Cathepsins and their involvement in immune responses
DOI: https://doi.org/10.4414/smw.2010.13042
Summary
The immune system is composed of an enormous variety of cells and molecules that generate a collective and coordinated response on exposure to foreign antigens, called the immune response. Within the immune response, endo-lysosomal proteases play a key role. In this review we cover specific roles of cathepsins in innate and adaptive immunity, as well as their implication in the pathogenesis of several diseases.
Introduction
Innate and adaptive immune responses are components of a defence mechanism which has become increasingly specialised with evolution. This immune system is able to generate numerous cells and molecules capable of specifically recognising and eliminating an apparently unlimited variety of foreign invaders by functioning cooperatively [1]. At inflammatory sites, accumulation of these specialised cells and molecules helps to remove invading agents efficiently, but may also amplify the inflammatory response by damaging surrounding tissue [2]. Therefore, and in particular under inflammatory conditions during which stimulated cells are releasing toxic mediators, the numbers of inflammatory cells must be tightly controlled.
To eliminate unwanted and potentially harmful cells from inflamed tissues without releasing hazardous intracellular contents, apoptosis, a crucial physiological form of programmed cell death, is conducted [3, 4]. The central component of apoptosis is a proteolytic amplifying cascade involving specialised proteases called cysteinyl-aspartate-cleaving proteases (= caspases) which cleave a wide range of cellular substrates [5].
Besides caspases, cathepsins have recently been shown to be associated with cell death regulation [6–12] and various other physiological and pathological processes, such as maturation of the MHC class II complex, bone remodelling, keratinocyte differentiation, tumour progression and metastasis, rheumatoid arthritis and osteoarthritis, as well as atherosclerosis [13, 14] (table 1). Thus, cathepsins appear to play a significant role in immune responses. In this review we discuss recent advances addressing the role of lysosomal proteases in the diverse aspects of the immune response, and also the involvement of cathepsins in the pathogenesis of diseases in which these proteases seem not to be properly under control.
|
Table 1Cathepsins and their functions in the immune system. |
|
Cathepsin
|
Class
|
Distribution
|
Function
|
Involvement in disease
|
| Cathepsin B |
Cysteine |
Ubiquitous |
Early neutrophil, T and B cell apoptosisTLRs signalling and TNF-alpha productionSelectors for peptide-MHC II complexes |
Inflammatory disordersAlzheimer’s diseaseCancer |
| Cathepsin C |
Cysteine |
Ubiquitous |
Activation of granzymes A and B |
Inflammatory disorders |
| Cathepsin D |
Aspartic |
Ubiquitous |
Early neutrophil, T and B cell apoptosisSelectors for peptide-MHC II complexesECM degradation |
Inflammatory disorders, cancerRheumatoid arthritis, Alzheimer’s disease |
| Cathepsin E |
Aspartic |
Immune cells |
Selectors for peptide-MHC II complexes |
Atopic dermatitis, dermatitis |
| Cathepsin F |
Cysteine |
Ubiquitous |
TLRs signalling |
Cancer |
| Cathepsin G |
Serine |
Neutrophil lysosomesHuman B cells (exogenous source) |
IL-8, IL-1beta and TNF-alpha activationIL-6 disactivationDestruction of the autoantigen MBP (in B cells) |
Inflammatory disorders |
| Cathepsin H |
Cysteine |
Ubiquitous |
ECM degradation |
Cancer |
| Cathepsin K |
Cysteine |
DCs, epithelial cells |
TLRs signallingECM degradation and bone remodeling |
Rheumatoid arthritis |
| Cathepsin L |
Cysteine |
Ubiquitous |
TLRs signallingSelectors for peptide-MHC II complexesNKT and CD4 T cells production,neuronal cell death and osteoclastic bone degradation |
Thymic pathologyAtherosclerosisRheumatoid arthritisCancer |
|
|
|
ECM degradation |
|
| Cathepsin S |
Cysteine |
APC |
TLRs signallingSelectors for peptide-MHC II complexesNKT cells production, ECM degradation |
Arthritis, atherosclerosisBronchial asthma, COPDPsoriasis, cancer |
| Cathepsin V |
Cysteine |
Ubiquitous |
Selectors for peptide-MHC II complexes,NKT and CD4 T cells production |
Thymic pathology, cancer |
| Cathepsin W |
Cysteine |
NK and CD8+ cytotoxic T cells |
Selectors for peptide-MHC II complexes |
Autoimmune atrophic gastritis |
| Cathepsin X |
Cysteine |
Ubiquitous |
T cell activation |
Cancer |
The cathepsin family
Lysosomes are membrane-bound organelles which represent the main degradative compartment in eukaryotic cells. Some cell types, mainly derived from the haematopoietic lineage, contain a specialised lysosomal compartment which can secrete its content into the extracellular environment in response to external stimuli [15]. These organelles have been commonly named “secretory lysosomes” or “lysosomal-related organelles” and include lytic granules, major histocompatibility (MHC) class II compartments, and basophil and azurophil granules [16]. Conventional and secretory lysosomes share similar proprieties, such as the internal acidic pH and presence of degradative proteins. However, secretory lysosomes are distinguished from conventional lysosomes by their ability to undergo regulated secretion [17].
Lysosomes contain a considerable number of proteases. Among them the best known are the cathepsins. The cathepsin family contains chiefly cysteine (Cys) proteases, although cathepsins A and G are serine proteases and cathepsins D and E are aspartic proteases. In humans eleven cysteine cathepsins are known: cathepsins B, C (J, dipeptidyl peptidase I or DPPI), F, H, K (O2), L, O, S, V (L2), X (P,Y,Z) and W (lymphopain) [18, 19]. They are all members of the papain family, which belongs to the clan CA (cathepsins) of cysteine proteases. The clan CA contains all the families of peptidases known to have structures similar to that of papain [20].
To prevent any uncontrolled proteolytic activity, cathepsins are kept tightly in check. All cathepsins are synthesised as inactive zymogens. The processing of the inactive zymogen into a catalytically active enzyme usually occurs within the lysosome by other active proteases or by autocatalysis under certain specific conditions, such as low pH or the presence of glycosaminoglycans [21]. The major regulators of cathepsin activity are endogenous protein inhibitors known as cystatins, stefins, thyropins, and serpins which bind tightly to the target enzyme, thereby preventing substrate hydrolysis [22]. At least for some cathepsins, activity can also be lost by degradation or by oxidation of the Cys residue in the catalytically active site [6].
Cathepsins cleave their substrate in a relatively unspecific manner. While cathepsins B and H function as exopeptidases and endopeptidases, cathepsins D, E, F, G, K, L, S and V are endopeptidases, and cathepsins A, C and X are exopeptidases. Following synthesis, cathepsins are glycosylated posttranslationally and transported to lysosomes via the mannose-6-phosphate receptor pathway, where their concentration can reach up to 1 mM [23]. Depending on the tissue, a number of cathepsins show significant differences in protein expression levels and ratios, suggesting that individual cathepsins may have highly specific cellular functions [24].
Besides their main function in lysosomal protein recycling, cathepsins play significant roles in a variety of physiological processes such as Ag processing, wound healing, bone remodelling, prohormone and proenzyme activation, hair cycle, reproduction and also in diseases such as cancer, bronchial asthma, atherosclerosis, periodontitis, rheumatoid arthritis (RA), and osteoarthritis [14, 18, 21] (table 1). The role of cathepsins in apoptosis was discovered quite recently [25, 26]. For their proapoptotic function, cathepsins must be released from the lysosome into the cytosol by either lysosomal destabilisation [27] or lysosomal membrane permeabilisation (LMP) [28]. So far a number of molecules have been found to target the lysosomal membrane and induce apoptosis in a caspase-dependent or -independent manner (reviewed in [1]).
Cathepsins and innate immunity
Lysosome-mediated apoptosis
Cathepsins have been shown to play significant roles in the physiological process of apoptosis. Moreover, the resolution of inflammation depends on apoptosis of inflammatory cells (e.g. neutrophils and eosinophils) and their subsequent clearance by phagocytes such as macrophages. Thus it is very likely that cathepsins are involved in innate immune responses.
Indeed, cathepsin D has been shown to be a key molecule of neutrophil apoptosis by directly activating the initiator caspase-8 [29] (fig. 1A). Interestingly, this newly identified pathway in neutrophils is blocked during inflammatory conditions and is crucial for the resolution of innate immune responses, as demonstrated by the observation that delayed neutrophil apoptosis due to cathepsin D deficiency amplifies and prolongs neutrophilic inflammation in vivo. Moreover, cathepsin B has also been shown to be involved in apoptosis of immune cells. Blomgran et al. reported that, during microbe-induced apoptosis of human neutrophils, ROS-dependent release of cathepsin B into cytosol induces cleavage of the pro-apoptotic Bcl-2 family member Bid, mitochondrial damage and subsequent caspase activation and apoptosis [30]. These two studies provide confirmation that cathepsins are important modulators of innate immune responses, and that azurophilic granules, in which cathepsins are expressed and stored, may represent a new target for the development of antiinflammatory drugs.
Toll-like receptor signalling
Unexpectedly, lysosomal cathepsins have also been shown to play a role in innate responses to microbes. Toll-like receptors (TLRs) 3, 7, 8 and 9 are expressed on endosomal membranes and recognise nucleic acids of microbes that have been phagocytosed by cells. Originally identified as an osteoclast-specific lysosomal protease, it has been shown that cathepsin K potently attenuates inflammatory autoimmune diseases [31]. Pharmacological or genetic inhibition of cathepsin K results in defective TLR9 signalling in dendritic cells in response to unmethylated CpG DNA, without affecting their antigen-presenting ability. Similarly, cathepsin B, L, S and F were shown to be required for TLR9 responses [32] (fig. 1B). Apparently cathepsins induce a proteolytic cleavage which is a prerequisite for TLR7 and TLR9 signalling [33, 34]. The ectodomains of TLR7 and TLR9 are cleaved in the endolysosome, in such a way that no full-length protein is detectable in the compartment where ligand is recognised. Notably, although both the full-length and cleaved forms of TLR9 are capable of binding ligand, only the processed form recruits the signalling adaptor MyD88 on activation. Moreover, the ectodomain cleavage represents a strategy to restrict receptor activation to endolysosomal compartments and prevent TLRs from responding to self nucleic acids [33]. Together these studies identify an important role for cathepsin-dependent lysosomal proteolysis in innate immunity.
Relationships between cytokines and cathepsins
Cathepsin activity has been shown to either directly activate or inhibit certain cytokines playing an important role in inflammatory responses and consequently in innate immunity. Neutrophil proteases, such as cathepsin G or proteinase-3, enhance the activity of interleukin (IL)-8, a strong neutrophil chemoattractant, activator and proinflammatory cytokine, by limited N-terminal truncation of 5 residues [35] (fig. 1C). In contrast, the same neutrophil proteases have been reported to reduce dramatically the bioactivity of IL-6 in fluids from inflammation sites [36]. More recently, in human fibroblasts, cathepsin L has been shown also to enhance the activity of IL-8 by limited N-terminal truncation, suggesting that cathepsin L secreted by human fibroblasts plays an important role in IL-8 processing at inflammatory sites [37]. Ha et al. found that cathepsin B was required for optimal posttranslational processing and production of TNF-alpha in response to the bacterial cell wall component lipopolysaccharide (LPS), suggesting that intracellular cathepsin B activity is involved in the TNF-alpha-containing vesicle trafficking to the plasma membrane [38]. In addition, neutrophil serine proteases, like cathepsin G, are able to activate the proinflammatory cytokines IL-1beta and TNF-alpha and various receptors, such as epidermal growth factor receptor and protease-activated receptors [39]. Neutrophil serine proteases, as well as cathepsins B and L, may therefore be important regulators of the inflammatory innate immune responses and may represent interesting targets for new therapeutic approaches against inflammatory disorders.
Activation of granule serine proteases
The effector functions of immune cells (e.g. neutrophils) depend on the activation of granule-localised serine proteases such as cathepsin G and proteinase-3 [40]. These enzymes are synthesised as inactive zymogens and are activated by the aminodipeptidase cathepsin C (also known as DPPI), which removes two N-terminal residues [41]. As expected, lack of cathepsin C was shown to be protective in some murine models of inflammation and sepsis due to attenuated neutrophil function [42, 43]. This indicates that cathepsin C may be an attractive drug target to control multiple inflammatory serine proteases. Nonetheless, it appears that efficient and sustained inhibition of cathepsin C, probably by cystatin F, is required to fully quench neutrophil granule protease activity [40, 44].
Cathepsins and adaptive immunity
Antigen processing and presentation
Cathepsins play important roles in antigen processing and presentation, suggesting that they are involved in adaptive immune responses. A specific adaptive immune response is dependent on the formation of appropriate peptides from foreign proteins and their consequent presentation on major histocompatibility class I or II (MHC I and II) complexes [24]. In this respect, endo-lysosomal proteases inside antigen presenting cells (APCs), such as dendritic cells (DCs) and macrophages, have been shown to be fundamental for effective adaptive immunity. These proteases degrade extracellular proteins which were internalised by APCs via endocytosis and/or phagocytosis into smaller peptides of appropriate size prior to peptide-MHC II complex formation and presentation to CD4+ T lymphocytes (fig. 2A). They are also able to initiate the removal of the MHC II-associated chaperone invariant chain (li) from MHC II [45]. A subtle balance exists between the formation of peptides with Ag epitopes and their complete destruction [46], and many different cathepsins (e.g. cathepsins S,
L, V, B, D and W) and their endogenous inhibitors (cystatin C and p41li) have been proposed as participating in this regulation [24]. These proteases and inhibitors are probably the major selectors for the repertoire of surface peptide-MHC II complexes [47].
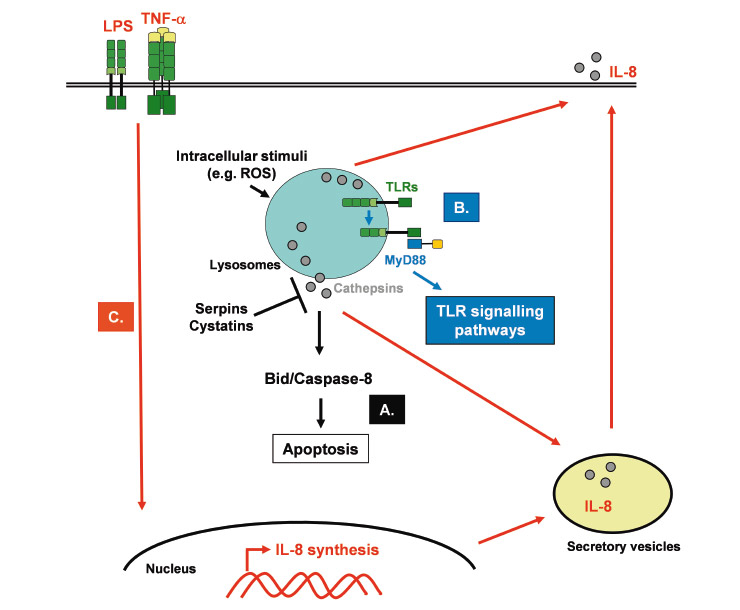
Figure 1
Roles of cathepsins in innate immunity. A. Depending on the cell type and the stimuli, cathepsins are rapidly released into the cytosol where they induce proteolysis of caspase-8 or the Bcl-2 family member Bid, which subsequently activates the enzymatic signalling cascade leading to apoptosis. Cysteine protease inhibitors, like serpins and cystatins, may regulate cathepsin activity in the cytosol. B. Lysosomal cathepsins induce proteolytic cleavage of TLRs (e.g. TLR7 and TLR9), which is a prerequisite for their signalling. The processed form then recruits the adaptor protein MyD88, which will in turn launch the different TLR signalling pathways. C. Inflammatory signals (e.g. LPS or TNF-α) induce the synthesis and secretion of cytokines and chemokines, such as IL-8. The secreted IL-8 precursor is converted into a mature form with enhanced neutrophil chemoattractant properties, by cathepsin activity.
Cathepsin S is without any doubt the major protease involved in MHC II Ag processing and presentation [21]. Indeed, cathepsin S null mice show a marked variation in generation of MHC II-bound li fragments and presentation, due to the substantially diminished li degradation in professional APCs where cathepsin S is abundantly expressed [48, 49]. In addition, endocytosis targets exogenous material selectively to cathepsin S in human DCs [50]. Enrichment of MHC II molecules within late endocytic structures has consistently been noted in splenic DCs of cathepsin S-deficient mice [51]. Cathepsin L-deficient mice exhibit a defect in MHC II-associated Ag presentation due to incomplete processing of li to CLIP in cortical thymic epithelial cells (cTECs) [52], which act as APCs for positive selection of T lymphocytes in the thymus. Further, in cathepsin L-deficient mice, the number of CD4+ T lymphocytes is diminished, confirming cathepsin L as an essential protease in MHC II Ag presentation in the thymus but not in bone marrow-derived APCs [52]. In humans, this proposed function of mouse cathepsin L is performed by its homologue cathepsin V [53].
Recent studies suggest that both cathepsin B and D are involved, but not essential for MHC II-mediated Ag presentation [47]. Cathepsin X, which is similar to cathepsin B, has been shown to mediate the function of β2-integrin receptors associated with cell adhesion during T cell activation [24] (fig. 2B). Loss or inhibition of cathepsin E has been reported also to compromise processing and presentation of antigens such as ovalbumin, myoglobin and tetanus toxin [54, 55]. Importantly, endo-lysosomal proteases can also destroy and generate T cell epitopes. They can attenuate protease activity, as for example in newly formed phagosomes, by raising phagosomal pH, which protects antigen for subsequent cross-presentation on class MHC I molecules [56]. DCs express reduced levels of endo-lysosomal proteases compared to macrophages, which appears to improve rather than impede their performance as APCs [57], suggesting that destructive processes could not only limit immunogenicity but may also compromise tolerance to self-proteins [58, 59]. Finally, endo-lysosomal proteases found in APCs may be complemented
60].
Relationships between cytokines and cathepsins
Certain cytokines regulate lysosomal protease activity. For instance, TNF-alpha and IL-1beta have been shown to increase the activity of cathepsin S and B in human DCs, leading to increased class II MHC dimer formation and T cell recognition [61] (fig. 2A). IL-6 and IL-10 also modulate protease activity, at least in part, by altering endosomal pH [61, 62]. Another study showed that IL-10, which was produced by bacillus Calmette-Guérin (BCG)-infected macrophages, reduced cathepsin S expression levels, overriding the ability of interferon (IFN)-gamma to enhance class II MHC levels and promote T cell recognition of infected cells [63]. In contrast, IL-10 and IL-6 were also shown to enhance the activity of various cathepsins, including cathepsin S [63] improving presentation and recognition of T cell epitopes in IL-6 treated DCs.
Activation of granule serine proteases
In NK cells and T lymphocytes, cathepsin C is involved in the activation of progranzymes, such as granzymes A and B, into proteolytically active enzymes. Granzymes A and B are neutral serine proteases expressed in the granules of NK cells and activated cytotoxic T lymphocytes (CTLs, also called CD8+ T cells), where they are involved in cytotoxic-lymphocyte-granule-mediated and perforindependent apoptosis of target cells [64]. Defects in cytotoxic T cell function have been reported in cathepsin C-deficient mice [64]. However, in the absence of cathepsin C, residual granzyme B, but not granzyme A, activity in cytotoxic T cells was observed and was sufficient to control some viral
infections, pointing to possible alternative mechanisms of granzyme activation [65]. Cathepsin W, also known as lymphopain, is selectively expressed in the endoplasmic reticulum (ER) and Golgi apparatus compartments of CD8+ T and NK cells, suggesting a function in cytotoxicity. However, CTLs from cathepsin W-deficient mice have shown that cathepsin W is not required for cytotoxic lymphocyte-induced target cell death [66]. The immunological roles of cathepsin W, however, require additional studies.
Lysosome-mediated apoptosis
Apoptosis in immune cells occurs as a mechanism to control appropriate or inappropriate immune responses. In a model of high dose tolerance during incubation of T cells with anti-thymocyte globulins (ATG), a rapid reduction in lymphocyte proliferation and an increase in the number of apoptotic cells was dependent on cytosolic cathepsin B activity [67, 68] (fig. 2C). Similarly, rapid apoptosis induction was mediated by cathepsins B and
L in CD4+ or CD8+ T lymphocytes incubated with high Ag concentrations via CD95/CD178 (Fas/FasL) interactions [69] or TNF receptor II/TNF interactions [70]. Germinal B cells are subject to cathepsin-mediated apoptosis through their antigen receptors [71]. In contrast, interactions with follicular DCs and CD40 ligation were shown to rescue B cells from LMP and apoptosis by impeding the release of lysosomal enzymes into cytosol [72]. At the end of an immune response, T cell contraction also results in massive apoptosis with
a small subset of highly specific memory cells surviving [40]. This process could be influenced by Spi2A, a cytosolic cysteine protease inhibitor recently shown to be upregulated in memory CD8+ T cells and to suppress cytosolic cathepsin B activity. Intracellular pathogens can also provoke cathepsin-mediated apoptosis. Indeed, CD4+ T cells harbouring HIV are susceptible to apoptosis via a mechanism involving the virally encoded protein Nef and release of lysosomal cysteine and aspartyl cathepsins B and D respectively [73]. Nevertheless, the mechanism of cathepsin release, by Nef or other stimuli, remains to be clarified.
Cathepsins and the pathogenesis of diseases
Due to altered expression (e.g. upregulation in cancer and metastasis progression), proteolytic activity (e.g. unbalanced amount between proteases and their endogenous inhibitors) and localisation (e.g. increased secretion outside the cells), deregulated cathepsins activity is thought to be a cause or contributing factor of diseases such as cancer, bronchial asthma, atherosclerosis, Alzheimer’s disease, rheumatoid arthritis (RA), and osteoarthritis.
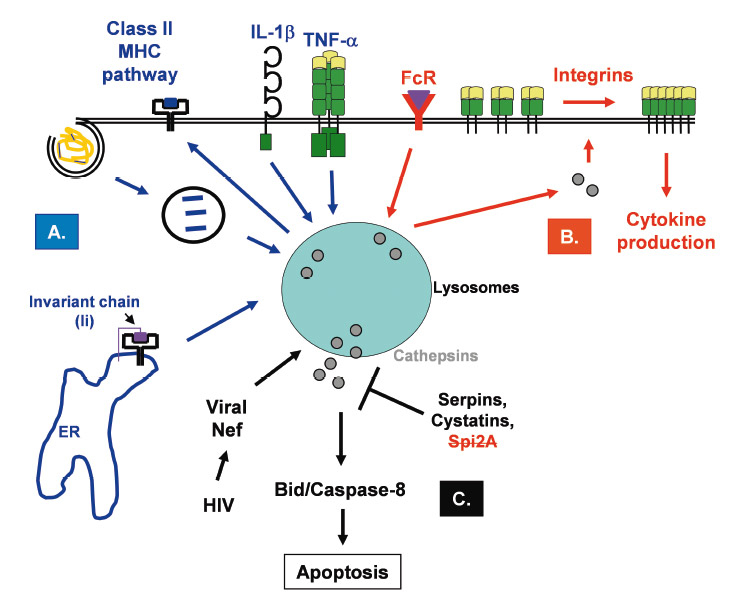
Figure 2
Roles of cathepsins in adaptive immunity. A. Lysosomal proteases remove the invariant chain (li) chaperone and introduce cleavages in endocytosed antigens which will consequently trigger unfolding and capture of processed antigen by newly synthesised class II MHC molecules. Moreover, cytokines (e.g. IL-1β, TNF-α) can modulate cathepsin activity and influence the class II MHC pathway. B. Activation of immune cells, for example following Fc receptors (FcR) ligation by immune complexes, induces conformational changes to surface receptors, such as β-integrins, increasing integrin affinity for its ligands. By an unexplained mechanism, cathepsins access the cytosol and target integrins at the plasma membrane. Both rearrangement of their cytoskeleton and ligand binding result in clustering of these molecules, thereby increasing cell-to-cell contact or adhesion to extracellular matrices. C. Following induction of apoptosis by different stimuli, such as the viral protein Nef, lysosomal cathepsins are released into the cytosol. Once in the cytosol, these proteases will induce the proteolysis of different targets (e.g. caspase-8, Bid, and anti-apoptotic Bcl-2 family members) and activate the enzymatic signalling cascade of apoptosis. In the cytosol, the cathepsin activity can be kept in check by the cysteine protease inhibitors, serpins and cystatins.
Cathepsin S is considered to be one of the prime targets for various immune system-related diseases. Cathepsin S secreted from tissue macrophages is involved in extracellular matrix (ECM) degradation, thereby contributing to the pathology of diseases such as atherosclerosis, arthritis, bronchial asthma and chronic obstructive pulmonary disease (COPD) [74, 75]. Chemical or genetic ablation of cathepsin S attenuates Th1-driven autoimmune disease in different mouse models (reviewed in [47]). Moreover, cathepsin S-deficient mice exhibit significantly diminished susceptibility to collagen-induced arthritis [48]. Pharmacological inhibition of cathepsin S by inhibitors such as N-morpholinurea-leucine-homophenylalanine-vinyl-sulfonephenol resulted in impaired li processing and Ag presentation [76]. At least one cathepsin S inhibitor is in clinical trials for the treatment of psoriasis (e.g. Celera was the first company to announce the entry into phase I clinical trials of an inhibitor of cathepsin S), [21] confirming that cathepsin S is a potential therapeutic target.
Cathepsin L-deficient mice show defects in skin and hair development [77, 78] and cardiac alterations [79]. Cathepsin L has also been associated with abdominal aortic aneurysm [80], atherosclerosis [81], neuronal cell death [82] and osteoclastic bone degradation [83]. Interestingly, cathepsin E-deficient mice are susceptible to development of atopic dermatitis (AD) due to an accumulation of both immunoglobulin (Ig)E and Th2 polarising cytokines. In humans AD was also linked to low levels of cathepsin E [84]. Moreover, cathepsin E-deficient mast cells accumulate the proform of carboxypeptidase A, which could be relevant for the development of dermatitis [85].
Cathepsin K is abundantly and selectively expressed in osteoclasts, where it is involved in ECM degradation and bone remodelling. Hence cathepsin K plays a critical role in the initiation and/or progression of RA, in which it has been found, in addition to cathepsins S, B and L, to be elevated in synovial fluids and the lining tissue inside arthritic joints [74, 86]. Apart from RA, cathepsin K plays a role in other pathological diseases involving bone and cartilage turnover, such as osteoporosis, osteoarthritis, osteopetrosis and pycnodysostosis [24]. Consequently, cathepsin K inhibitors are in clinical trials for the treatment of osteoporosis and RA [14, 87].
Cathepsin D is a ubiquitously expressed lysosomal protease which has been shown to be involved in proteolytic degradation, cell invasion and apoptosis. In humans, as well as in mice and sheep, cathepsin D deficiency is known to cause congenital neuronal ceroid-lipofuscinosis (NCL), a devastating inherited neurodegenerative disorder of unknown metabolic basis [88, 89]. Cathepsin D, as well as cathepsin B, has been shown to be involved in the pathology of Alzheimer’s disease [90, 91]. In addition, cathepsins B and D were previously reported to play a role in innate immune responses and inflammation [29, 30]. Interestingly, cathepsin C plays a critical role in neutrophil recruitment during the development of experimental abdominal aortic aneurysm, which is associated with chronic inflammation and destructive remodelling of aortic wall connective tissue [92]. Furthermore, cathepsin C-deficient mice are largely resistant to collagen-induced arthritis [93]. Finally, human insufficiency of cathepsin C, resulting in Papillon-Lefèvre syndrome, is characterised by periodontitis and skin infections [94].
Once secreted, cathepsins such as cathepsins B, D, L and S, are known to induce degradation of the extracellular matrix and to participate in tumour progression and metastasis. However, cancer cells show transformation-induced changes of the lysosomal compartment which, besides their pro-oncogenic effects, may sensitise cells to the lysosomal death pathway, thereby allowing cell death to occur even in cancer cells with multiple defects in the classical apoptosis signalling pathways [95, 96]. Lysosomes of tumour cells may therefore be targets for anti-tumour therapy employing agents able to induce LMP and provoke cathepsin-mediated cytotoxicity. In addition, silencing of naturally occurring cathepsin inhibitors, such as serpins and cystatins, which attenuate the cytosolic activity of cathepsins and hence LMP-driven apoptosis, may induce apoptosis and be consequently therapeutically useful in autoimmune lymphocyte populations and tumour cells [40].
In contrast, an increase in apoptosis may be pathogenic in the case of hereditary deficiencies in lysosomal enzymes. Indeed, loss-of-function mutations of cystatin B, a cytosolic inhibitor of lysosomal cysteine cathepsins, cause Unverricht-Lundborg syndrome, an autosomal recessive inherited form of epilepsy [97]. Similarly, cystatin B knockout mice exhibit signs of apoptosis affecting cerebellar granule cells [98]. Finally, ceramidase deficiency causes Farber’s disorder due to an accumulation of ceramide, a pro-apoptotic second messenger known to induce LMP and to inhibit the respiratory chain which in turn induces mitochondrial outer membrane permeabilisation (MOMP) and apoptosis [99]. Table 1 summarises the involvement of cathepsins in the pathogenesis of diseases.
Concluding remarks
Cathepsins represent a very important part of the immune system and need to be properly kept under control to avoid pathological damage to cells or tissues. Due to their emerging role in activation of innate and adaptive immune responses, which may result in diseases such as chronic inflammation and autoimmune disorders, cathepsins and their inhibitors constitute attractive therapeutic targets. In this context, further investigations regarding the mechanism(s) of cathepsin release from lysosomes and their cytosolic targets are required. Moreover, future clarification of the molecular mechanisms controlling lysosomal membrane stability under inflammatory conditions, as well as approaches to control the levels of endogenous cathepsin inhibitors, are central themes of current research. The information gleaned therefrom may help to formulate new therapeutic applications designed to either prevent or induce cathepsin release.
Correspondence to:
Sébastien Conus Ph.D.
Institute of Pharmacology
University of Bern
Friedbühlstrasse 49
3010 Bern
Switzerland
sebastien.conus@pki.unibe.ch
References
1 Conus S, Simon H-U. Cathepsins: key modulators of cell death and inflammatory responses. Biochem Pharmacol. 2008;76(11):1374–82.
2 Weiss SJ. Tissue destruction by neutrophils. N Engl J Med. 1989;320(6):365–77.
3 Savill J, Fadok V. Corpse clearance defines the meaning of cell death. Nature. 2000;407(6805):784–8.
4 Simon H-U. Neutrophil apoptosis pathways and their modifications in inflammation. Immunol Rev. 2003;193(1):101–10.
5 Lamkanfi M, Festjens N, Declercq W, Berghe TV, Vandenabeele P. Caspases in cell survival, proliferation and differentiation. Cell Death Differ. 2006;14(1):44–55.
6 Turk B, Turk D, Turk V. Lysosomal cysteine proteases: more than scavengers. Biochem Biophys Acta. 2000;1477(1-2):98–111.
7 Foghsgaard L, Wissing D, Mauch D, Lademann U, Bastholm L, Boes M, et al. Cathepsin B acts as a dominant execution protease in tumor cell apoptosis induced by tumor necrosis factor. J Cell Biol. 2001;153(5):999–1010.
8 Guicciardi ME, Miyoshi H, Bronk SF, Gores GJ. Cathepsin B knockout mice are resistant to tumor necrosis factor-{alpha}-mediated hepatocyte apoptosis and liver injury: implications for therapeutic applications. Am J Pathol. 2001;159(6):2045–54.
9 Salvesen GS. A lysosomal protease enters the death scene. J Clin Invest. 2001;107(1):21–3.
10 Roberg K, Kagedal K, Ollinger K. Microinjection of cathepsin D induces caspase-dependent apoptosis in fibroblasts. Am J Pathol. 2002;161(1):89–96.
11 Turk B, Stoka V, Rozman-Pungercar J, Cirman T, Droga-Mazovec G, Oresic K, et al. Apoptotic pathways: involvement of lysosomal proteases. Biol Chem. 2002;383:1035–44.
12 Lockshin RA, Zakeri Z. Caspase-independent cell death? Oncogene. 2004;23(16):2766–73.
13 Friedrichs B, Tepel C, Reinheckel T, Deussing J, von Figura K, Herzog V, et al. Thyroid functions of mouse cathepsins B, K, and L. J Clin Invest. 2003;111(11):1733–45.
14 Vasiljeva O, Reinheckel T, Peters C, Turk D, Turk V, Turk B. Emerging roles of cysteine cathepsins in disease and their potential as drug targets. Curr Pharm Des. 2007;13:387–403.
15 Holt OJ, Gallo F, Griffiths GM. Regulating secretory lysosomes. J Biochem. 2006;140(1):7–12.
16 Dell’Angelica EC, Mullis C, Caplan S, Bonifacino JS. Lysosome-related organelles. FASEB J. 2000;14(10):1265–78.
17 Blott EJ, Griffiths GM. Secretory lysosomes. Nat Rev Mol Cell Biol. 2002;3(2):122–31.
18 Turk V, Turk B, Turk D. Lysosomal cysteine proteases: facts and opportunities. EMBO J. 2001;20(17):4629–33.
19 Rossi A, Deveraux Q, Turk B, Sali A. Comprehensive search for cysteine cathepsins in the human genome. Biol Chem. 2004;385(5):363–72.
20 Barrett AJ, Rawlings ND, Woessner JF. Handbook of proteolytic enzymes. (New York, USA: Academic Press) 2004.
21 Zavasnik-Bergant T, Turk B. Cysteine cathepsins in the immune response. Tissue Antigens. 2006;67(5):349–55.
22 Turk B, Turk D, Salvesen GS. Regulating cysteine protease activity: essential role of protease inhibitors as guardians and regulators. Curr Pharm Des. 2002;8(18):1623–37.
23 Chwieralski CE, Welte T, Bühling F. Cathepsin-regulated apoptosis. Apoptosis. 2006;11(2):143–9.
24 Zavasnik-Bergant T, Turk B. Cysteine proteases: destruction ability versus immunomodulation capacity in immune cells. Biol Chem. 2007;388(11):1141–9.
25 Guicciardi ME, Deussing J, Miyoshi H, Bronk SF, Svingen PA, Peters C, et al. Cathepsin B contributes to TNF-alpha-mediated hepatocyte apoptosis by promoting mitochondrial release of cytochrome c. J Clin Invest. 2000;106(9):1127–37.
26 Stoka V, Turk B, Schendel SL, Kim TH, Cirman T, Snipas SJ, et al. Lysosomal protease pathways to apoptosis. Cleavage of Bid, not pro-caspases, is the most likely route. J Biol Chem. 2001;276(5):3149–57.
27 Li W, Yuan X, Nordgren G, Dalen H, Dubowchik GM, Firestone RA, et al. Induction of cell death by the lysosomotropic detergent MSDH. FEBS Lett. 2000;470(1):35–9.
28 Boya P, Andreau K, Poncet D, Zamzami N, Perfettini J-L, Metivier D, et al. Lysosomal membrane permeabilization induces cell death in a mitochondrion-dependent fashion. J Exp Med. 2003;197(10):1323–34.
29 Conus S, Perozzo R, Reinheckel T, Peters C, Scapozza L, Yousefi S, et al. Caspase-8 is activated by cathepsin D initiating neutrophil apoptosis during the resolution of inflammation. J Exp Med. 2008;205(3):685–98.
30 Blomgran R, Zheng L, Stendahl O. Cathepsin-cleaved Bid promotes apoptosis in human neutrophils via oxidative stress-induced lysosomal membrane permeabilization. J Leukoc Biol. 2007;81(5):1213–23.
31 Asagiri M, Hirai T, Kunigami T, Kamano S, Gober H-J, Okamoto K, et al. Cathepsin K-dependent Toll-like receptor 9 signalling revealed in experimental arthritis. Science. 2008;319(5863):624–7.
32 Matsumoto F, Saitoh S, Fukui R, Kobayashi T, Tanimura N, Konno K, et al. Cathepsins are required for Toll-like receptor 9 responses. Biochem Biophys Res Commun. 2008;367(3):693–9.
33 Ewald SE, Lee BL, Lau L, Wickliffe KE, Shi G-P, Chapman HA, et al. The ectodomain of Toll-like receptor 9 is cleaved to generate a functional receptor. Nature. 2008;456(7222):658–62.
34 Park B, Brinkmann MM, Spooner E, Lee CC, Kim Y-M, Ploegh HL. Proteolytic cleavage in an endolysosomal compartment is required for activation of Toll-like receptor 9. Nat Immunol. 2008;9(12):1407–414.
35 Padrines M, Wolf M, Walz A, Baggiolini M. Interleukin-8 processing by neutrophil elastase, cathepsin G and proteinase-3. FEBS Lett. 1994;352(2):231–5.
36 Bank U, Küpper B, Reinhold D, Hoffmann T, Ansorge S. Evidence for a crucial role of neutrophil-derived serine proteases in the inactivation of interleukin-6 at sites of inflammation. FEBS Lett. 1999;461(3):235–40.
37 Ohashi K, Naruto M, Nakaki T, Sano E. Identification of interleukin-8 converting enzyme as cathepsin L. Biochem Biophys Acta. 2003;1649(1):30–9.
38 Ha S-D, Martins A, Khazaie K, Han J, Chan BM, Kim SO. Cathepsin B is involved in the trafficking of TNF-{alpha}-containing vesicles to the plasma membrane in macrophages. J Immunol. 2008;181(1):690–7.
39 Meyer-Hoffert U. Neutrophil-derived serine proteases modulate innate immune responses. Front Biosci. 2009;14:3409–18.
40 Colbert JD, Matthews SP, Miller G, Watts C. Diverse regulatory roles for lysosomal proteases in the immune response. Eur J Immunol. 2009;39(11):2955-65.
41 Salvesen G, Enghild JJ. An unusual specificity in the activation of neutrophil serine proteinase zymogens. Biochemistry. 1990;29(22):5304–8.
42 Adkison AM, Raptis SZ, Kelley DG, Pham CT. Dipeptidyl peptidase I activates neutrophil-derived serine proteases and regulates the development of acute experimental arthritis. J Clin Invest. 2002;109(3):363–71.
43 Mallen-St. Clair J, Pham CT, Villalta SA, Caughey GH, Wolters PJ. Mast cell dipeptidyl peptidase I mediates survival from sepsis. J Clin Invest. 2004;113(4):628–34.
44 Methot N, Guay D, Rubin J, Ethier D, Ortega K, Wong S, et al. In vivo inhibition of serine protease processing requires a high fractional inhibition of cathepsin C. Mol Pharmacol. 2008;73(6):1857–65.
45 Honey K, Rudensky AY. Lysosomal cysteine proteases regulate antigen presentation. Nat Rev Immunol. 2003;3(6):472–82.
46 Villadangos JA, Schnorrer P, Wilson NS. Control of MHC class II antigen presentation in dendritic cells: a balance between creative and destructive forces. Immunol Rev. 2005;207(1):191–205.
47 Chapman HA. Endosomal proteases in antigen presentation. Curr Opin Immunol. 2006;18(1):78–84.
48 Nakagawa TY, Brissette WH, Lira PD, Griffiths RJ, Petrushova N, Stock J, et al. Impaired invariant chain degradation and antigen presentation and diminished collagen-induced arthritis in cathepsin S null mice. Immunity. 1999;10(2):207–17.
49 Shi G-P, Villadangos JA, Dranoff G, Small C, Gu L, Haley KJ, et al. Cathepsin S required for normal MHC Class II peptide loading and germinal center development. Immunity. 1999;10(2):197–206.
50 Reich M, van Swieten PF, Sommandas V, Kraus M, Fischer R, Weber E, et al. Endocytosis targets exogenous material selectively to cathepsin S in live human dendritic cells, while cell-penetrating peptides mediate nonselective transport to cysteine cathepsins. J Leukoc Biol. 2007;81(4):990–1001.
51 Driessen C, Bryant RA, Lennon-Dumenil A-M, Villadangos JA, Bryant PW, Shi G-P, et al. Cathepsin S controls the trafficking and maturation of MHC class II molecules in dendritic cells. J Cell Biol. 1999;147(4):775–90.
52 Nakagawa T, Roth W, Wong P, Nelson A, Farr A, Deussing J, et al. Cathepsin L: critical role in li degradation and CD4 T cell selection in the thymus. Science. 1998;280(5362):450–3.
53 Tolosa E, Li W, Yasuda Y, Wienhold W, Denzin LK, Lautwein A, et al. Cathepsin V is involved in the degradation of invariant chain in human thymus and is overexpressed in myasthenia gravis. J Clin Invest. 2003;112(4):517–26.
54 Kakehashi H, Nishioku T, Tsukuba T, Kadowaki T, Nakamura S, Yamamoto K. Differential regulation of the nature and functions of dendritic cells and macrophages by cathepsin E. J Immunol. 2007;179(9):5728–37.
55 Burster T, Reich M, Zaidi N, Voelter W, Boehm BO, Kalbacher H. Cathepsin E regulates the presentation of tetanus toxin C-fragment in PMA activated primary human B cells. Biochem Biophys Res Commun. 2008;377(4):1299–303.
56 Savina A, Jancic C, Hugues S, Guermonprez P, Vargas P, Moura IC, et al. NOX2 controls phagosomal pH to regulate antigen processing during crosspresentation by dendritic cells. Cell. 2006;126(1):205–18.
57 Delamarre L, Pack M, Chang H, Mellman I, Trombetta ES. Differential lysosomal proteolysis in antigen-presenting cells determines antigen fate. Science. 2005;307(5715):1630–4.
58 Zou J, Henderson L, Thomas V, Swan P, Turner AN, Phelps RG. Presentation of the goodpasture autoantigen requires proteolytic unlocking steps that destroy prominent T cell epitopes. J Am Soc Nephrol. 2007;18(3):771–9.
59 Manoury B, Mazzeo D, Fugger L, Viner N, Ponsford M, Streeter H, et al. Destructive processing by asparagine endopeptidase limits presentation of a dominant T cell epitope in MBP. Nat Immunol. 2002;3(2):169–74.
60 Burster T, Beck A, Tolosa E, Marin-Esteban V, Rotzschke O, Falk K, et al. Cathepsin G, and not the asparagine-specific endoprotease, controls the processing of myelin basic protein in lysosomes from human B lymphocytes. J Immunol. 2004;172(9):5495–503.
61 Fiebiger E, Meraner P, Weber E, Fang IF, Stingl G, Ploegh H, et al. Cytokines regulate proteolysis in major histocompatibility complex class II-dependent antigen presentation by dendritic cells. J Exp Med. 2001;193(8):881–92.
62 Drakesmith H, O’Neil D, Schneider SC, Binks M, Medd P, Sercarz E, et al. In vivo priming of T cells against cryptic determinants by dendritic cells exposed to interleukin 6 and native antigen. Proc Natl Acad Sci. USA 1998;95(25):14903–8.
63 Sendide K, Deghmane A-E, Pechkovsky D, Av-Gay Y, Talal A, Hmama Z. Mycobacterium bovis BCG attenuates surface expression of mature class II molecules through IL-10-dependent inhibition of cathepsin S. J Immunol. 2005;175(8):5324–32.
64 Pham CT, Ley TJ. Dipeptidyl peptidase I is required for the processing and activation of granzymes A and B in vivo. Proc Natl Acad Sci. USA 1999;96(15):8627–32.
65 Sutton VR, Waterhouse NJ, Browne KA, Sedelies K, Ciccone A, Anthony D, et al. Residual active granzyme B in cathepsin C-null lymphocytes is sufficient for perforin-dependent target cell apoptosis. J Cell Biol. 2007;176(4):425–33.
66 Ondr JK, Pham CT. Characterization of murine cathepsin W and its role in cell-mediated cytotoxicity. J Biol Chem. 2004;279(26):27525–33.
67 Michallet M-C, Saltel F, Preville X, Flacher M, Revillard J-P, Genestier L. Cathepsin B-dependent apoptosis triggered by antithymocyte globulins: a novel mechanism of T-cell depletion. Blood. 2003;102(10):3719–26.
68 Michallet M-C, Saltel F, Flacher M, Revillard J-P, Genestier L. Cathepsin-dependent apoptosis triggered by supraoptimal activation of T lymphocytes: a possible mechanism of high dose tolerance. J Immunol. 2004;172(9):5405–14.
69 Kishimoto H, Sprent J. Strong TCR ligation without costimulation causes rapid onset of Fas-dependent apoptosis of naive murine CD4+ T cells. J Immunol. 1999;163(4):1817–26.
70 Alexander-Miller MA, Leggatt GR, Sarin A, Berzofsky JA. Role of antigen, CD8, and cytotoxic T lymphocyte (CTL) avidity in high dose antigen induction of apoptosis of effector CTL. J Exp Med. 1996;184(2):485–92.
71 van Eijk M, de Groot C. Germinal center B cell apoptosis requires both caspase and cathepsin activity. J Immunol. 1999;163(5):2478–82.
72 van Nierop K, Muller FJ, Stap J, Van Noorden CJ, van Eijk M, de Groot C. Lysosomal destabilization contributes to apoptosis of germinal center B-lymphocytes. J Histochem Cytochem. 2006;54(12):1425–35.
73 Laforge M, Petit F, Estaquier J, Senik A. Commitment to apoptosis in CD4+ T lymphocytes productively infected with human immunodeficiency virus type 1 is initiated by lysosomal membrane permeabilization, itself induced by the isolated expression of the viral protein Nef. J Virol. 2007;81(20):11426–40.
74 Reddy VY, Zhang QY, Weiss SJ. Pericellular mobilization of the tissue-destructive cysteine proteases, cathepsin B, L and S, by human monocyte-derived macrophages. Proc Natl Acad Sci. USA 1995;92(9):3849–53.
75 Thurmond RL, Sun S, Karlsson L, Edwards JP. Cathepsin S inhibitors as novel immunomodulators. Curr Opin Investig Drugs. 2005;6(5):473–82.
76 Pierre P, Mellman I. Developmental regulation of invariant chain proteolysis controls MHC class II trafficking in mouse dendritic cells. Cell. 1998;93(7):1135–45.
77 Roth W, Deussing JA, Botchkarev VA, Pauly-Evers M, Saftig P, Hafner A, et al. Cathepsin L deficiency as molecular defect of furless: hyperproliferation of keratinocytes and pertubation of hair follicle cycling. FASEB J. 2000;14(13):2075–86.
78 Hagemann S, Günther T, Dennemärker J, Lohmüller T, Brömme D, Schüle R, et al. The human cysteine protease cathepsin V can compensate for murine cathepsin L in mouse epidermis and hair follicles. Eur J Cell Biol. 2004;83(11-12):775–80.
79 Stypmann J, Gläser K, Roth W, Tobin DJ, Petermann I, Matthias R, et al. Dilated cardiomyopathy in mice deficient for the lysosomal cysteine peptidase cathepsin L. Proc Natl Acad Sci. USA 2002;99(9):6234–9.
80 Liu J, Sukhova GK, Yang J-T, Sun J, Ma L, Ren A, et al. Cathepsin L expression and regulation in human abdominal aortic aneurysm, atherosclerosis, and vascular cells. Atherosclerosis. 2006;184(2):302–11.
81 Kitamoto S, Sukhova GK, Sun J, Yang M, Libby P, Love V, et al. Cathepsin L deficiency reduces diet-induced atherosclerosis in low-density lipoprotein receptor-knockout mice. Circulation. 2007;115(15):2065–75.
82 Sevenich L, Pennacchio LA, Peters C, Reinheckel T. Human cathepsin L rescues the neurodegeneration and lethality in cathepsin B/L double-deficient mice. Biol Chem. 2006;387(7):885–91.
83 Everts V, Korper W, Hoeben KA, Jansen ID, Bromme D, Cleutjens KB, et al. Osteoclastic bone degradation and the role of different cysteine proteinases and matrix metalloproteinases: differences between calvaria and long bone. J Bone Miner Res. 2006;21(9):1399–408.
84 Tsukuba T, Okamoto K, Okamoto Y, Yanagawa M, Kohmura K, Yasuda Y, et al. Association of cathepsin E deficiency with development of atopic dermatitis. J Biochem. 2003;134(6):
893–902.
85 Henningsson F, Yamamoto K, Saftig P, Reinheckel T, Peters C, Knight SD, et al. A role for cathepsin E in the processing of mast-cell carboxypeptidase A. J Cell Sci. 2005;118(9):2035–42.
86 Yasuda Y, Kaleta J, Brömme D. The role of cathepsins in osteoporosis and arthritis: rationale for the design of new therapeutics. Adv Drug Deliv Rev. 2005;57(7):973–93.
87 Grabowska U, Chambers TJ, Shiroo M. Recent development in cathepsin K inhibitor design. Curr Opin Drug Discov Devel. 2005;8(5):619–30.
88 Siintola E, Partanen S, Stromme P, Haapanen A, Haltia M, Maehlen J, et al. Cathepsin D deficiency underlies congenital human neuronal ceroid-lipofuscinosis. Brain. 2006;129(6):1438–45.
89 Steinfeld R, Reinhardt K, Schreiber K, Hillebrand M, Kraetzner R, Brück W, et al. Cathepsin D deficiency is associated with a human neurodegenerative disorder. Am J Hum Genet. 2006;78(6):988–98.
90 Mueller-Steiner S, Zhou Y, Arai H, Roberson ED, Sun B, Chen J, et al. Antiamyloidogenic and neuroprotective functions of cathepsin B: implications for Alzheimer’s disease. Neuron. 2006;51(6):703–14.
91 Benes P, Vetvicka V, Fusek M. Cathepsin D – many functions of one aspartic protease. Crit Rev Oncol Hematol. 2008;68(1):12–28.
92 Pagano MB, Bartoli MA, Ennis TL, Mao D, Simmons PM, Thompson RW, et al. Critical role of dipeptidyl peptidase I in neutrophil recruitment during the development of experimental abdominal aortic aneurysms. Proc Natl Acad Sci. 2007;104(8):2855–60.
93 Hu Y, Pham CT. Dipeptidyl peptidase I regulates the development of collagen-induced arthritis. Arthritis Rheum. 2005;52(8):2553–8.
94 Pham CT, Ivanovich JL, Raptis SZ, Zehnbauer B, Ley TJ. Papillon-Lefèvre syndrome: correlating the molecular, cellular, and clinical consequences of cathepsin C/dipeptidyl peptidase I deficiency in humans. J Immunol. 2004;173(12):7277–81.
95 Jäättelä M. Multiple cell death pathways as regulators of tumour initiation and progression. Oncogene. 2004;23(16):2746–56.
96 Kirkegaard T, Jäättelä M. Lysosomal involvement in cell death and cancer. Biochem Biophys Acta. 2009;1793(4):746–54.
97 Pennacchio LA, Lehesjoki AE, Stone NE, Willour VL, Virtaneva K, Miao J, et al. Mutations in the gene encoding cystatin B in progressive myoclonous epilepsy (EPM1). Science. 1996;271(5256):1731–4.
98 Pennacchio LA, Bouley DM, Higgins KM, Scott MP, Noebels JL, Myers RM. Progressive ataxia, myoclonic epilepsy and cerebellar apoptosis in cystatin B-deficient mice. Nat Genet. 1998;20(3):251–8.
99 Farina F, Cappello F, Todaro M, Bucchieri F, Peri G, Zummo G, et al. Involvement of caspase-3 and GD3 ganglioside in ceramide-induced apoptosis in Farber disease. J Histochem
Cytochem. 2000;48(1):57–62.

