alfa-2b plus ribavirin in patients with chronic hepatitis C infected with genotype 1 and low pretreatment viremia. J Hepatol. 2006;44:97–103.
41 Zeuzem S, Hultcrantz R, Bourliere M, et al. Peginterferon alfa-2b plus ribavirin for treatment of chronic hepatitis C in previously untreated patients infected with HCV genotypes 2 or 3. J Hepatol. 2004;40:993–9.
42 Dore GJ, Torriani FJ, Rodriguez-Torres M, et al. Baseline factors prognostic of sustained virological response in patients with HIV-hepatitis C virus co-infection. Aids. 2007;21:1555–9.
43 Bernstein D, Kleinman L, Barker CM, et al. Relationship of health-related quality of life to treatment adherence and sustained response in chronic hepatitis C patients. Hepatology. 2002;35:704–8.
44 Foster GR, Goldin RD, Thomas HC. Chronic hepatitis C virus infection causes a significant reduction in quality of life in the absence of cirrhosis. Hepatology. 1998;27:209–12.
45 Hauser W, Zimmer C, Schiedermaier P, et al. Biopsychosocial predictors of health-related quality of life in patients with chronic hepatitis C. Psychosom Med. 2004;66:954–8.
46 Helbling B, Overbeck K, Gonvers JJ, et al. Host- rather than virus-related factors reduce health-related quality of life in hepatitis C virus infection. Gut. 2008;57:1597–603.
47 Kramer L, Hofer H, Bauer E, et al. Relative impact of fatigue and subclinical cognitive brain dysfunction on health-related quality of life in chronic hepatitis C infection. Aids. 2005;19(Suppl 3):S85–92.
48 Rodger AJ, Jolley D, Thompson SC, et al. The impact of diagnosis of hepatitis C virus on quality of life. Hepatology. 1999;30:1299–301.
49 von Wagner M, Lee JH, Kronenberger B, et al. Impaired health-related quality of life in patients with chronic hepatitis C and persistently normal aminotransferase levels. J Viral Hepat. 2006;13:828-34.
50 Hays RD, Cunningham WE, Sherbourne CD, et al. Health-related quality of life in patients with human immunodeficiency virus infection in the United States: results from the HIV Cost and Services Utilization Study. Am J Med. 2000;108:714–22.
51 Miners AH, Sabin CA, Mocroft A, et al. Health-related quality of life in individuals infected with HIV in the era of HAART. HIV Clin Trials. 2001;2:484–92.
52 Sherbourne CD, Hays RD, Fleishman JA, et al. Impact of psychiatric conditions on health-related quality of life in persons with HIV infection. Am J Psychiatry. 2000;157:248–54.
The authors wish to thank Roche Pharma Switzerland for supporting this project.