Figure 1
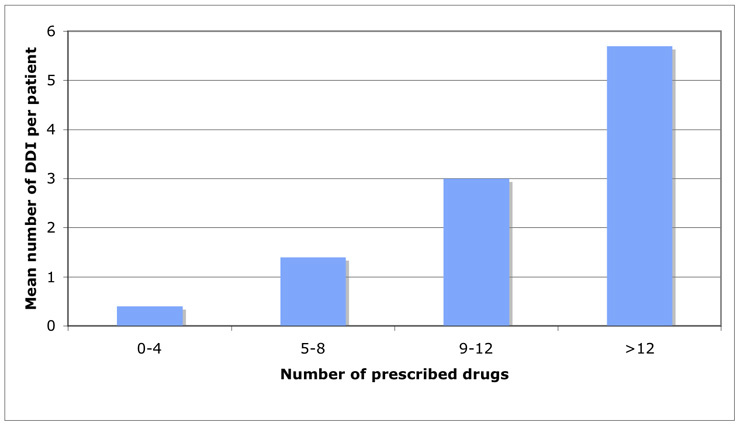
Correlation of number of prescribed drugs and number of drug-drug interactions.
DOI: https://doi.org/10.4414/smw.2010.13043
About 5% of all adverse drug reactions in hospitals are caused by drug-drug interactions (DDI), the majority of which are avoidable [1–2]. Up to 10% of all hospitalised patients have at least one adverse drug reaction after discharge [3–5]. Change of medication, addition of new drugs during a hospital stay and a lack of therapeutic or nursing care after discharge are among the most important risk factors for drug related problems. Some studies show that 40–70% of patients at discharge have a potential adverse drug interaction combination [6–10]. For patients it is therefore of great importance that discharge medication has the lowest risk of potential DDI and that doctors are aware of possibly preventable, drug-related complications.
The aim of this prospective study was the evaluation of the prevalence of potential DDI and the assessment of their clinical relevance in a patient’s discharge medication in the medical wards of a community teaching hospital. Relevant clinical information was reported to the treating physicians in a written standardissed form before patient’s discharge.
The study was conducted in the medical wards of the Ospedale Regionale di Lugano, sede Civico between November 2007 and July 2008. Prescribed drugs from 200 patients at discharge were analysed for interactions using a commercially available software (Pharmavista®), which scored best in a recent study evaluating four different interaction programs [11]. Pharmavista® classifies interactions in 5 categories: major, moderate, minor, insignificant and exotic source. In this study, only the first three types (major, moderate, minor) of interactions were collected, the latter two were not considered (insignificant, exotic source). Major interactions are defined as interactions that may be life threatening, cause intoxication or permanent damage – the drug-drug combination should therefore be avoided. Moderate interactions are defined as those that frequently cause therapeutic complications – the drug combination is continued but with careful monitoring of the patient. Minor interactions are associated with an increase or a reduction of drug efficacy, especially in patients with risk factors. All the major, moderate and minor interactions identified by Pharmavista® were discussed by a team composed of two pharmacists and a physician with the support of other literature sources (e.g. Stockley’s Interaction Alerts®, Lexi-Interact®, Micromedex® Drug-Interactions). Consequently, some of the interactions were considered to be of very low or no clinical relevance and were not taken into consideration. For example, a physician can prescribe a combination of two antihypertensive drugs, e.g. an ACE-inhibitor and a diuretic, to better control blood pressure. Even if such a combination is associated with the risk of arterial hypotension, from a clinical point of view it is not useful to warn the physician of this potential DDI.
Information on medications was collected up to 24 hours before patients’ discharge. After having screened all therapy drugs, by means of the software (Pharmavista®), a recommendation was formulated for DDI considered of potential clinical relevance.
The written recommendation was given to the physician as rapid feedback before discharge, providing information about the potential clinical consequence, the expected adverse drug reactions, the degree of severity and how to manage the (potential) DDI. The physicians were asked to forward the feedback information to the general practitioners with the referral letter. Only for DDI of major severity were the physicians asked for feedback about the acceptance of the recommendation and whether the alert led to any therapeutic consequence (e.g. monitoring or change of a specific drug). For the DDI of moderate and minor severity, the outcome of the provided recommendation was not evaluated.
Data including age, sex, medication at discharge, detected DDI, and degree of severity, were entered in a Microsoft Office Access database (Microsoft Corporation), which was specifically developed for the purpose of the study. Descriptive statistics included mean, minimum, maximum, range, 95% confidence intervals (95% CI) for continuous data (age, number of prescribed drugs) and frequencies for ordinal and nominal data.
Differences in number of interactions between males and females, as well as between age classes (≤65 years vs. >65 years) were tested for statistical significance using a two-tailed t-test, assuming non-equal variances. Statistical analysis was conducted using the statistical programme SPSS® (SPSS Inc., Chicago, Illinois).
In this prospective study we evaluated the potential DDI among prescribed drugs in 200 patients at hospital discharge. The median age of the patients was 69 years (range 54–85); 53% were women (n = 107) and 47% (n = 93) were men.

Figure 1
Correlation of number of prescribed drugs and number of drug-drug interactions.
Among all patients, 62.5% (n = 125) had at least one potential DDI, whereas 37.5% (n = 75) had no potential DDI.
In total, 373 potential DDI were identified, corresponding to 1.9 potential DDI per patient (range 0–13). From these 373 potential DDI, 60% (n = 223) were of minor severity, 38% (n = 143) of moderate severity and 2% (n = 7) of major severity (table 1). Two (antiarrhythmic – antibiotics and antiarrhythmic – neuroleptics) out of the seven DDI with the highest severity score were identified and led to a modification of the drug prescriptions (withdrawal or replacement of a drug), whereas the other five did not result in a modification of the therapy, but in close monitoring of the patient.
73% (n = 272) of the overall detected interactions were considered clinically relevant and resulted in a written warning for the treating physician. 100% (n = 7/7) of major interactions, 88% (n = 126/143) of moderate interactions and 62% (n = 139/223) of minor interactions resulted in a warning.
Compared to the younger patients (≤65 years), older patients (>65 years) had an increased number of minor DDI (0.48 vs. 1.4 DDI/patient) and moderate DDI (0.38 vs 0.87 DDI/patient); DDI of major severity were detected only in the older patient group. However differences between age groups were not statistically significant (t = 0.524 m, p = 0.601, 2-tailed). No statistical differences were seen in the number of interactions between males (n = 1.7 DDI/patient) and females (n = 2.1 DDI/patient) (t = –0–581, p = 0.562, 2-tailed).
Patients had, on average, 7.1 different prescribed drugs (range 1–20); older patients (>65 years) had, on average, a higher number of prescribed drugs (n = 7.1) than younger ones (n = 5.6). The total number of DDI/patient increased with the number of prescribed drugs: 0.4 DDI/patient in the patient group with 0–4 prescribed drugs, 1.4 DDI/patient in the 5–8 drugs group, 3.0 DDI/patient in the 9–12 drugs group, 5.7 DDI/patient in the >12 drugs group (fig. 1).
Oral anticoagulants were the most frequently implicated drugs with 13.5% of all DDI, followed by beta-blockers (8.8%), antiplatelet drugs (7.3%), diuretics (6.8%), ACE-inhibitors (6.6%) and antidiabetic agents (6.2%). Table 2 summarises in detail the most frequent DDI.
| Table 1Detected DDI of major severity. | ||
| Major severity DDI | Number of patients with major interactions | Potential adverse effect |
| Beta-Sympathomimetic - non cardioselective beta-blockersalbutamol – carvedilolsalbutamol – sotalol | 21 | Beta-blockers may reduce the therapeutic effect of beta2-Agonists, risk of asthma. Particularly relevant with nonselective beta-blockers. |
| K+ salt - K+ sparing diureticsKCl – spironolactone | 2 | Potassium salts may enhance the hyperkalaemic effect of potassium-sparing diuretics, risk of hyperkalaemia. |
| Antiarrhythmic – Antibioticssotalol – moxifloxacin | 1 | QTc-prolonging agents may enhance the adverse/toxic effect of other QTc-prolonging agents. The effect can be additive, leading to a torsade de pointes. |
| Antiarrhythmic – Neurolepticsotalol – quetiapine | 1 | QTc-prolonging agents may enhance the adverse/toxic effect of other QTc-prolonging agents. The effect can be additive, leading to Torsade de pointes. |
| Table 2Most prevalent drug-drug interactions (DDI). | ||
| Type of interaction | Number of patients with a specific DDI | Potential adverse effect |
| Antidiabetic agent – ACE-inhibitor | 20 | Hypoglycaemia, if necessary adjust the dosage of the antidiabetic drug. |
| ACE-inhibitor – Potassium wasting diuretic | 19 | Diuretics may enhance the hypotensive effect of ACE inhibitors. Especially postural hypotension at the beginning of the therapy (first dose hypotension). |
| Oral anticoagulant – Proton pump blocker | 18 | Proton pump inhibitors may enhance the anticoagulant effect of vitamin-K antagonists. |
| Oral anticoagulant – Statin | 17 | HMG-CoA reductase inhibitors may enhance the anticoagulant effect of vitamin-K antagonists. |
| Oral anticoagulant – Low dose salicylate | 15 | Salicylates may enhance the anticoagulant effect of vitamin-K antagonists, risk of bleeding. |
| Anticoagulant – Selective serotonin reuptake inhibitors | 15 | Selective serotonin reuptake inhibitors may enhance the anticoagulant effect of vitamin-K antagonists. Additive effects, risk of bleeding. |
| Potassium diuretic – Corticosteroid | 13 | Corticosteroids may enhance the hypokalaemic effect of loop diuretics, risk of hypokalaemia. |
| Beta-sympathomimetic – Cardioselective beta-blocker | 12 | Beta-blockers may decrease the therapeutic effect of beta2-agonists, risk of asthma. Cardioselective beta-blockers have a lower potential for significant bronchoconstriction, particularly at lower doses. |
Our study highlights the high prevalence (62.5% of patients) of potential harmful drug-combinations prescribed at hospital discharge; each patient had on average 1.9 DDI (all types of severity included). These findings confirm the results of similar previous studies, in which potential DDI were found in 40–70% of patients [6–10].
In our study population, the number of moderate or major DDI was 0.75 per patient. This is slightly higher than results shown in previous similar studies, which reported a prevalence between 0.49 and 0.60 DDI per patient [11, 13]. This difference cannot just be explained by the number of prescribed drugs (which were the same in our and the comparator studies) and may therefore have arisen by chance due to the small sample size. However, this finding emphasises the need for a careful physician’s review of prescribed drug combinations at hospital discharge.
40% of all detected DDI were considered of major or moderate severity, whereas 60% were considered to be of minor severity. Despite this, the majority of all the detected DDI (73%) were considered to be clinically relevant and resulted in a written recommendation for the prescribing physician (100% of the major DDI, 88% of moderate DDI and 62% of minor DDI).
3.5% of the patients included in our study had a drug combination with potential for a DDI of major clinical relevance. This prevalence is lower that the 8.8% found by Egger et al., a study which had a larger patient population and was conducted in a different hospital setting (University hospital) [6].
Our data suggest a positive correlation between the number of prescribed drugs as well as increasing patient age and number of potential DDI, similarly to a previous study showing that both variables are indeed two of the major, if not the most important, risk factors for DDI [6]. This finding is however limited by the lack of stratification by age and number of prescribed drugs in our study.
As already shown by other similar studies [14], oral anticoagulants were the most frequently involved drugs among all detected DDI and were implicated in 13.5% of all cases. These potential DDI were almost all of moderate degree and involved either a pharmacokinetic mechanism, leading to decreased hepatic metabolism and therefore to a higher concentration of oral anticoagulants, or a pharmacodynamic additive effect. This kind of risk can be managed and requires appropriate prescribing, monitoring, and patient education.
Interestingly, among all the major interactions, only two lead to a therapeutic change (antiarrhythmic – antibiotics and antiarrhythmic – neuroleptics); whereas in the other five cases (beta-sympathomimetic – non-cardioselective beta-blocker and potassium salt – potassium sparing diuretics) a close monitoring of the patient was considered sufficient from a clinical point of view, without the need of changing the established therapy. This fact, already shown in previous studies [13], underscores the importance of a comprehensive clinical evaluation of a given DDI.
The clinical management of potential DDI (not only those detected in this study) generally implies monitoring of symptoms related to a possible side effect and laboratory parameters, such as serum-creatinine, INR and blood-glucose, in order to prevent potentially serious adverse patient outcomes.
Our study was particularly useful for the development of a close collaboration between clinicians and the hospital pharmacists in a community teaching hospital. In modern medicine, complex therapeutic schemes with multiple drug combinations have become the rule. Therefore, the training of clinicians by clinical pharmacists in the evaluation of pharmacokinetic and pharmacodynamic interactions is of paramount importance. In contrast to many previous studies, limited to the epidemiology of potential DDI, we tried to go a step further by providing the treating physician with information that may lead to treatment modification or, at least, to specific patient monitoring in order to identify early potential harmful DDI.
We also acknowledge several limitations of our study. Firstly, the sample size was not large enough to detect significant differences in the subgroup analysis. Secondly, our study focused on potential DDI and did not address the question of how many of the detected potential DDI were known by the physician and if some of the patients were already under close clinical monitoring. We are also not aware of all the reported DDI that eventually led to a treatment modification or to clinically relevant consequences in a given patient, except for those of major severity. In order to quantify the impact of the detected DDI, which was also dependent on specific patient related risk factors, it would be necessary to design a prospective study, including a follow-up phase after discharge [6–12]. Finally, the software program used for the detection of DDI (Pharmavista®), did not take into consideration the overall clinical state of the individual patients, thus leading to a possible overestimation of the risk for potential manifestations of DDI [6].
The systematic review of therapy at discharge using the software Pharmavista® provides identification of a significant number of potential DDI, the majority of which can be reported as written recommendations to the physician before patient’s discharge. Drug interaction programs for the detection of DDI, combined with pharmacological expertise, as well as the knowledge of important patient-related risk factors, may be valuable for decreasing the number of potentially harmful drug-combinations, and therefore contribute to an increase in patient safety. A close collaboration between treating physicians and clinical pharmacists can help to prevent and manage the risks related to drug therapy. However, in order to appraise the real relevance of such pharmacological expertise, it is necessary to monitor the impact of every given recommendation on each patient.
The authors wish to express thanks to the personnel of the involved medical ward for collaborating to this study and to Dr. Orlando Petrini for the statistical analysis.
1 Fijn R, et al. Hospital prescribing errors: epidemiological assessment of predictors. Br J Clin Pharmacol. 2002;53(3):326–31.
2 Classen DC, et al. Adverse drug events in hospitalized patients: excess length of stay, extra cost, and attributable mortality. JAMA. 1997;277(4):301–6.
3 Forster AJ, et al. Adverse drug events occurring following hospital discharge. J Gen Intern Med. 2005;20:317–23.
4 Forster AJ, et al. Adverse events affecting medical patients following discharge from hospital CMAJ. 2004;170(3):345–9.
5 Forster AJ. Can you prevent adverse drug events after hospital discharge? CMAJ. 2006;174(7):921–2.
6 Egger S, et al. Potential drug-drug interactions in the medication of medical patients at hospital discharge. Eur J Clin Pharmacol. 2003;58:773–8.
7 Di Castri A, et al. Interactions médicamenteuses: études de 409 ordonnances établies a l’issue d’une hospitalisation gériatrique. Therapie. 1995;50:259–64.
8 Koehler GI, et al. Drug-drug interactions in medical patients: effects of in-hospital treatment and relation to multiple drug use. Int J Clin Pharmacol Ther. 2000;38:504–13.
9 Bonetti PO, et al. Potentielle Arzneimittelinteraktionen und Verordnungshaufigkeit von Medikamenten mit speziellem Instruktionsbedarf bei Spitalaustritt. Schweiz Rundsch Med Prax. 2000;89:182–9.
10 Kruse W, et al. Potentielle Medikamentenwechselwirkungen in der Behandlung multimorbider Hochbetagter. Z Gerontol. 1988;21:164–8.
11 Vonbach P, et al. Evaluation of frequently used drug interaction screening programs, Pharm World Sci. 2008;30:367–74.
12 Jankel CA, et al. Epidemiology of drug-drug interactions as a cause of hospital admissions. Drug Saf. 1993;9:51–9.
13 Vonbach P, et al. Recognition and management of potential drug-drug interactions in patients on internal medicine wards, Eur J Clin Pharmacol. 2007;63:1075–83.
14 Vàzquez L, et al. Drug interactions in the prescription of medical patients at hospital discharge. An Med Interna. 2005;22:69–75.
15 Straubhaar B, et al. The prevalence of potential drug-drug interactions in patients with heart failure at hospital discharge. Drug Safety. 2006;29(1):79–90.
The authors declare no financial or commercial conflict of interest.